Back to Journals » Infection and Drug Resistance » Volume 16
Risk Factors for Carbapenem-Resistant Enterobacteriaceae Colonization and the Effect on Clinical Outcomes and Prognosis in Allogeneic Hematopoietic Stem Cell Transplanted Patients
Authors Wu WQ , Zhang YQ, Xu J, Tang ZX, Li SJ, Wei XY, Li L, Wu HQ, Ma X, Liu JS, Wu DP, Wu XJ
Received 2 June 2023
Accepted for publication 14 September 2023
Published 25 October 2023 Volume 2023:16 Pages 6821—6831
DOI https://doi.org/10.2147/IDR.S424048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Wen-Qi Wu,1– 3,* Yu-Qi Zhang,1– 3,* Jie Xu,1– 3,* Zai-Xiang Tang,4 Shi-Jia Li,1– 3 Xi-Ya Wei,1– 3 Ling Li,1– 3 He-Qing Wu,1– 3 Xiao Ma,1– 3 Ji-Sheng Liu,1,5 De-Pei Wu,1– 3 Xiao-Jin Wu1– 3
1Department of Hematology, the First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, Suzhou, People’s Republic of China; 3Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, People’s Republic of China; 4Department of Epidemiology and Statistics, School of Public Health, Faculty of Medicine, Soochow University, Suzhou, People’s Republic of China; 5Department of Otolaryngology Head and Neck Surgery, the First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Jin Wu; De-Pei Wu, Email [email protected]; [email protected]
Purpose: The current study assesses which are the main risk factors, clinical outcome and prognosis following the colonization of CRE in patients that underwent allo-HSCT.
Patients and Methods: A total of 343 patients subjected to allo-HSCT in the period comprised between June 2021 and June 2022 were enrolled in this retrospective study. The CRE colonization was diagnosed by clinical history and routine microbial culture of perirectal swab. In this regard, a clinical prediction model was designed based on independent risk factors underlying the pre-transplantation CRE colonization using a backward stepwise logistic regression, followed by the evaluation of its discrimination and calibration efficacies, along with clinical usefulness. Furthermore, univariate and multivariate Cox regression analyses were then conducted to assess the risk factors for post-transplantation clinical outcomes.
Results: Out of 343 patients enrolled in this study, 135 (39.3%) reported CRE colonization. The independent risk factor variables for CRE colonization were incorporated into the nomogram to build a prediction model, which showed an area under the curve of 0.767 (95% CI: 0.716– 0.818), and well-fitted calibration curves (χ 2 = 1.737, P = 0.9788). The patients with CRE colonization reported a significantly lower platelet engraftment rate with a higher risk of post-transplantation BSI when compared with the non-CRE colonization group (P = 0.02 and P < 0.001; respectively). The non-relapse mortality (NRM) value was higher in the CRE patients (P < 0.05), consistently with a survival probability that was thus significantly lower for the same timeframe (P < 0.05).
Conclusion: A reliable clinical prediction model for pre-transplantation CRE colonization was developed that demonstrated that the CRE colonization negatively affects platelet engraftment and survival outcomes following allo-HSCT.
Keywords: CRE, Enterobacteriaceae colonization, perirectal swab, allo-HSCT, risk factors, prognosis
Introduction
Hematopoietic stem cell transplantation (HSCT) represents one of the last treatments for malignant hematologic diseases; however, patients are more predisposed to infectious complications due to the immune suppression and high-dose of chemotherapy that may lead to bone marrow suppression and mucosal damage.1 The infection complication is the third most common cause of death in allogeneic (allo)-HSCT patients within the next 100 days post allo-HSCT,2 with bloodstream infections (BSI) caused by carbapenem-resistant Enterobacteriaceae (CRE) responsible for more than 50% of deaths reported.2–4 With a long-term therapy and relevant use of carbapenem antibiotics developed in the recent years, the resistance rates increased, with gram-negative bacteria being the main resistant strains detected. The CRE resistance mechanism is mainly related to the production of carbapenemase, which can hydrolyze several β-lactam antibiotics, including carbapenem.5 Patients undergoing allo-HSCT reported longer hospital stays, prolonged neutropenia, and a repeated exposure to antibiotics that significantly may increase the risk of developing BSI, pneumonia, and death, making treatment significantly more challenging.6,7 Recent studies demonstrated that common complications of allo-HSCT, including infections and mucositis, are significantly related to changes of the composition of the gut microbiota.8,9 However, limited research was conducted to assess the risk factors for CRE colonization.
To this aim, the risk factors for CRE colonization and its impact on clinical outcomes along with prognosis were evaluated from 343 allo-HSCT patients that did not report any bacterial infection before the transplantation.
Materials and Methods
Study Subjects and Design
Patients admitted for allo-HSCT at the First Affiliated Hospital of Soochow University between June 2021 and June 2022 that did not show any symptoms of infection and that on a voluntary basis were subject to CRE screening were included in the study. The follow-up cutoff time considered was the patient’s last hospitalization time. All individuals signed a written informed consent before the enrollment, which was conducted in accordance with the Declaration of Helsinki and approved by the Faculty Hospital Ethics Committee at the First Affiliated Hospital of Soochow University.
Each patient provided a perirectal swab to investigate the presence of CRE colonization within 48 hours of hospitalization for the initial screening, and weekly thereafter up to the day of the stem cell transfusion. To collect perirectal swabs, a swab soaked in aseptic saline was inserted approximately 4 to 5 cm into the anus. The swab tip was then swiped to collect visible feces, after which it was placed into a sterile tube and promptly submitted for examination within a 30-minute window. The rectal swabs were directly inoculated onto China Blue agar plates containing carbapenem as a selective agent for CRE screening. Based on the microbial culture and carbapenem antimicrobial susceptibility results, patients were classified into 2 groups: CRE colonized and non-CRE colonized. CRE colonized was defined as the isolation of CRE from a rectal swab in patients not showing any clinical symptom, while the non-CRE colonized included patients were those in which neither CRE nor any other pathogens were cultured from perirectal swabs. For patients with multiple incidences of a CRE-positive swab, only the first one was analyzed. Perirectal swab screening within 48 hours of admission was used as baseline screening, and patients exhibiting positive results were excluded from the study. A few patients were also excluded if important clinical information were unavailable.
Data Collection
The following data were collected: age, sex, transplantation types, conditioning regimens, stem cell source, preliminary diagnosis, disease status, antithymocyte globulin (ATG) usage, human leukocyte antigen (HLA) antibody positivity, blood type of donor and recipient, rituximab application, plasma exchange, history of BSI, doses of mononuclear cells (MNCs) and CD34+ cells, diagnosis of pulmonary, gastrointestinal, and perianal infections, carbapenem antibiotic application within 30 days before allo-HSCT. The date in which allo-HSCT was performed was recorded to accurately calculate the time up to the post-transplantation infections and related complications like BSI, cytomegalovirus (CMV) or Epstein–Barr virus (EBV) reactivation, graft-versus-host disease (GVHD), and thrombotic microangiopathy (TMA).
Definition of Observation Index
The presence of CRE was determined by measuring the minimum inhibitory concentration (MIC) using Vitek 2 compact 60 (AST-GN67 &AST-XN04, bioMérieux, France), and resistance to carbapenems was defined as follows: meropenem and/or imipenem MIC ≥ 4 µg/mL (CLSI M100 32nd). The disc diffusion method was used to test each CRE strain for meropenem and imipenem. The neutrophil engraftment was defined when the absolute value of peripheral blood neutrophils was >0.5×109/L for three consecutive days without growth factors, while platelet engraftment was defined when platelet count was >20×109/L for seven consecutive days without blood transfusion. CMV or EBV reactivation was observed when a CMV DNA viral load >100 copies/mL or an EBV DNA viral load >100 copies/mL were detected at any time after allo-HSCT. Post-transplantation BSI was diagnosed when at least one pathogenic bacterium was isolated by blood culture, and it was monitored since the day it received conditioning. Relapse was defined when blast cells were ≥5% in the bone marrow or when blast cells reappeared in the peripheral blood or in any site outside the bone marrow, including extramedullary relapse. Progression-free survival (PFS) was defined as the time from transplantation to the first disease progression or death from any cause. Non-relapse mortality (NRM) refers to death after transplantation without disease relapse or progression, while the overall survival (OS) was the interval between allo-HSCT and death or the last day of follow-up.
Statistical Analysis
Descriptive statistics were used to characterize the categorical and numerical variables. The first ones were presented as counts and percentages and analyzed by Pearson’s chi-square test or Fisher’s exact test, while the continuous variables were summarized as median and interquartile range (IQR) and they were analyzed by Mann–Whitney U-test. A logistic regression model was used for CRE colonization prediction model (CREP-model) before allo-HSCT. A backward stepwise logistic regression analysis was conducted using significant variables for CRE colonization on univariate analysis to assess the factors discriminating the CRE-colonized patients before allo-HSCT. Receiver operating characteristic (ROC) curve analysis was used to calculate the optimal cutoff values determined by maximizing the Youden index (sensitivity+specificity-1). The predictive performance of the model was measured by the area under curve (AUC) and calibration using 1000 bootstrap samples to decrease the overfit bias. Decision curve analysis (DCA) was used to calculate the net benefit, while the clinical impact curve (CIC) demonstrated the clinical effectiveness of the model. Survival estimates were calculated using the Kaplan–Meier method, and curves were compared using the Log rank test. The clinical outcomes, including engraftment, post-transplantation BSI, infection (CMV/EBV), GVHD, TMA, relapse and NRM were analyzed with the cumulative incidence function and Cox proportional hazards regression models. All analyses were performed using R software version 4.1.1. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Baseline Characteristics and Colonization Status
A total of 343 patients with no symptoms of infection before the transplantation were screened for CRE colonization (Figure 1). Overall, 135 out of 343 perirectal swabs were CRE positive, with a colonization rate of 39.3%. The colonizing microbiota was composed of all gram-negative bacteria, such as Klebsiella pneumoniae (41%), Escherichia coli (26%), and Enterobacter cloacae (17%). The baseline and clinical characteristics of the two groups are reported in Table 1. Patients with CRE colonization reported statistically more gastrointestinal, pulmonary and perianal infections, along with carbapenem antibiotic application within the 30 days before transplantation. Further, the CRE-colonized patient rate was statistically associated with male gender, infusion with a greater number of MNCs and with a not complete remission (CR).
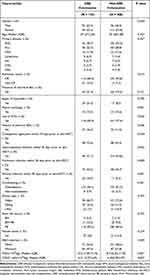 |
Table 1 Baseline Characteristics |
 |
Figure 1 The procedure of perirectal swab screening in allogeneic hematopoietic stem cell transplanted patients from June 2021 to June 2022. |
CRE Colonization Prediction Model Development and Evaluation
All variables significantly associated with CRE colonization were analyzed with a backward stepwise multivariate logistic regression. Our data showed that gender (odds ratio [OR] = 2.94; 95% CI: 1.76–4.9; P < 0.001), pulmonary infection within 30 days before transplantation (OR = 2.38; 95% CI: 1.42–3.90; P = 0.001), gastrointestinal infection within 30 days before transplantation (OR = 1.82; 95% CI 1.08–3.05; P = 0.024), perianal infection within 30 days before transplantation (OR = 2.58; 95% CI; 1.11–5.99; P = 0.027), and carbapenem antibiotic application within 30 days before transplantation (OR = 3.72; 95% CI; 2.22–6.23; P < 0.001) resulted to be independent risk factors for CRE colonization as shown in Supplementary Table 1. To establish a clinical prediction model, these five independently associated risk factors were used to conduct a CRE colonization risk estimation nomogram (Figure 2A), and the final model was validated using the bootstrap method based on 1000 repetitions. The nomogram demonstrated a good accuracy in the estimation of the risk of CRE colonization, with an AUC of 0.767 (95% CI: 0.716–0.818) (Figure 2B). In addition, calibration plots graphically reported a good agreement between the risk estimated by the nomogram and actual perirectal swab results (Hosmer–Lemeshow goodness-of-fit test: χ2 = 1.737, P = 0.9788) (Figure 2C). Since threshold probability ranges from 0.1 to 0.9, DCA curve showed that the nomogram led to a greater net benefit than either the treat-all-patients scheme or the treat-none scheme (Figure 2D). Based on the CIC curve results, the number of subjects at a higher risk of CRE colonization based on the nomogram highly matched the number of true-positive cases when the threshold probability was above 75% (Figure 2E).
Impact of CRE Colonization on the Engraftment and Clinical Events
No significant differences were found in the engraftment of neutrophils between the two groups (HR = 0.87; 95% CI: 0.70–1.08; P = 0.12) (Figure 3A), while multivariate cox regression analysis showed that CRE colonization negatively affected the engraftment of platelets in Supplementary Table 2 (HR = 0.66; 95% CI: 0.52–0.83; P < 0.001) (Figure 3B). However, CRE colonization did not affect the incidence of EBV or CMV reactivation (HR = 1.62; 95% CI: 0.73–3.63; P = 0.23 and HR = 1.11; 95% CI: 0.79–1.55; P = 0.55; respectively) (Figure 3C and D), acute GVHD (aGVHD), chronic GVHD (cGVHD) and TMA (HR = 1.06; 95% CI: 0.72–1.56; P = 0.74; HR = 0.84; 95% CI: 0.51–1.37; P = 0.48 and HR = 1.09; 95% CI: 0.18–6.51; P = 0.93; respectively) (Figure 3E–G).
CRE Colonization and Post-Transplantation BSI
Out of the 343 patients of this study, 48 (14%) patients developed post-transplantation BSI, with 39 of which (11.4%) induced by CRE colonization. The culture results for blood and perirectal swab were consistent, with a BSI rate which was 4.3 times (39/9) higher when compared with not-CRE colonized group (P < 0.001) (Figure 3H). The multivariate cox analysis results showed that CRE colonization (HR = 5.71; 95% CI: 2.66–12.30; P < 0.001) was an independent risk factor for post-transplantation BSI as shown in Table 2.
 |
Table 2 Univariate and Multivariate Analysis for Risk Factors Associated with Post-Transplantation BSI |
Impact of CRE Colonization on the Prognosis of Patients
We further investigated the early and long-term prognosis of transplant recipients: patients with pre-transplantation CRE colonization showed higher NRM values (P < 0.001) and lower OS rates (P = 0.0034) within 100 days after transplantation than patients without pre-transplantation CRE colonization (Figure 4A and B). After a median follow-up of 163 days, 25 (18.5%) out of 135 patients with pre-transplantation CRE colonization died, mainly for secondary infections (18/25). Among patients without pre-transplantation CRE colonization, after a median follow-up of 188 days, 18 (8.7%) out of 208 patients died, mainly for disease relapse (10/18). By the end of the follow-up period, the cumulative incidences of NRM (P = 0.0021) and relapse (P = 0.014) were significantly higher in the CRE colonization group than in the non-CRE colonization group (Figure 4C and D). In addition, patients with CRE colonization had lower PFS rates (P < 0.001) and lower OS rates (P = 0.005) (Figure 4E and F). Cox regression analysis showed that pre-transplantation CRE colonization led to an increasing in NRM at 100 days after transplantation and in the long term as shown in Supplementary Table 3 and Supplementary Table 4 (HR = 5.50; 95% CI: 1.53–19.73; P = 0.009 and HR = 2.68; 95% CI: 1.13–9.9; P = 0.029; respectively). A significant difference in recurrence rates between the two groups was observed, mainly due to differences in the baseline levels of pre-transplantation remission status (HR = 8.30; 95% CI, 3.80–18.12; P < 0.001), which were not associated with CRE colonization as shown in Supplementary Table 5 (HR = 1.94; 95% CI, 0.91–4.45; P = 0.086). CRE colonization resulted in a shorter 100-day survival, PFS rate and total survival time after transplantation in Supplementary Table 6–8 (HR = 3.28; 95% CI: 1.14–11.59; P = 0.007; HR = 1.89; 95% CI: 1.02–3.49; P = 0.044 and HR = 2.31; 95% CI: 1.37–3.90; P = 0.002; respectively).
Discussion
The human gut represents an ecosystem colonized by many commensal bacteria, with about 1000–1150 species of approximately 100 trillion of bacteria, a number far greater than human cells.10 A healthy gut microbiota is able to prevent invasion of pathogen bacteria, a phenomenon known as colonization resistance,11 which can be, however, gradually lost with a long-term treatment of carbapenem antibiotics in clinical settings.12,13 In this regard, the harsh pre-transplantation preparative regimens can cause dysbiosis of the gut microbiome, with consequent outgrowth of less favorable or pathogenic gut bacteria, such as different species of Enterobacteriaceae.14 In recent years, the detection rate of CRE is increasing, representing a growing global public health problem.15–17 Indeed, infections caused by CRE during the transplantation period represent an important cause of high mortality rates observed following the treatment.
Previous studies developed several clinical predictive models about the risk of CRE infections. In this regard, Ju Yeon Song established a clinical prediction model for the risk of CRE colonization in patients admitted to the intensive care unit,18 while Jia Liu developed a predictive risk model for the risk of BSI in hematological patients with CRE isolated from perianal swabs in order to promptly adopt preventive strategies.19 However, few predictive models about the risk of CRE colonization in allo-HSCT recipients have been developed so far. In our study, we demonstrated that pulmonary infection, gastrointestinal infection, perianal infection, and exposure to carbapenem antibiotics within 30 days before allo-HSCT were all independent risk factors for CRE colonization. Furthermore, chemotherapy was associated with a prolonged neutropenia and with many complications such as several infections,20 like the pulmonary, gastrointestinal and perianal.21 Consistent with the model introduced by Sonis, both inflammation and apoptosis of the mucosal barrier may cause the loss of its integrity, thereby promoting bacterial translocation, which is a predisposing factor for CRE colonization.22 In the case of co-infections, carbapenem antibiotics are used empirically for long periods of time, leading to a detrimental effect on the intestinal microbiota. A study on the intestinal tract bacterial diversity concluded that treatment based on intravenous vancomycin, metronidazole, or β-lactams as well as treatment based on aggressive conditioning regimens for HSCT resulted in a lower diversity in the gut microbiome, leading to colonization by unfavorable species.23 Weijie Cao also found that pulmonary disease, perianal infection and carbapenem application in the previous 3 months were independent risk factors for rectal CRE colonization.2 In addition, our study showed that also male gender represents another independent risk factor for CRE colonization. This may be related to the hormone levels, as already demonstrated in an animal study on the influence of intestinal flora on the properties of bile acid.24,25 However, another study aimed to investigate the effects of gender and body mass index on the composition of the intestinal flora and revealed that although there were no significant differences in the diversity and overall composition of intestinal flora based on gender, the relative abundance of specific taxa was different between males and females.26 Therefore, novel strategies to assess the risk of colonization by pathogenic bacteria prior to allo-HSCT still need to be implemented.
The rectal colonization of CRE predisposes to bacterial translocation leading to the following endogenous CRE infections. Jessica found that the overall risk for CRE colonization progressing to CRE infections was 16.5%.27 High-dose chemotherapy or intestinal GVHD may cause damage to the intestinal mucosal barrier and subsequent translocation of bacteria to the peripheral blood, leading to BSI.28 BSI is a fatal complication occurring in the early stage of allo-HSCT that can lead to a mortality of 40–60%.29–32 In this study, we found that 28.9% (39/135) of patients with pre-transplantation CRE colonization developed a post-transplantation BSI, at a rate significantly higher than non-colonized patients, according to a retrospective, single-center study conducted in Poland.25 Furthermore, platelet reconstitution also required more time in patients with pre-transplantation CRE colonization, while no significant effect was observed on granulocyte reconstitution. Consistent with previous research, we found that bacterial colonization can affect the platelet engraftment.33 Additionally, our findings demonstrated that pre-transplantation CRE colonization was associated with an increased NRM, decreased PFS and decreased OS, indicating the aggressivity of CRE colonization and the need for a powerful antibiotic therapeutic strategy. Jaroslaw Bilinski found that gut colonization by resistant bacteria impairs OS after allo-HSCT and that the decrease in OS is caused by an increased NRM, which was due to a higher incidence of hard-to-treat infection.25 It was also reported that the colonization of multidrug-resistant bacteria (MDRB), including CRE and Vancomycin-Resistant Enterococci (VRE), is associated with lower OS rates in patients receiving transplantation, mainly due to a higher NRM, with infections as the main cause of death.11,34,35 Also, our multivariable analysis demonstrated that pre-transplantation CRE colonization was an independent risk factor for NRM or OS within 100 days and in the long term after allo-HSCT. Therefore, patients at high risk should be actively monitored, isolated, and treated to reduce the CRE colonization, the incidence of bloodstream infections and deaths.
This study explored the risk factors within patients with pre-transplantation CRE colonization and established a clinical prediction model to guide clinical decisions. However, being the present study a single-center retrospective study, it could be likely that the colonization of the intestinal flora may be geographically influenced, and therefore external and further validation of our findings is needed. In addition, neither fingerprinting nor genotyping analysis of the colonization and infection strains was carried out in our laboratory. We hope that, in the future, further research would be done to compare the colonized and infected CRE by the molecular analysis. Meanwhile, the comprehensive eradication of the carrier state in CRE-positive patients has yet to be widely established. This area undoubtedly holds promise for our future research endeavors.
Conclusions
CRE colonization is an emerging and public health problem, and the pre-transplantation CRE colonization can negatively affect the post-transplantation prognosis. The surveillance on CRE colonization can have an impact on the control and prevention of the spread of CRE. The clinical prediction models developed in this study to predict the CRE colonization before allo-HSCT can justify the early intervention in patients at high risk to improve the survival rates. Altogether, our data indicated that an increased awareness of the risks associated with pre-transplantation bacterial colonization is urgently warranted. Indeed, due to a high rate of mortality associated with CRE infections, further research and eradication attempts of CRE infections should be objects of future studies.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81974001 and 82170222); the Jiangsu Natural Science Foundation (BK20211070); The Key Disease Program of Suzhou (LCZX202101); National Science and Technology Major Project (2017ZX09304021); National Key R&D Program of China (2019YFC0840604, 2017YFA0104502); Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); Jiangsu Provincial Key Medical Center (YXZXA2016002); Research project of Jiangsu Provincial Health Commission (ZD2021008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Isles NS, Mu A, Kwong JC, Howden BP, Stinear TP. Gut microbiome signatures and host colonization with multidrug-resistant bacteria. Trends Microbiol. 2022;30(9):853–865. doi:10.1016/j.tim.2022.01.013
2. Cao W, Zhang J, Bian Z, et al. Active Screening of Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae for Subsequent Bloodstream Infection in Allogeneic Hematopoietic Stem Cell Transplantation. Infect Drug Resist. 2022;15:5993–6006. doi:10.2147/IDR.S387615
3. Trecarichi EM, Pagano L, Candoni A, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21(4):337–343. doi:10.1016/j.cmi.2014.11.022
4. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203. doi:10.1186/s12879-017-2297-9
5. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Therapeutic Advances Infectious Dis. 2016;3(1):15–21. doi:10.1177/2049936115621709
6. Misch EA, Andes DR. Bacterial Infections in the Stem Cell Transplant Recipient and Hematologic Malignancy Patient. Infect Dis Clin North Am. 2019;33(2):399–445. doi:10.1016/j.idc.2019.02.011
7. Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081. doi:10.1002/ajh.24489
8. de Molla VC, Heidrich V, Bruno JS, et al. Disruption of the oral microbiota is associated with a higher risk of relapse after allogeneic hematopoietic stem cell transplantation. Sci Rep. 2021;11(1):17552.
9. Heidrich V, Bruno JS, Knebel FH, et al. Dental Biofilm Microbiota Dysbiosis Is Associated With the Risk of Acute Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2021;12:692225. doi:10.3389/fimmu.2021.692225
10. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821
11. Su F, Luo Y, Yu J, et al. Tandem fecal microbiota transplantation cycles in an allogeneic hematopoietic stem cell transplant recipient targeting carbapenem-resistant Enterobacteriaceae colonization: a case report and literature review. Eur J Med Res. 2021;26(1):37. doi:10.1186/s40001-021-00508-8
12. Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–295. doi:10.1038/nrc.2018.10
13. Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279(1):90–105. doi:10.1111/imr.12563
14. Chong PP, Koh AY. The gut microbiota in transplant patients. Blood Rev. 2020;39:100614. doi:10.1016/j.blre.2019.100614
15. Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: the Impact and Evolution of a Global Menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi:10.1093/infdis/jiw282
16. Tang SSL, Chee E, Teo JQ, Chlebicki MP, Kwa ALH. Incidence of a subsequent carbapenem-resistant Enterobacteriaceae infection after previous colonisation or infection: a prospective cohort study. Int J Antimicrob Agents. 2021;57(6):106340. doi:10.1016/j.ijantimicag.2021.106340
17. Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58(9):1274–1283. doi:10.1093/cid/ciu052
18. Song JY, Jeong IS. Development of a risk prediction model of carbapenem-resistant Enterobacteriaceae colonization among patients in intensive care units. Am J Infect Control. 2018;46(11):1240–1244. doi:10.1016/j.ajic.2018.05.001
19. Liu J, Zhang H, Feng D, et al. Development of a Risk Prediction Model of Subsequent Bloodstream Infection After Carbapenem-Resistant Enterobacteriaceae Isolated from Perianal Swabs in Hematological Patients. Infect Drug Resist. 2023;16:1297–1312. doi:10.2147/IDR.S400939
20. Clark O, Lyman G, Castro AA, Clark L, Djulbegovic B. Colony stimulating factors for chemotherapy induced febrile neutropenia. Cochrane Database Systematic Rev. 2003;3(3):CD003039.
21. van Vliet MJ, Harmsen HJM, de Bont ESJM, Tissing WJE. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6(5):e1000879. doi:10.1371/journal.ppat.1000879
22. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4(4):277–284. doi:10.1038/nrc1318
23. Taur Y, Jenq RR, Perales M-A, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182. doi:10.1182/blood-2014-02-554725
24. Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi:10.1080/19490976.2016.1203502
25. Bilinski J, Robak K, Peric Z, et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: a Retrospective, Single-Center Study. Biol Blood Marrow Transplant. 2016;22(6):1087–1093. doi:10.1016/j.bbmt.2016.02.009
26. Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS One. 2016;11(5):e0154090. doi:10.1371/journal.pone.0154090
27. Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539–543. doi:10.1016/j.ajic.2015.12.005
28. Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol Mol Biol Rev. 2019;83(3). doi:10.1128/MMBR.00007-19
29. Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi:10.1038/sj.bmt.1705690
30. Dandoy CE, Haslam D, Lane A, et al. Healthcare Burden, Risk Factors, and Outcomes of Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections after Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(9):1671–1677. doi:10.1016/j.bbmt.2016.06.002
31. Orasch C, Weisser M, Mertz D, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(3):521–526. doi:10.1038/bmt.2009.187
32. Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M. Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis. 2014;16(1):106–114. doi:10.1111/tid.12175
33. Ustun C, Young J-AH, Papanicolaou GA, et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54(8):1254–1265. doi:10.1038/s41409-018-0401-4
34. Tanaka Y, Kurosawa S, Tajima K, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(4):553–559. doi:10.1038/bmt.2015.330
35. Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40(3):271–278. doi:10.1007/s15010-011-0229-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



