Back to Journals » Patient Related Outcome Measures » Volume 14
Risk Factors and Prognosis in Anti-NMDA Receptor Encephalitis Patients with Disturbance of Consciousness
Authors Gong Z , Lao D, Huang F, Lv S , Mao F, Huang W
Received 6 March 2023
Accepted for publication 4 June 2023
Published 14 June 2023 Volume 2023:14 Pages 181—192
DOI https://doi.org/10.2147/PROM.S411260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lynne Nemeth
Zhuowei Gong, Dayuan Lao, Fang Huang, Sirao Lv, Fengping Mao, Wen Huang
Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
Correspondence: Wen Huang, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Nanning, Guangxi, 530021, People’s Republic of China, Tel +86-771-5356504, Email [email protected]
Purpose: Disturbance of consciousness is common in patients with severe anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. However, little is known about it. This study aimed to analyze the clinical manifestations and prognostic factors of anti-NMDAR encephalitis with disturbance of consciousness.
Methods: In this retrospective study, the clinical features, treatment results, and long-term outcomes of anti-NMDAR encephalitis patients with disturbance of consciousness were analyzed, and multivariate logistic regression was used to analyze the factors affecting their prognosis.
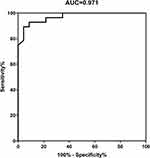
Results: In the group with disturbance of consciousness, the incidences of seizures, involuntary movements, pulmonary infection, mechanical ventilation, intensive care unit (ICU) admission, neutrophil-lymphocyte ratio (NLR), abnormal cerebrospinal fluid index, plasma exchange, and immunosuppressive therapy were higher than those in the group without disturbance of consciousness (all P< 0.05). During the follow-up period (median: 36 months, range: 12– 78 months), the modified Rankin scale (mRS) score, the maximum mRS score during hospitalization, the mRS score at discharge, and the mRS score at 12 months after discharge were higher in the disturbance of consciousness group (all P < 0.001). However, there was no significant difference in long-term outcomes and recurrence between the two groups. Multivariate logistic regression analysis showed that mechanical ventilation, elevated IgG index, and delayed immunotherapy were independent risk factors for poor outcomes in patients with anti-NMDAR encephalitis with disturbance of consciousness at 12 months (odds ratio: 22.591, 39.868, 1.195). The receiver operating characteristics (ROC) curve analysis showed that the area under the curve (AUC) of mechanical ventilation, elevated IgG index, and delayed immunotherapy was 0.971 (95% CI=0.934– 1.000, P< 0.001).
Conclusion: Mechanical ventilation, elevated IgG index, and delayed immunotherapy may be the influencing factors of poor prognosis of anti-NMDAR encephalitis patients with disturbance of consciousness. Although their condition is relatively serious, most patients with anti-NMDAR encephalitis with disturbance of consciousness will achieve favorable long-term outcomes after long-term treatment.
Keywords: anti-NMDAR encephalitis, disturbance of consciousness, severity of disease, prognosis
Introduction
Anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is a widely recognized and discussed autoimmune disease of the central nervous system (CNS).1 It is characterized by the abnormal presence of autoantibodies against NMDAR in cerebrospinal fluid (CSF). These autoantibodies can mediate the cross-linking and internalization of NMDAR, which interferes with synaptic transmission and leads to symptoms of the disease.2 The main clinical manifestations of anti-NMDAR encephalitis include psychiatric disturbance, seizures, speech disorders, abnormal movement, and so forth. In severe cases, disturbance of consciousness, central hypopnea, and autonomic dysfunction may occur.3 The characteristic is the presence of antibodies targeting the GluN1 subunit of NMDAR in cerebrospinal fluid. The main treatment measures for anti-NMDAR encephalitis are immunotherapy and tumor resection, and commonly used first-line immunotherapy methods include corticosteroids, immunoglobulins, and plasma exchange. Patients who not responding to first-line treatment are provided with second-line treatment. However, despite established treatment methods, 17–33% of patients still have poor results.4–6 Disturbance of consciousness is one of the main clinical manifestations of anti-NMDAR encephalitis, the incidence of consciousness disorders in patients with anti-NMDAR encephalitis is approximately 50%-80%.4,7,8 Previous studies have reported that Disturbance of consciousness is an independent risk factor for the poor short-term outcomes of anti-NMDAR encephalitis.9–11 However, studies have yet to focus on the analysis of the clinical features, long-term prognosis and potential influencing factors of anti-NMDAR encephalitis with disturbance of consciousness. In addition, whether these patients need more complex immunotherapy and symptomatic treatment also requires further study. Therefore, this study aimed to investigate the clinical characteristics, immunotherapy, prognosis and predictive factors of anti-NMDAR encephalitis patients with disturbance of consciousness. This will help clinicians better understand the clinical characteristics and prognosis of anti-NMDAR encephalitis patients with consciousness impairment, and develop personalized treatment plans.
Patients and Methods
Study Population
We identified a total of 111 patients with anti-NMDAR encephalitis diagnosed in the First Affiliated Hospital of Guangxi Medical University between April 2014 and September 2021. We have ruled out pediatric cases. 2 patients were lost during follow-up. Therefore, 96 eligible adult patients were included in the present study. According to the diagnostic criteria of anti-NMDAR encephalitis,12 the inclusion criteria were as follows: (1) after reasonable exclusion of other diseases, one or more of the six types of symptoms appeared quickly (less than 3 months), including psychiatric disturbance, seizures, speech dysfunction, disturbance of consciousness, abnormal movement, and autonomic dysfunction; (2) positive detection of NMDAR antibody in CSF (cell-based assay). The exclusion criteria were: (1) patients with intracranial infection; (2) patients with encephalopathy secondary to systemic inflammatory response syndrome; (3) patients diagnosed with brain trauma, epilepsy and/or other nervous system disease before the onset of encephalitis; (4) patients with positive serum and/or cerebrospinal fluid laboratory tests for another type of autoimmune encephalitis; (5) patients lacking critical clinical data. All patients underwent detailed physical examination, laboratory and imaging studies, and immunotherapy after admission. Studies were approved by the Medical Ethics Review Committee of the First Affiliated Hospital of Guangxi Medical University. Written informed consent for studies was obtained from patients and/or their families.
Data Collection
Clinical data collected included gender, age, prodromal symptoms, major clinical manifestations and complications, neutrophil-to-lymphocyte ratio (NLR), CSF findings, serum and CSF antibody titers (cell-based assay, CBA), magnetic resonance imaging (MRI), electroencephalogram (EEG) examinations, the time from the onset to the initiation of immunotherapy, the time from initial immunotherapy to improvement, and the method of immunotherapy. The Glasgow Coma Scale (GCS) was used to evaluate the level of consciousness of patients, including somnolence, sopor, coma, confusion, delirium and mutism. A GCS score of less than 15 indicated disturbance of consciousness.13 According to whether there were symptoms of disturbance of consciousness in the course of the disease, the patients were divided into two groups: one group had disturbance of consciousness and the other group did not.
CSF findings included CSF cell count, CSF protein, oligoclonal bands, albumin-CSF/serum-quotient (Qalb), immunoglobulin G (IgG) index, and 24-hour intrathecal immunoglobulin G synthesis rate (24-h intrathecal IgG). The IgG index was calculated according to the formula IgG index =(IgG CSF/IgG serum)/(Albumin CSF/Albumin serum), and the 24-h intrathecal IgG was calculated according to the Tourtellotte formula.14 The abnormal values were defined as CSF pleocytosis >5×106/L, CSF protein >450 mg/L, IgG index >0.7, 24-h intrathecal IgG >3.3 mg/dL, QAlb >7.00, and positive oligoclonal bands.
All patients received immunotherapy, symptomatic, and supportive treatment. Tumor resection was performed for those with tumors. Immunotherapy includes pulsed intravenous glucocorticoids, intravenous immunoglobulin (IVIg), plasma exchange (PE), and immunosuppressants (cyclophosphamide, mycophenolate mofetil, or azathioprine). The modified Rankin scale (mRS) score was used for prognosis evaluation.2 The mRS scores at admission, maximum mRS scores during hospitalization, mRS scores at discharge and every 6 months after discharge were recorded. The prognosis after discharge was evaluated by a neurologist during outpatient clinic or telephone follow-up. According to the evaluation criteria, the prognosis was good for mRS scores 0–2 points and poor for 3–6 points. The recurrence was defined as the onset of new symptoms or deterioration after at least 2 months of stabilization or improvement.15
Statistical Analysis
IBM SPSS 23.0 was used for statistical analysis and figures were generated with GraphPad Prism 7. The normal distribution data were expressed as mean ± standard deviation, while non-normal distribution data were expressed as median (interquartile range, IQR). The countable data were represented as counts (percentage) [n (%)], and the chi-square analysis with continuity correction or Fisher’s exact test for categorical variables where applicable, and the Mann–Whitney U-test for continuous variables. Multivariate logistic regression was used to analyze the factors affecting the prognosis of anti-NMDAR encephalitis patients with disturbance of consciousness. P values < 0.05 were considered to be statistically significant.
Results
The Features of Disturbance of Consciousness in Anti-NMDAR Encephalitis
Of the 96 patients with anti-NMDAR encephalitis, 51 (53.1%) had disturbance of consciousness, including somnolence (8 patients, 15.7%), sopor (3 patients, 5.9%), coma (8 patients, 15.7%), confusion (25 patients, 49%), delirium (3 patients, 5.9%), and mutism (4 patients, 7.8%), as indicated in Table 1. The median GCS score of 51 patients with disturbance of consciousness was 11 (IQR 10–12) on admission and 13 (IQR 10–15) on discharge. The median time from the onset to disturbance of consciousness was 18 days (IQR 14–25) and the median duration of disturbance of consciousness was 29 days (IQR 16–37). The disturbance of consciousness lasted more than 7 days in 39.2% and more than 30 days in 47.1% of patients.
 |
Table 1 The Clinical Manifestation and Incidence of Disturbance of Consciousness |
The Clinical Features in Anti-NMDAR Encephalitis Patients with Disturbance of Consciousness
In total, 96 patients with anti-NMDAR encephalitis were enrolled in this study, including 44 males (45.8%) and 52 females (54.2%). Patients were aged from 18 to 76, with a median age of 24 years (IQR 21–34). 50 patients (52.1%) had prodromal symptoms such as fevers and headaches. The most common clinical manifestation was psychiatric disturbance (90.6%), followed by seizures (69.8%), involuntary movement (51%), speech dysfunction (22.9%), autonomic dysfunction (13.5%). 6 (6.3%) patients had ovarian teratoma, of which 5 patients had disturbance of consciousness. All of them underwent teratoma resection after admission.
51 patients were in the group with disturbance of consciousness, compared with 45 patients in the group without disturbance of consciousness. The clinical manifestations of the two groups were compared as shown in Table 2. Significant differences were observed in seizures, involuntary movements, mechanical ventilation, pulmonary infection, and ICU admission. Compared with the patients without disturbance of consciousness, those with disturbance of consciousness were more likely to have seizures (82.4% vs 55.6%, P=0.004), involuntary movements (72.5% vs 26.7%, P<0.001), pulmonary infection (58.8% vs 20%, P < 0.001), mechanical ventilation (37.3% vs 2.2%, P<0.001), and ICU admission (37.3% vs 4.4%, P<0.001). No statistical difference was observed in gender, age, prodromal symptoms, speech disorders, psychiatric disturbances, sleep disorders, autonomic dysfunction, and tumors between the two groups (P>0.05).
 |
Table 2 The Clinical Characteristics of Patients with or Without Disturbance of Consciousness |
The auxiliary examination results showed that among 96 patients with anti-NMDAR encephalitis, 84 patients (87.5%) had abnormal CSF findings. 50 (52.1%) had CSF pleocytosis, 28 (29.2%) patients had elevated CSF protein, 47 (49%) had elevated IgG index, 72 (75%) patients had elevated 24-h intrathecal IgG, 15 (15.6%) had blood-brain barrier disruption (QAlb >7), and 9 (9.4%) patients had positive oligoclonal bands. The proportion of CSF protein elevation was higher in the patients with disturbance of consciousness than those in the patients without disturbance of consciousness (P<0.05). 79 (82.3%) of the 96 patients underwent brain MRIs. 42 (53.2%) had abnormal MRI findings, mainly involving the bilateral temporal lobe, frontal, occipital lobe, lateral ventricle, and hippocampal cortical gyrus. All 96 patients underwent EEGs. 81 (84.4%) had an abnormal EEG, with focal and diffuse slow waves being the most common. Only 8 (8.3%) patients had the extreme delta brush, and there were no statistical differences between the two groups. The NLR in the disturbance of consciousness group was higher than that in the group without disturbance of consciousness (P<0.001). All patients (100%) were positive for anti-NMDAR antibodies of CSF, while only 68 cases (70.8%) were positive for anti-NMDAR antibodies of serum.
The disease severity and immunotherapy between the two groups were found significant differences. Compared to the group without disturbance of consciousness. The patients with disturbance of consciousness were more seriously ill. The admission mRS scores (median of 4 vs 3, P<0.001), the maximum mRS scores during hospitalization (median of 4 vs 3, P<0.001), and the discharge mRS scores (median of 4 vs 2, P<0.001) for patients with disturbance of consciousness were also higher (Figure 1). All patients received first-line immunotherapy, of which 94 (97.9%) patients received steroid therapy, 54 (56.3%) patients received IVIg, and 17 (17.7%) patients received PE. Of the 34 (35.4%) patients who received immunosuppressant treatment, 14 (14.6%) patients received cyclophosphamide, 8 (8.3%) patients received mycophenolate mofetil, and 12 (12.5%) patients received azathioprine. Compared to the patients without disturbance of consciousness, the proportion of receiving PE and immunosuppressant in patients with disturbance of consciousness was higher (P<0.05) and the time from initial immunotherapy to improvement was longer (P<0.001).
Neurological Functional Outcome and Factors Associated with Poor Long-Term Functional Outcome
The median follow-up period was 36 months (range 12–78 months), and the median mRS score at discharge was 4 and 2, respectively, among those with or without disturbance of consciousness. In order to investigate the long-term prognosis of the patients, mRS scores were evaluated every 6 months. A total of 2 patients were lost during the follow-ups, and both were in the group without disturbance of consciousness. The long-term follow-up results showed that 78 (83%) of patients showed good neurological outcomes (mRS ≤ 2), with a long-term mortality rate of 3.2%, most of which died of disease progression and complications. Six patients had ovarian teratoma and all patients underwent resection.
After follow-up for at least 12 months, the influencing factors of poor prognosis in patients with anti-NMDAR encephalitis with disturbance of consciousness were analyzed, as shown in Table 3. Compared with the patients with good prognosis (mRS ≤ 2), the patients with poor prognosis had a higher proportion of mechanical ventilation, ICU admission, elevated IgG index, and delayed immunotherapy. Multivariate logistic regression analysis of these indicators, as shown in Table 4. The results showed that mechanical ventilation, elevated IgG index, and delayed immunotherapy were independent risk factors for poor prognosis in patients with anti-NMDAR encephalitis with disturbance of consciousness. In order to evaluate the role of the above three parameters in predicting the prognosis of patients with NMDAR encephalitis with disturbance of consciousness, ROC curve analysis was carried out. The results show that the area under the curve (AUC) is 0.971 (95% CI= 0.934–1.000, P<0.001) (Figure 2). Although there was no significant difference in the rates of ICU admission between the two groups, this parameter was higher in patients with poor prognoses than in patients with good prognoses.
 |
Table 3 Predictors of Poor Neurologic Outcome (mRS>2) in Anti-NMDAR Encephalitis Patients with Disturbance of Consciousness at the 12-Month Follow-Up |
 |
Table 4 Factors Associated with Prognosis in Anti-NMDAR Encephalitis Patients with Disturbance of Consciousness |
Discussion
Anti-NMDAR encephalitis is an autoimmune inflammatory disease of the central nervous system, which can be reversed by early active treatment.4,16 Disturbance of consciousness is one of the common clinical manifestations in patients with anti-NMDAR encephalitis. Studies have shown that the proportion of disturbance of consciousness in severe anti-NMDAR encephalitis is as high as 89.7%.7 Disturbance of consciousness can occur at different stages of the disease, sometimes even throughout the disease, and may even lead to a poor overall prognosis.9,11 Therefore, actively exploring the clinical characteristics and prognostic factors of anti-NMDAR encephalitis patients with disturbance of consciousness can provide a reference for clinical prevention and treatment.
The incidence of consciousness disorders in patients with anti-NMDAR encephalitis is approximately 50%-80%.4,7,8 51 (53.1%) patients in this study had disturbance of consciousness, which was also consistent with previous studies. Anti-NMDAR encephalitis is an autoimmune inflammatory disease mediated by self-antibodies. NMDA receptors form neural connections in the brain, and most connections to the external world and internal experiences rely on the normal function of NMDA receptors. Antibodies cause dysfunction of the NMDA receptors, leading to severe neurological damage and affecting the normal functioning of the brain.17 Consciousness impairment might be due to damage and deactivation of the cerebral cortex, responsible for perception, movement, language, and high-level cognition. When this area is affected by inflammation, it may stop receiving and processing information, leading to consciousness disorders.18,19 Our results showed that the manifestations of disturbance of consciousness in anti-NMDAR encephalitis patients varied. The type of disturbance of consciousness with the highest incidence was confusion, followed by coma, somnolence, mutism, delirium, and sopor. Notably, after early, aggressive, combined immunotherapy and life support, the symptoms of disturbance of consciousness in most patients were alleviated.
The initial condition of anti-NMDAR encephalitis patients with disturbance of consciousness is more serious, the trend of deterioration is obvious, and the prognosis is relatively poor.
Compared with the unconscious disorder group, the disturbance of consciousness group had a higher proportion of seizures and involuntary movement and required ICU admissions and mechanical ventilation more frequently. In addition, their admission mRS score, the maximum mRS score during hospitalization, and the mRS score at discharge are also higher, which means that the condition is more serious at admission, is more likely to worsen during treatment, and the short-term prognosis is relatively poor. The reason may be that with the occurrence and progress of disturbance of consciousness, patients need to stay in bed or endotracheal intubation for a long time, resulting in more complications, and the patient’s condition tends to worsen, thus increasing disease unpredictability and the risk of poor prognosis.20,21 In addition, respiratory and urinary dysfunction also increased the risk of pulmonary infection and urinary tract infection. In this study, although there was no significant difference in urinary tract infection, abnormal liver function, deep venous thrombosis, sleep disturbance, and tumor between the two groups, the proportion of patients in the disturbance of consciousness group was higher than that in the group without disturbance of consciousness. It is worth mentioning that this study also showed that the percentage of abnormal CSF indexes in patients with anti-NMDAR encephalitis with disturbance of consciousness was higher than that in patients without disturbance of consciousness. The analysis of biochemical and immune parameters of cerebrospinal fluid can reflect the inflammation and intrathecal synthesis of the central nervous system. Elevated cerebrospinal fluid proteins are commonly used to evaluate abnormal immune inflammatory responses in the central nervous system.22 In this study, the proportion of elevated CSF protein in patients with disturbance of consciousness was higher, which indirectly indicated that the central immune response of anti-NMDAR encephalitis patients with disturbance of consciousness was more serious, and the disease may be progressing gradually, which is consistent with previous studies.23
The treatment time of anti-NMDAR encephalitis patients with disturbance of consciousness is longer and more difficult, so the combined immunotherapy for primary diseases should be strengthened.
In this study, the time from onset to the initiation of immunotherapy was similar between the patients with or without disturbance of consciousness (18 days, IQR12-33 vs 20 days, IQR11.5–32). However, the time from immunotherapy to initiation of improvement was longer (32 days, IQR24-38 vs 20 days, IQR17-26.5). Patients with anti-NMDAR encephalitis who experience consciousness disorders have a high degree of severity. The appearance of consciousness disorder symptoms suggests that there may be damage and inactivation of the cerebral cortex, which may affect advanced functions such as thinking, perception, movement and language.19 Consciousness disorder is one of the manifestations of disease progress,23 so the treatment time is longer. Therefore, doctors should closely monitor the consciousness of patients with anti-NMDAR encephalitis during hospitalization and rule out any potential causes of consciousness disturbance as soon as possible. At present, the main treatment of anti-NMDAR encephalitis is immunotherapy and tumor resection. The commonly used first-line immunotherapy includes steroid therapy combined with IVIg or PE, and the second-line immunotherapy recommends the use of immunosuppressant.4,24 Studies have shown that early treatment is a predictor of a good outcome,4 and some researchers suggest that patients who do not improve 10 days after first-line treatment should be treated with immunosuppressant therapy.2,8,25 In our study, the number of patients receiving PE and immunosuppressant therapy in the disturbance of consciousness group was higher than that in the group without disturbance of consciousness, there were significant differences in the choice of immunotherapy and the intensity of combined immunotherapy between the two groups, suggesting that patients in the disturbance of consciousness group received more active combined immunotherapy. In addition, early treatment is defined as starting immunotherapy within 30 days of onset.26 36 patients (70.1%) in the consciousness disorder group received immunotherapy in the early stages of the disease. After receiving hormone combined with PE or hormone combined with immunosuppressive therapy in the early stage, the symptoms of disturbance of consciousness in most patients can be alleviated. Among the consciousness disorder group, 39 patients (76.5%) achieved good long-term prognosis. And there was no significant difference in long-term prognosis between the groups with or without consciousness disorders. In the past, it was reported that the neurological function of patients with disturbance of consciousness completely returned to normal after treatment.27 In addition, long-term follow-up shows that although immunotherapy takes a long time for patients with disturbance of consciousness to begin to improve, most patients with anti-NMDAR encephalitis with disturbance of consciousness will eventually achieve favorable long-term results. This enlightens us that for anti-NMDAR encephalitis patients with disturbance of consciousness, combined immunotherapy and life support should be given as soon as possible, even if the condition is very critical and the treatment time is long. The patients may eventually have a good prognosis.
Mechanical ventilation, elevated IgG index, and delayed immunotherapy are independent prognostic factors in patients with anti-NMDAR encephalitis with disturbance of consciousness.
In this study, during the follow-up of 12 months after discharge, the mRS score in the disturbance of consciousness group was still significantly higher than that in the group without disturbance of consciousness (P < 0.001). Therefore, multivariate logistic regression analysis was used to analyze the factors affecting the long-term prognosis of patients with anti-NMDAR encephalitis with disturbance of consciousness. The patients were divided into two groups (good prognosis mRS≤2 and poor prognosis mRS>2). Logical regression analysis showed that some factors were independently related to the long-term outcome of patients with anti-NMDAR encephalitis with disturbance of consciousness, namely mechanical ventilation, elevated IgG index, and delayed immunotherapy. Using ROC analysis, it was found that the AUC of using the above three parameters to predict the prognosis was 0.971, indicating that it had a good reference value. Some studies have found that NMDAR N1 subunit knockout mice died of respiratory failure one day after birth, indicating that NMDAR is involved in the central regulation of respiration,28,29 which may be the reason for central hypopnea in patients with anti-NMDAR encephalitis. Patients with central hypopnea usually need mechanical ventilation to maintain vital signs, which leads to more serious outcomes and long-term mortality. Similar results have been reported in encephalitis of various causes. The more serious outcome is related to the need for long-term respiratory support.21,30 The IgG index is often used to monitor intrathecal immunoglobulin synthesis, which is an important index to evaluate the intensity of the central immune response. The more obvious the abnormal intrathecal synthesis, the stronger the central immune response. IgG in CSF is an immune response in which antibodies are produced locally in the central nervous system. Normally, the content of IgG in CSF is low, and IgG is not synthesized in the central nervous system. There are two sources of IgG in CSF: one is immunoglobulin synthesized in the sheath, and the other is synthesized in the liver through blood circulation through BBB into the sheath. When humoral immunity occurs, intrathecal IgG will increase accordingly, such a mechanism could involve cervical lymph nodes as key sites of antigen drainage and immune response initiation.31 Since the IgG index fully takes into account the influence of IgG in CSF caused by the increase of serum IgG and the destruction of BBB, it suggests that the accuracy of abnormal intrathecal synthesis is higher.22 The researchers found that intrathecal IgG synthesis increased in the middle and later stages of anti-NMDAR encephalitis.32 At present, there are few reports on the IgG index of cerebrospinal fluid in patients with anti-NMDAR encephalitis. Previous similar reports have shown that the increase in intrathecal IgG synthesis is significantly correlated with QAlb, while QALB is associated with poor short-term prognosis.33 This study shows that the IgG index can objectively reflect the degree of central inflammatory reaction and is an independent risk factor for poor prognosis in patients with anti-NMDAR encephalitis with disturbance of consciousness.
Some previous studies have found that the predictor of good functional outcomes is the lack of ICU admission and early treatment.4 Active targeted immunotherapy plays a key role in rapidly improving nervous system symptoms, promoting neurofunctional recovery, and controlling disease progression.23 Previous studies have also shown that the prognosis of AE patients receiving immunotherapy is good, and the shorter the time from onset to immunotherapy, the better the prognosis.34 Delayed immunotherapy will aggravate the irreversible damage caused by disturbance of consciousness, which has a significant effect on the prognosis. Therefore, for patients with anti-NMDAR encephalitis with disturbance of consciousness, immunotherapy should be given as soon as possible on the basis of symptomatic treatment, so as to shorten the time from onset to immunotherapy as far as possible, so as to strengthen the curative effect and improve the prognosis.
In summary, the novelty of this study is to describe the clinical characteristics of patients with anti-NMDAR encephalitis with disturbance of consciousness and to identify three risk factors that may affect their prognosis. Studying the disturbance of consciousness of anti-NMDAR encephalitis may help clinicians to better understand anti-NMDAR encephalitis, provide personalized treatment for these patients, evaluate the efficacy of treatment and prognosis. However, the main limitations of our study include the single-center, small sample, and retrospective of data, The longer follow-up time makes it more difficult to expand the samples. Although the sample size is sufficient for multivariate logistic regression for risk factor analysis, prospective studies with multiple centers and large samples are still needed. In the future, more specific questionnaires and detailed assessments (such as Modified Barthel Index Evaluation Scale, Clinical Scale for Autoimmune Encephalitis) can be used to better evaluate and detect potential risk factors for the prognosis in anti-NMDAR encephalitis patients with disturbance of consciousness.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, Guangxi, China. Written informed consents were obtained from all the patients enrolled in this study. All procedures were carried out in compliance with the Declaration of Helsinki. The authors confirm that all patient information is confidential.
Acknowledgments
The authors cordially thank the participants and their families.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (82060236) and the Natural Science Foundation of Guangxi Province (CN) (2019GXNSFDA245032).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dalmau J, Graus F, Ropper AH. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–851. doi:10.1056/NEJMra1708712
2. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi:10.1016/s1474-4422(10)70253-2
3. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045–1057. doi:10.1016/s1474-4422(19)30244-3
4. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi:10.1016/s1474-4422(12)70310-1
5. Nosadini M, Eyre M, Molteni E, et al. Use and safety of immunotherapeutic management of N-Methyl-d-Aspartate receptor antibody encephalitis: a meta-analysis. JAMA Neurol. 2021;78(11):1333–1344. doi:10.1001/jamaneurol.2021.3188
6. Lim JA, Lee ST, Jung KH, et al. Anti-N-methyl-d-aspartate receptor encephalitis in Korea: clinical features, treatment, and outcome. J Clin Neurol. 2014;10(2):157–161. doi:10.3988/jcn.2014.10.2.157
7. Zhang Y, Liu G, Jiang M, Chen W, He Y, Su Y. Clinical characteristics and prognosis of severe Anti-N-methyl-D-aspartate receptor encephalitis patients. Neurocrit Care. 2018;29(2):264–272. doi:10.1007/s12028-018-0536-6
8. Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655–1667. doi:10.1093/brain/awq113
9. Aungsumart S, Ha A, Apiwattanakul M. Abnormal level of consciousness predicts outcomes of patients with anti-NMDA encephalitis. J Clin Neurosci. 2019;62:184–187. doi:10.1016/j.jocn.2018.11.033
10. Qiao S, Wu HK, Liu LL, et al. Characteristics and prognosis of autoimmune encephalitis in the east of China: a multi-center study. Front Neurol. 2021;12:642078. doi:10.3389/fneur.2021.642078
11. Mo Y, Wang L, Zhu L, et al. Analysis of risk factors for a poor prognosis in patients with Anti-N-Methyl-D-aspartate receptor encephalitis and construction of a prognostic composite score. J Clin Neurol. 2020;16(3):438–447. doi:10.3988/jcn.2020.16.3.438
12. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi:10.1016/s1474-4422(15)00401-9
13. Mehta R, Chinthapalli K. Glasgow coma scale explained. BMJ. 2019;365:l1296. doi:10.1136/bmj.l1296
14. Conrad AJ, Chiang EY, Andeen LE, et al. Quantitation of intrathecal measles virus IgG antibody synthesis rate: subacute sclerosing panencephalitis and multiple sclerosis. J Neuroimmunol. 1994;54(1–2):99–108. doi:10.1016/0165-5728(94)90236-4
15. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi:10.1161/01.str.19.5.604
16. McKeon A. The importance of early and sustained treatment of a common autoimmune encephalitis. Lancet Neurol. 2013;12(2):123–125. doi:10.1016/s1474-4422(12)70319-8
17. Goldsmith PJ. NMDAR PAMs: multiple chemotypes for multiple binding sites. Curr Top Med Chem. 2019;19(24):2239–2253. doi:10.2174/1568026619666191011095341
18. Cruse D, Young GB. The complexity of disorders of consciousness. Clin Neurophysiol. 2016;127(2):1001–1002. doi:10.1016/j.clinph.2015.08.018
19. Webb AC. Consciousness and the cerebral cortex. Br J Anaesth. 1983;55(3):209–219. doi:10.1093/bja/55.3.209
20. Chi X, Wang W, Huang C, et al. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand. 2017;136(4):298–304. doi:10.1111/ane.12723
21. Lin J, Xiang Q, Liu X, Li J. Risk factors and prognosis in patients with Anti-N-Methyl-D-Aspartate receptor encephalitis requiring prolonged mechanical ventilation. Front Neurol. 2022;13:814673. doi:10.3389/fneur.2022.814673
22. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184(2):101–122. doi:10.1016/s0022-510x(00)00501-3
23. Vernino S, Geschwind M, Boeve B. Autoimmune encephalopathies. Neurologist. 2007;13(3):140–147. doi:10.1097/01.nrl.0000259483.70041.55
24. Florance-Ryan N, Dalmau J. Update on anti-N-methyl-D-aspartate receptor encephalitis in children and adolescents. Curr Opin Pediatr. 2010;22(6):739–744. doi:10.1097/MOP.0b013e3283402d2f
25. Mann AP, Grebenciucova E, Lukas RV. Anti-N-methyl-D-aspartate-receptor encephalitis: diagnosis, optimal management, and challenges. Ther Clin Risk Manag. 2014;10:517–525. doi:10.2147/tcrm.S61967
26. Armangue T, Titulaer MJ, Málaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162(4):850–856.e2. doi:10.1016/j.jpeds.2012.10.011
27. Liba Z, Sebronova V, Komarek V, Sediva A, Sedlacek P. Prevalence and treatment of anti-NMDA receptor encephalitis. Lancet Neurol. 2013;12(5):424–425. doi:10.1016/s1474-4422(13)70070-x
28. Waters KA, Machaalani R. Role of NMDA receptors in development of respiratory control. Respir Physiol Neurobiol. 2005;149(1–3):123–130. doi:10.1016/j.resp.2005.03.009
29. Liu Q, Wong-Riley MT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neuroscience. 2010;171(3):637–654. doi:10.1016/j.neuroscience.2010.09.055
30. Lin KL, Lin JJ. Neurocritical care for Anti-NMDA receptor encephalitis. Biomed J. 2020;43(3):251–258. doi:10.1016/j.bj.2020.04.002
31. Al-Diwani A, Theorell J, Damato V, et al. Cervical lymph nodes and ovarian teratomas as germinal centres in NMDA receptor-antibody encephalitis. Brain. 2022;145(8):2742–2754. doi:10.1093/brain/awac088
32. Wang R, Guan HZ, Ren HT, Wang W, Hong Z, Zhou D. CSF findings in patients with anti-N-methyl-D-aspartate receptor-encephalitis. Seizure. 2015;29:137–142. doi:10.1016/j.seizure.2015.04.005
33. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of Anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604. doi:10.1016/j.msard.2020.102604
34. Trewin BP, Freeman I, Ramanathan S, Irani SR. Immunotherapy in autoimmune encephalitis. Curr Opin Neurol. 2022;35(3):399–414. doi:10.1097/wco.0000000000001048
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.