Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Risk Factors for Subclinical Diabetic Peripheral Neuropathy in Type 2 Diabetes Mellitus
Authors Gao L, Qin J, Chen Y, Jiang W , Zhu D , Zhou X, Ding J, Qiu H, Zhou Y, Dong Q, Guan Y
Received 1 September 2023
Accepted for publication 21 December 2023
Published 25 January 2024 Volume 2024:17 Pages 417—426
DOI https://doi.org/10.2147/DMSO.S433024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Li Gao,1,* Jiexing Qin,1,* Ying Chen,1 Wenqun Jiang,2 Desheng Zhu,1 Xiajun Zhou,1 Jie Ding,1 Huiying Qiu,1 Yan Zhou,1 Qing Dong,1 Yangtai Guan1
1Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Laboratory Medicine, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Dong; Yangtai Guan, Department of Neurology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To investigate the risk factors associated with subclinical diabetic peripheral neuropathy (sDPN) in patients with type 2 diabetes mellitus (T2DM).
Patients and Methods: This cross-sectional, retrospective study involved 311 patients with T2DM who were successively admitted from January 2018 to December 2021 without any neurological symptoms. All participants underwent a nerve conduction study (NCS), and those asymptomatic patients with abnormal nerve conduction were diagnosed with sDPN. Differences between groups were evaluated by the chi-squared, Wilcoxon, or Fisher’s exact test. Binary logistic regression analysis was performed to determine the independent risk factors for sDPN. Receiver operating characteristic (ROC) curves were constructed, and the areas under curves (AUCs) were detected.
Results: Among 311 asymptomatic patients with T2DM, 142 (45.7%) with abnormal nerve conduction were diagnosed with sDPN. Patients with sDPN significantly differed from those without diabetic peripheral neuropathy (DPN) in age, history of hypertension, duration of diabetes, anemia, neutrophil-to-lymphocyte ratio, fasting C-peptide level, serum creatinine level, and albuminuria (all p< 0.05). Furthermore, the duration of diabetes (odds ratio [OR]: 1.062, 95% confidence interval [CI]: 1.016– 1.110), fasting C-peptide level (OR: 2.427, 95% CI: 1.126– 5.231), and presence of albuminuria (OR: 2.481, 95% CI: 1.406– 4.380) were independently associated with the development of sDPN (all p< 0.05). The AUCs for fasting C-peptide level, duration of diabetes, and the two factors combined were 0.6229 (95% CI: 0.5603– 0.6855, p=0.0002), 0.6738 (95% CI: 0.6142– 0.7333, p< 0.0001), and 0.6808 (95% CI: 0.6212– 0.7404, p< 0.0001), respectively.
Conclusion: For patients with T2DM and longer duration of diabetes, lower fasting C-peptide levels, and presence with albuminuria, the risk for developing DPN is higher even if they have no clinical signs or symptoms. Identifying potential risk factors for the development of sDPN and effectively controlling them early are critical for the successful management of DPN.
Keywords: type 2 diabetes mellitus, subclinical diabetic peripheral neuropathy, nerve conduction study, fasting C-peptide, albuminuria, risk factor
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most prevalent and disabling complications of diabetes mellitus (DM), which occurs in more than half of affected individuals.1 Chronic hyperglycemia and various pathophysiological changes have been reported to cause damage to the nervous system.2 The most common symptoms of DPN are limb pain and numbness, which eventually lead to foot ulcer, gangrene, and amputation, causing great pain to patients and leading to long-term disability. However, early manifestations of DPN are often overlooked until it is well established, at which point it is often irreversible.
Subclinical DPN (sDPN) refers to the existence of neurological abnormalities in a nerve conduction study (NCS) or measurement of small fiber neuropathies, but without any neurological symptoms or signs.3 Some previous studies reported that more than 25% patients with T2DM may develop DPN, although up to half of them may remain asymptomatic.4,5 Clinically, the diagnosis of DPN often depends on the presence of patient-reported symptoms and physical signs, thus delaying the detection of sDPN. However, the use of NCS can increase the diagnostic yield of sDPN, from one-third at baseline to nearly two-thirds during the follow-up of 5 years.6 Therefore, identifying sDPN at an early stage and detecting the predisposing factors for its development may slow, stop, or even reverse the progression of DPN and avoid the occurrence of DPN-related morbidity and complications.
Some recent studies have addressed that multiple risk factors such as age, disease duration, poor glycemic control, hyperlipidemia, and glycosylated hemoglobin A1c (HbA1c) level may affect the development and progression of DPN.7,8 However, the predictive risk factors for the development of sDPN have not been clearly elucidated yet. Thus, identifying the potential risk factors for the development of sDPN and effectively controlling them at an early stage are critical for the successful management of DPN. In this study, we intend to evaluate the prevalence of sDPN and to identify the risk factors for sDPN in asymptomatic patients with T2DM, which may provide critical information on the spectrum of risk factors of DPN, as well as to help in risk stratification and develop personalized approaches to DPN prevention.
Materials and Methods
Patients
The study was carried out at Shanghai Ren Ji Hospital, which is a major teaching hospital of the medical school of Shanghai Jiao Tong University. We recruited consecutive patients with T2DM admitted to the Department of Endocrinology between January 2018 and December 2021. This study was approved by the Ethics Committee of Biomedical Research of the Ren Ji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (KY2020-056). Written consent was waived because the data were anonymous and retrospective. The waiver would not adversely affect the rights and welfare of the participants as the study involved no more than minimal risk. The waiver of consent was approved by ethics committee. All clinical investigations were carried out in conformity with the principles outlined in the Helsinki Declaration.
The clinical, laboratory, and NCS records of all the included patients were retrospectively reviewed. To achieve data integrity, we included patients with NCS and complete blood count data, intending to identify potential risk factors for the development and progression of sDPN. T2DM was diagnosed according to criteria established by the American Diabetes Association in 2020.9 Patients were excluded if they were diagnosed with type 1 diabetes mellitus (T1DM), gestational diabetes, or other types of diabetes due to specific causes. Patients with peripheral neuropathy due to other causes such as (i) hematological diseases (including M-albuminemia), autoimmune disease (including dermatomyositis), or malignant tumor; (ii) diagnosed with systemic disease with advanced liver or renal disease; (iii) a history of cervical spondylosis or lumbar spondylosis; and (iv) nerve lesions caused by drugs (such as chemotherapeutic and antifungal drugs), trauma, toxins, or inherited neuropathy were also excluded from the study.
The participants were assessed on admission to detect the presence of diabetic neuropathy symptoms (DNSs). The DNS score is a four-item symptom score developed previously,10 comprising the symptoms of numbness, paresthesia, neuropathic pain, and unsteadiness in walking, which has high predictive value for the presence of peripheral neuropathy in diabetes. The presence of one symptom is scored as 1 point; the maximum score is 4 points, and a score of ≥1 is considered positive for DPN. Patients were specifically questioned about neuropathic symptoms based on their DNS scores and underwent clinical neurological examinations with standard protocols.
Data Collection
Demographic and Anthropometric Data
Demographic and anthropometric data, including age, sex, history of smoking or alcohol drinking, duration of diabetes, height, waist-to-hip ratio (WHR), body mass index (BMI), and past medical history of hypertension, were extracted from electronic medical records. According to the World Health Organization (WHO) criteria, a BMI of <18.5 kg/m2 was considered underweight, and that of ≥25 kg/m2 was considered overweight or obese. WHR was defined as the ratio of waist circumference to hip circumference according to the Geneva 2008 WHO Expert Consultation, with normal cut-off values of 0.9 in men and 0.8 in women. A history of hypertension was defined as a history of antihypertensive drug use before hospital admission.
Biochemical Data
Laboratory test data of participants were also taken from hospital records. Fasting blood samples were obtained from the overnight fasting state and were detected using standard hematological and biochemical tests, including those for measuring the hemoglobin (Hb), fasting plasma glucose (FPG), HbA1c, fasting C-peptide, triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine, blood urea nitrogen, uric acid, vitamin B12 (B12), and folate levels; white blood cell (WBC) counts; lymphocyte counts; and neutrophil counts and platelet indices. The neutrophil-to-lymphocyte ratio (NLR) was defined as the ratio of neutrophil counts to lymphocyte counts. The levels of FPG were determined using standard enzymatic methods. The concentrations of HbA1c were analyzed with high pressure-liquid chromatography, while lipid profiles and renal function indices were examined on an autoanalyzer with an enzymatic assay.
An early morning spot urine specimen was collected to assess the urinary albumin-to-creatinine ratio (UACR), which was measured by dividing the respective concentrations of urine albumin by urine creatinine during morning urine collection. The levels of urinary albumin and creatinine were measured by an immunoturbidimetric technique and the standard enzymatic method. A UACR of 30–299 mg/g was defined as microalbuminuria and that of ≥300 mg/g was defined as overt proteinuria or macroalbuminuria. In addition, anemia was defined as a Hb level of <13 g/dL for men and that of <12 g/dL for women according to WHO guidelines. In our laboratory, serum B12 (normal range: 180–914 pg/mL) and folate (normal range: 3.1–19.9 ng/mL) levels were measured with an automated immunoassay device. A low B12 level was defined as a concentration of <180 pg/mL, while a low folate level was defined as a concentration of <3.1 ng/mL. The normal reference range for fasting C-peptide levels is 0.78–5.19 ng/mL, and a low C-peptide level is defined as a concentration of <0.78 ng/mL. The normal reference ranges are ≤1.7 mmol/L for TG levels, 0.9–2.0 mmol/L for HDL levels, and <3.4 mmol/L for LDL levels.
Nerve Conduction Study
All patients underwent conventional NCS. Stimulation of the ulnar, median, tibial, and common peroneal motor nerves, and that of the ulnar, median, superficial peroneal, and sural sensory nerves in both limbs were conducted with a Keypoint 9031A070 electromyography machine (Keypoint 9031A070; Tonsbakken 16–18, DK-2740, Skovlunde, Denmark). The electrophysiological test was done by clinical neurophysiologists using standard techniques. The parameters of amplitudes, distal latencies, and conduction velocities were recorded. The normal reference values used are referred to a previous epidemiological survey conducted in the Chinese population.11 The threshold for slowed nerve conduction velocity (NCV) was set at <20% of the control NCV. DPN was diagnosed when patients showed relevant abnormalities in the NCS, according to the 2010 Toronto Expert Consensus.3 The environment of examination room should be kept quiet, and there were no interference sources in the examination room. The room temperature was kept at 18–25°C, and the local skin temperature was kept constant (28–30°C).
Statistical Analyses
All data analyses were performed with SPSS version 25 (IBM, Armonk, NY). Continuous variables were analyzed using the t- or Wilcoxon test according to the data distribution. Categorical variables were analyzed using the chi-squared or Fisher’s exact test. Numerical variables were expressed as medians with interquartile range (IQRs), and categorical variables as frequencies and percentages.
Binary logistic regression was conducted to investigate the associated independent factors with sDPN. Then, the baseline characteristics that appear to be associated with the prevalence of sDPN (indicated by p<0.2 on univariate analysis) were included in the multivariable model. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated to determine the ability of each independent factor contributing to sDPN. Receiver operating characteristic (ROC) curves were performed, and the areas under curves (AUCs) were detected. All statistical comparisons were two-sided. A p-value <0.05 was considered to be statistically significant.
Results
Baseline Characteristics of the Study Population
A total of 520 patients with T2DM underwent NCS, and 311 (193 male and 118 female individuals) were enrolled as their DNS scores were 0. The demographic and clinical characteristics of all participants are presented in Table 1. Among the 311 patients with a DNS score of 0, 142 (45.7%) were enrolled into the sDPN group based on the presence of abnormalities on the NCS, and 169 (54.3%) were enrolled into the control group as their NCS results were normal. The mean age of the 311 patients was 62.0 (54.0, 68.0) years, the mean duration of T2DM was 5.5 (0.4, 12.0) years, the mean body height was 165.0 (160.0, 173.0) cm, and the mean BMI was 24.9 (22.9, 27.1) kg/m2.
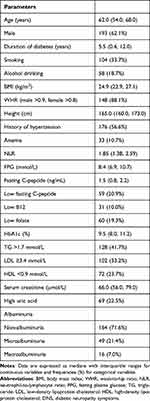 |
Table 1 Baseline Characteristics of Participants with a DNS Score of 0 (n=311) |
Of the 142 patients with sDPN, 77 (54.2%) had polyneuropathy, 53 (37.3%) had mononeuropathy (including injuries to the median nerve, ulnar nerve, and common peroneal nerve at the head of the fibula), and 20 (14.1%) had mononeuropathy multiplex according to electrophysiologic parameters.
Comparisons of Parameters Between the non-DPN and sDPN Groups
The demographic and clinical characteristics of participants in the non-DPN and sDPN groups are shown in Table 2. Patients with sDPN showed significant differences compared to those without DPN with respect to age, duration of diabetes, history of hypertension, anemia, NLR, fasting C-peptide level, serum creatinine level, and albuminuria. When compared with those in the non-DPN group, patients in the sDPN group were significantly older, had a longer duration of disease, and a higher prevalence of hypertension history. Moreover, higher NLR, lower fasting C-peptide level, higher serum creatinine level, higher albuminuria rate, and more severe anemia were also observed in patients with sDPN (all p<0.05).
 |
Table 2 Comparison of Parameters Between Patients with sDPN and those without DPN |
However, no significant differences were found regarding sex, alcohol drinking, smoking, WHR, BMI, height, or FPG, HbA1c, uric acid, B12, folate, TG, HDL, and LDL levels (all p>0.05).
Binary Logistic Regression Analysis of sDPN
To further determine the association of independent factors with sDPN, a binary logistic regression was conducted. As shown in Table 3, only those baseline characteristics that appeared to be associated with the prevalence of sDPN (indicated by p<0.2 on univariate analysis) were included into the multivariable model, comprising age, duration of diabetes, history of hypertension, NLR, anemia, LDL level, TG level, serum creatinine level, fasting C-peptide level, and albuminuria. We found that sDPN was significantly associated with the duration of diabetes (OR: 1.062, 95% CI: 1.016–1.110), fasting C-peptide level (OR: 2.427, 95% CI: 1.126–5.231), and presence of albuminuria (OR: 2.481, 95% CI: 1.406–4.380).
 |
Table 3 Binary Logistic Regression Analysis of sDPN |
Sensitivity, Specificity, and ROC Analysis
The effect of a low fasting C-peptide level and longer duration of diabetes on predicting the development of sDPN was analyzed using ROC curves (Figure 1). The AUCs for a low fasting C-peptide level, longer duration of diabetes, and the two factors combined were 0.6229 (95% CI: 0.5603–0.6855, p=0.0002), 0.6738 (95% CI: 0.6142–0.7333, p<0.0001), and 0.6808 (95% CI: 0.6212–0.7404, p<0.0001), respectively. The cut-off value for the fasting C-peptide level was 1.675 ng/mL, with a sensitivity of 66.9% and specificity of 56.2%. The cut-off value for the duration of diabetes was 2.5 years, with a sensitivity of 81.7% and specificity of 49.7%. Considering that albuminuria is a categorical variable, we did not perform ROC analysis.
 |
Figure 1 ROC curves obtained for the low fasting C-peptide level (a), duration of diabetes (b) and the two factors combined (c). Abbreviations: ROC, receiver operating curve; AUC, area under curve. |
Discussion
In this retrospective study, we assessed the presence of sDPN in 311 asymptomatic patients with T2DM using NCS and found that 142 (45.7%) had abnormal nerve conduction and were diagnosed with sDPN. Several different patterns of neuropathy were observed in our cohort, including polyneuropathy (54.2%), mononeuropathy (37.3%), and mononeuropathy multiplex (14.1%). Furthermore, we found that a longer duration of diabetes, a lower fasting C-peptide level, and the presence of albuminuria were independently associated with sDPN in patients with T2DM.
The epidemiology of sDPN in patients with diabetes remains poorly defined, and the prevalence of sDPN in patients with T2DM shows considerable variability in the literature. In 2017, Unmar et al12 reported that the prevalence of sDPN was 17.1% in 240 patients with T2DM and that this disease accounted for 33.1% of all DPN cases. In a cross-sectional study of Danish patients, Gylfadottir et al found that only 10 (2.5%) patients had sDPN in a cohort of 389 patients recently diagnosed with T2DM.13 In contrast, Bertora et al14 and Akbar et al15 found an sDPN prevalence rate of 57% in asymptomatic patients with T2DM; however, their samples consisted of only asymptomatic patients, which may account for the higher frequency of sDPN. Consistent with the findings of these two studies, 45.7% of the asymptomatic patients in our study presented abnormalities on NCS and were diagnosed with sDPN. The variation in the prevalence of sDPN in patients with T2DM may be attributed to different study populations, duration of disease, extent of glycemic control, variable methodologies applied to assess clinical neuropathy, and comorbidities determining the severity of neuropathy. Considering the high prevalence rate of sDPN in asymptomatic patients, NCSs are recommended for screening at regular intervals in clinical practice. If the sDPN can be detected earlier, the necessary measures can be taken to preclude its progression and decrease the related serious consequences.
NCS has been regarded as the most consistent method to detect DPN among studies conducted to evaluate the prevalence and patterns of neuropathy in patients with diabetes. Several different patterns of neuropathy have been reported to be present in patients with diabetes. Among these, the most common pattern is distal symmetric polyneuropathy, which presents with a “stocking and glove” distribution affecting the hands and lower limbs. In addition, sensory polyneuropathy is the most classical presentation of neuropathy in patients with diabetes. However, patterns of nerve injury in sDPN are seldom reported. In 2015, de Souza et al16 reported that individuals with presymptomatic diabetics had significantly slowed motor NCV than normal subjects. Toopchizadeh et al17 found that the most frequent electrophysiologic findings of sDPN were low sural amplitude, unobtainable H-reflex, and median sensory responses in children and adolescents with T1DM in Iran. In our study, different neuropathy patterns were also observed in patients with sDPN, including polyneuropathy, mononeuropathy, and mononeuropathy multiplex, suggesting diversity in sensory symptoms in patients with sDPN.
Longer duration of diabetes, higher HbA1c level, dyslipidemia, and existence of comorbidities, including hypertension, cardiovascular disease, and kidney disease are widely reported as risk factors of DPN.2,8,18 Some studies observed that NLR and hypoglycemic episodes showed significant independent associations with DPN in T2DM.19,20 However, the risk factors for sDPN are seldom studied and vary between studies. Unmar et al12 reported that sDPN was associated with age, height, HbA1c level, and presence of atherosclerosis and diabetic retinopathy. Another study showed that height, older age of onset, and poor metabolic control were related to the development of sDPN in children with newly diagnosed T1DM. Of those risk factors, sustained hyperglycemia and duration of diabetes are evidenced to be more critical factors over a 5-year follow-up.21 In this study, we evaluated parameters based on previous epidemiological data and the factors suggested to be associated with the underlying pathogenesis of DPN. Our findings indicate that patients with sDPN had older age, a longer duration of diabetes and greater prevalence of hypertension history than those without sDPN. Furthermore, higher NLR, lower fasting C-peptide level, higher serum creatinine level, higher albuminuria rate, and more severe anemia were observed in sDPN group. After adjusting for potential confounding factors, longer duration of diabetes, low fasting C-peptide level, and presence of microalbuminuria were proved to be independently associated with sDPN.
Older age and longer duration of diabetes have been previously confirmed as risk factors in studies into DPN and sDPN.12,22 Similarly, patients with sDPN in our study tended to be older and have a longer duration of diabetes than those without DPN, and disease duration was further proved to be an independent risk factor of sDPN. Advanced age and disease duration imply poor metabolic control and sustained damage to the peripheral nerves, which may account for the higher prevalence of neuropathy.
The pathophysiology of DPN is complex, which not only related to hyperglycemia but also involved the vascular and several other metabolic mechanisms, particularly in patients with T2DM. Metabolic factors such as abdominal obesity, hypertension, and dyslipidemia are consistently related to DPN in patients with T2DM and in selected T1DM cohorts.5 In our study, patients with sDPN had a greater prevalence of hypertension history, but the parameters of WHR, BMI, height, and uric acid, TG, HDL, and LDL levels were not significantly associated with sDPN. Furthermore, we did not observe a significant correlation between the HbA1c level and sDPN, as previously reported. One reason could be attributed to our small sample size. However, patients with sDPN are still in the early stage of disease, who may have good metabolic control and a short duration of diabetes.23 Chronic inflammatory response has been implicated as an underlying mechanism contributing to the progression of DPN as well. Inflammatory factors such as NLR have been reported to be a new risk factor of DPN in patients with T2DM.19 Accordingly, we also found that patients with sDPN had a higher NLR, further suggesting that DPN is an inflammation-related disease and that the inflammatory response continuously exists even in early-stage DPN.
C-peptide is an integral component of insulin biosynthesis, which is secreted together with insulin in equimolar amounts and has a substantially longer half-life. C-peptide has lots of protective effects, such as increasing expression of Na+-K+-ATPase, improving endoneurial blood flow, and stimulating some transcription factors exerting antiapoptotic, anti-inflammatory, and cytoprotective effects.24 The beneficial action of C-peptide on DPN in T1DM has been confirmed by different studies.24–26 However, the role of C-peptide is not well-defined in T2DM. Some previous studies demonstrated that C-peptide had a protective effect on DPN,27,28 while others have not proved such an effect29 or indeed observed a contrary result.30 There are currently no definite studies assessing the correlation between C-peptide and sDPN in T2DM. In this study, we found that a lower C-peptide lower is an independent risk factor for the development of sDPN, which may suggest a potentially beneficial role of C-peptide on DPN in T2DM. However, we could not conclude the causal relationship between serum C-peptide levels and sDPN in T2DM. Large prospective studies should be performed to determine the relationship between C-peptide and sDPN.
Diabetic nephropathy is a common microvascular complication of diabetes, and changes in proteinuria and urinary protein excretion have been widely used as indicators to evaluate the progression of renal disease. Early cohort studies have demonstrated that albuminuria reflects inflammatory states and acts as an independent predictor of DPN in patients with diabetes.31,32 In this study, we found that the UACR was independently associated with sDPN after adjusting for potential confounding factors, suggesting that the presence of albuminuria might portend sDPN in people with diabetes and actions should be taken to hinder the progression from sDPN to DPN.
There are some limitations of our study. Although the sample size was larger than some previous studies,12,13 but was not powered enough to determine a causal correlation between risk factors and sDPN and lacked repeat testing to assess the progression of DPN. Additionally, as we intended to identify the risk factors of sDPN, the electrophysiological parameters of NCS were not included in this study. Furthermore, routine NCS is not able to determine dysfunction of small nociceptive fibers or large fibers by detecting vibration and autonomic sensations. Therefore, a more ideal screening process should be developed to include testing for these modalities as well.
Conclusion
In this study, we found that the prevalence rate of sDPN was 45.7% in patients with T2DM. A longer duration of diabetes, a lower fasting C-peptide level, and the presence of albuminuria were independently associated with sDPN. The pathogenesis of sDPN remains unknown, leading to the lack of a specific and effective clinical treatment. Hence, initial screening with NCSs for early diagnosis and timely reduction of multiple risk factors are key to reducing DPN-related comorbidities and mortality.
Acknowledgments
The work was supported by grants from the Fundamental Research Funds for the Central Universities (YG2023QNB10), National Natural Science Foundation of China (81801298), NSFC Promotion Program of Ren Ji Hospital affiliated to Shanghai Jiao Tong University School of Medicine (RJTJ22-MS-011).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–948. doi:10.1016/S2213-8587(19)30081-6
2. Wu B, Niu Z, Hu F. Study on risk factors of peripheral neuropathy in type 2 diabetes mellitus and establishment of prediction model. Diabetes Metab J. 2021;45(4):526–538. doi:10.4093/dmj.2020.0100
3. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
4. Lu Y, Xing P, Cai X, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: estimates of the INTERPRET-DD study. Front Public Health. 2020;8:534372. doi:10.3389/fpubh.2020.534372
5. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. doi:10.1038/s41572-019-0092-1
6. Walter-Höliner I, Barbarini DS, Lütschg J, et al. High prevalence and incidence of diabetic peripheral neuropathy in children and adolescents with type 1 diabetes mellitus: results from a five-year prospective cohort study. Pediatr Neurol. 2018;80:51–60. doi:10.1016/j.pediatrneurol.2017.11.017
7. Levitt Katz LE, White NH, El Ghormli L; Group TS. Risk factors for diabetic peripheral neuropathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care. 2021;45(5):1065–1072. doi:10.2337/dc21-1074
8. Andersen ST, Witte DR, Dalsgaard E-M, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care. 2018;41(5):1068–1075. doi:10.2337/dc17-2062
9. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi:10.2337/dc20-S002
10. Meijer J-WG, Bosma E, Lefrandt JD, et al. Clinical diagnosis of diabetic polyneuropathy with the diabetic neuropathy symptom and diabetic neuropathy examination scores. Diabetes Care. 2003;26(3):697–701. doi:10.2337/diacare.26.3.697
11. Tang XF, Yang T, Yang BX, et al. Electromyographic findings in normal Chinese. Analysis of 310 subjects. Chin Med J. 1984;97(8):613–622.
12. Unmar Y, Zafar MI, Gao F. Factors associated with peripheral neuropathy in type 2 diabetes: subclinical versus confirmed neuropathy. J Huazhong Univ Sci Technolog Med Sci. 2017;37(3):337–342. doi:10.1007/s11596-017-1737-5
13. Gylfadottir SS, Itani M, Krøigård T, et al. Diagnosis and prevalence of diabetic polyneuropathy: a cross-sectional study of Danish patients with type 2 diabetes. Eur J Neurol. 2020;27(12):2575–2585. doi:10.1111/ene.14469
14. Bertora P, Valla P, Dezuanni E, et al. Prevalence of subclinical neuropathy in diabetic patients: assessment by study of conduction velocity distribution within motor and sensory nerve fibres. J Neurol. 1998;245(2):81–86. doi:10.1007/s004150050182
15. Akbar DH, Mira SA, Zawawi TH, et al. Subclinical diabetic neuropathy: a common complication in Saudi diabetics. Saudi Med J. 2000;21(5):433–437.
16. de Souza RJ, de Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J Diabetes Complications. 2015;29(6):811–817. doi:10.1016/j.jdiacomp.2015.05.009
17. Toopchizadeh V, Shiva S, Khiabani N-Y, et al. Electrophysiologic pattern and prevalence of subclinical peripheral neuropathy in children and adolescents with type I diabetes mellitus in Iran. Saudi Med J. 2016;37(3):299–303. doi:10.15537/smj.2016.3.13625
18. Bjerg L, Nicolaisen SK, Christensen DH, et al. Diabetic polyneuropathy early in type 2 diabetes is associated with higher incidence rate of cardiovascular disease: results from two Danish cohort studies. Diabetes Care. 2021;44(7):1714–1721. doi:10.2337/dc21-0010
19. Liu S, Zheng H, Zhu X, et al. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabet Res Clin Pract. 2017;130:90–97. doi:10.1016/j.diabres.2017.05.008
20. Pai YW, Lin CH, Lee IT, et al. Hypoglycaemic episodes and risk of diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab. 2019;45(4):395–398. doi:10.1016/j.diabet.2017.09.009
21. Lee -S-S, Han H-S, Kim H. A 5-yr follow-up nerve conduction study for the detection of subclinical diabetic neuropathy in children with newly diagnosed insulin-dependent diabetes mellitus. Pediatr Diabetes. 2010;11(8):521–528. doi:10.1111/j.1399-5448.2009.00636.x
22. Liu X, Xu Y, An M, et al. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. 2019;14(2):e0212574. doi:10.1371/journal.pone.0212574
23. Kallinikou D, Soldatou A, Tsentidis C, et al. Diabetic neuropathy in children and adolescents with type 1 diabetes mellitus: diagnosis, pathogenesis, and associated genetic markers. Diabetes Metab Res Rev. 2019;35(7):e3178. doi:10.1002/dmrr.3178
24. Wahren J, Foyt H, Daniels M, et al. Long-acting C-peptide and neuropathy in type 1 diabetes: a 12-month clinical trial. Diabetes Care. 2016;39(4):596–602. doi:10.2337/dc15-2068
25. Panero F, Novelli G, Zucco C, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32(2):301–305. doi:10.2337/dc08-1241
26. Gubitosi-Klug RA, Braffett BH, Hitt S, et al. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021;131(3):e143011. doi:10.1172/JCI143011
27. Qiao X, Zheng H, Zhang S, et al. C-peptide is independent associated with diabetic peripheral neuropathy: a community-based study. Diabetol Metab Syndr. 2017;9(1):12. doi:10.1186/s13098-017-0208-2
28. Zhao L, Ma J, Wang S, et al. Relationship between β-cell function, metabolic control, and microvascular complications in type 2 diabetes mellitus. Diabetes Technol Ther. 2015;17(1):29–34. doi:10.1089/dia.2014.0214
29. Sari R, Balci MK. Relationship between C peptide and chronic complications in type-2 diabetes mellitus. J Natl Med Assoc. 2005;97(8):1113–1118.
30. Gottsäter A, Ahmed M, Fernlund P, et al. Autonomic neuropathy in Type 2 diabetic patients is associated with hyperinsulinaemia and hypertriglyceridaemia. Diabet Med. 1999;16(1):49–54. doi:10.1046/j.1464-5491.1999.00001.x
31. Bell DS, Ketchum CH, Robinson CA, et al. Microalbuminuria associated with diabetic neuropathy. Diabetes Care. 1992;15(4):528–531. doi:10.2337/diacare.15.4.528
32. Zhang Y, Jiang Y, Shen X, et al. Can both normal and mildly abnormal albuminuria and glomerular filtration rate be a danger signal for diabetic peripheral neuropathy in type 2 diabetes mellitus? Neurol Sci. 2017;38(8):1381–1390. doi:10.1007/s10072-017-2946-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
