Back to Journals » Journal of Pain Research » Volume 16
Risk Factors Analysis of Phantom Limb Pain in Amputees with Malignant Tumors
Authors Huo X , Huang P, Di H, Ma T, Jiang S, Yao J, Huang L
Received 5 August 2023
Accepted for publication 14 November 2023
Published 21 November 2023 Volume 2023:16 Pages 3979—3992
DOI https://doi.org/10.2147/JPR.S433996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Natalie Strand
Xiulin Huo,1,2 Peiying Huang,1 Hexuan Di,3 Tianxiao Ma,3 Sufang Jiang,1 Jie Yao,4 Lining Huang1
1Department of Anesthesiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 3Department of Orthopaedic Surgery, The Key Laboratory of Orthopedic Biomechanics of Hebei Province, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 4Department of Anesthesiology, The First Affiliated Hospital of Hebei North University, Zhangjiakou, Hebei, People’s Republic of China
Correspondence: Lining Huang, Department of Anesthesiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China, Email [email protected]
Purpose: Postamputation neuropathic pain is a common disease in patients with malignant tumor amputation, seriously affecting amputees’ quality of life and mental health. The objective of this study was to identify independent risk factors for phantom limb pain in patients with tumor amputation and to construct a risk prediction model.
Methods: Patients who underwent amputation due to malignant tumors from 2013 to 2023 were retrospectively analyzed and divided into phantom limb pain group and non-phantom limb pain group. To determine which preoperative factors would affect the occurrence of phantom limb pain, we searched for candidate factors by univariate analysis and used multivariate logistic regression analysis to identify independent factors and construct a predictive model. The receiver operating characteristic curve (ROC) was drawn to further evaluate the accuracy of the prediction model in evaluating the phantom limb pain after amputation of bone and soft tissue tumors.
Results: Multivariate analysis showed that age (OR, 1.054; 95% CI, 1.027 to 1.080), preoperative pain (OR, 5.773; 95% CI, 2.362 to 14.104), number of surgeries (OR, 3.425; 95% CI, 1.505 to 7.795), amputation site (OR, 5.848; 95% CI, 1.837 to 18.620), amputation level (OR, 8.031; 95% CI, 2.491 to 25.888) were independent risk factors for phantom limb pain for bone and soft tissue tumors. The the area under the curve (AUC) of this model was 0.834.
Conclusion: Risk factors for postoperative phantom limb pain were the site of amputation, proximal amputation, preoperative pain, multiple amputations, and older age. These factors will help surgeons to individualize and stratify phantom limb pain and help patients with risk counseling. In particular, an informed clinical decision targeting those modifiable factors can be considered when needed.
Keywords: neuropathic pain, phantom limb pain, bone and soft tissue tumors, amputation, risk factors, prediction model
Introduction
As early as the 16th century, Dr. Ambroise Pare, one of the founders of modern surgery, found that some of his patients could still feel the presence of their limbs and pain in those areas months after they had been amputated.1–3 In the 19th century, American neurologist Silas Weir Mitchell named the condition “phantom limb”, and the pain that the patient felt was called “phantom limb pain(PLP)”.4 A phantom limb is a phenomenon involving human neural induction rather than a patient’s imagination. Phantom limb pain is a very common neuropathic pain.5 According to statistics, up to 98% of amputated patients will have phantom limb sensation, and even about 50–80% of amputated patients will experience phantom limb pain.6–8 The prevalence of severe phantom limb pain(PLP)ranges from 5% to 10%.9,10 Neuropathic pain after amputation has a new name, that is, painful disability. Due to its huge impact on the quality of life and social function of patients, it is also a disease with the highest burden in the world. Phantom limb pain is often accompanied by emotional problems,11 such as low mood, anxiety, lack of speech, social loss, loss of confidence, etc., that is, amputation syndrome.12 These negative emotions in turn aggravate phantom limb pain.5 Although we have said goodbye to the era of treating phantom limb patients as mental patients, phantom limb pain after amputation is still not paid attention to by doctors and families.
Possibly due to the insufficient number of patients, the etiology of PLP is often unclassified in the published literature, and detailed analyses targeting a single cause are rarely performed. In addition to trauma, diabetes, and vascular atherosclerotic occlusion, upper and lower limb amputation caused by bone and soft tissue tumors is the first. However, phantom limb pain caused by amputation of bone and soft tissue tumors(BSTT) is rarely reported based on previous studies.8,13 Some studies have suggested that a history of diabetes is an influencing factor of phantom limb pain after amputation,14,15 and tumor amputation has also been suggested as a risk factor for phantom limb pain.13 The results of studies on different populations may not be applicable to amputees with BSTT. Management of neuropathic pain is particularly important for patients with amputation for malignancy, since adolescents make up a large proportion of this population and thus may have more recreational or social interaction needs than patients who are older or have amputation due to diabetes or vascular insufficiency. Therefore, it is necessary to conduct a separate study on phantom limb pain after amputation in people with BSTT.
Although the disease is easily diagnosed, the cause and risk factors for PLP are unknown and, accordingly, its prevention and treatment are inconsistent at all levels of healthcare.5,16–19 Review of previous literature, there is a paucity of studies on the etiology, prevalence, risk factors, and predictive models of PLP in China. In particular, there is a lack of detailed data on the incidence, risk factors, and predictive models of PLP after amputation for malignant tumors in Chinese patients. This is an obvious gap in the literature, as China has a population of more than 1.41 billion, accounting for 17% of the global population. The incidence and risk factors for PLP may differ in Western and Chinese countries due to differences in ethnicity, genetic background, national culture, and healthcare settings. Postoperative pain is often ignored by patients’ families and doctors, so some patients choose to tolerate the pain. Many studies conducted in China have found misconceptions about analgesics and insufficient emphasis on acute or chronic pain.20,21
BSTT have special clinical characteristics.22,23 The treatment of malignant BSTT is a worldwide problem. Compared with malignant tumors of other tissues and organs, the incidence of malignant BSTT is very low. The lack of understanding of malignant BSTT by primary doctors and patients leads to incorrect diagnosis and treatment. Before amputation, most of the patients had undergone long-term conservative treatment or multiple surgical treatments, chronic pain experience, or even pathological fractures. Malignant tumor is a systemic chronic disease with high mortality and disability rate, which brings heavy psychological and economic burden to patients and their families. Specific pathological types of malignant tumors have potential neurotropic characteristics and potential neurological damage caused by preoperative neoadjuvant chemotherapy. Therefore, it is very necessary to conduct systematic research on these factors.
Preoperative pain is considered to be a risk factor for PLP, however, there is a lack of relevant studies and predictive model value of preoperative pain in the malignant tumor population.8,10 Therefore, a retrospective study was performed to study the presence of preoperative pain and its predictive efficiency for postoperative phantom limb pain. Amputation site and level may also be a risk factor, and systematic studies of upper versus lower limb amputation and proximal versus distal amputation populations are urgently needed. The mechanism of phantom limb pain has not been clarified, and current studies believe that it mainly includes the central mechanism and peripheral mechanism.5,19,24 The risk factors for PLP in this study may be due to the role of central mechanisms such as cortical functional reorganization, central sensitization, and pain memory.25–27 It is undeniable that detailed epidemiological studies can provide more clues to reveal the mechanism of the phantom limb phenomenon.
Materials and Methods
Study Design
The clinical data of patients with bone and soft tissue malignant tumor amputation admitted to the Third Affiliated Hospital of Hebei Medical University, a regional comprehensive medical treatment center in North China from 2013 to 2023 were retrospectively analyzed. Data were collected retrospectively through electronic medical records. Phantom pain information can be accessed through the Electronic medical record (EMR), outpatient medical record, telephone follow-up, and readmission EMR (via a unique identification number). Patients were divided into phantom limb pain group and non-phantom limb pain group according to whether they had phantom limb pain or not.
The study was supervised and approved by the institutional internal review boards of the participating institutions (No.2023–055-1), in accordance with the Declaration of Helsinki, and consent was waived because this was an observational study without intervention. All data is recorded and analyzed anonymously in order to protect patient privacy. Patients and the public were not directly involved in the development of this clinical trial.
Study Participants
The study cohort consisted of 168 amputated patients with bone and soft tissue tumors (98 males and 70 females; amputation sites: upper extremity 27, lower extremity 141, amputation age 9–75 years old) and 2935 amputated patient records from the orthopedic ward database. All 2935 records were screened in advance and included the following inclusion criteria: amputation for bone and soft-tissue tumors and complete electronic medical records. Patients were also excluded based on the following criteria: inability to communicate with patients such as a history of mental disorders or dementia, incarceration, loss of patients or family members to follow-up (complete medical record documentation was not available, and telephone numbers and home addresses were not available.), comorbidity that resulted in moderate-to-severe functional limitations, refusal to participate in the study, and children younger than 6 years of age. Finally, 168 eligible records were obtained for analysis. The specific process of the study is shown in the figure (Figure 1).
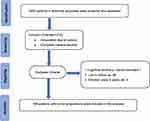 |
Figure 1 Flowchart of patient eligibility screening. |
Perioperative Treatment and Surgical Procedure
Our hospital has a special inpatient ward for bone and soft tissue cancer and a special inpatient ward for orthopedic diseases. Patients in the ward are assessed by a multidisciplinary team that includes at least two orthopedic surgeons, pathologists, internal medicine consultants responsible for the patient’s perioperative management, and an attending anesthesiologist and nurse. X-ray film, computed tomography, isotope whole body bone scan, nuclear magnetic resonance imaging and puncture biopsy were routinely taken before surgery. All patients were treated with amputation for malignant tumors in accordance with international treatment guidelines. Surgery is performed under general or regional anesthesia. These patients were subject to regular outpatient reviews or telephone interviews with patients or their families.
General Indications of Amputation During the Study Period
In this study, orthopedic oncologists routinely resorted to amputation to control malignancy in limbs that they considered unsalvageable. Usually, Amputation usually occurs when the tumor volume is large and causes limb dysfunction, or when the tumor continues to expand and penetrate key limb structures after chemotherapy, and the amputation is often accompanied by impaired blood perfusion or impaired sensitivity resulting in loss of limb function. In recent years, with the development of adjuvant therapy, especially neoadjuvant chemotherapy, the current treatment of malignant bone tumors is mainly based on surgery, chemotherapy, and radiotherapy, combined with a comprehensive treatment plan such as biological therapy, and immunotherapy. The survival rate and limb preservation rate of limb malignant tumors have been greatly improved. The surgical approach to salvage reconstruction and resection techniques have evolved, and we must acknowledge the fact that, even under similar indications, a greater proportion of patients are undergoing limb-salvage surgery in recent years than in previous years.
Primary and Secondary Study Goals
The main objective of this study was to identify independent risk factors for phantom limb pain in tumor patients and to establish a risk prediction model. Patients undergoing amputation due to malignant tumors from 2013 to 2023 were retrospectively analyzed and divided into phantom limb pain group and non-phantom limb pain group. In order to determine which preoperative factors would affect the occurrence of phantom limb pain, we searched for candidate factors through univariate analysis and used multivariate logistic regression analysis to identify independent factors and construct predictive models. The receiver operating characteristic curve (ROC) was drawn to further evaluate the accuracy of the prediction model in evaluating the phantom limb pain after amputation of bone and soft tissue tumors. These factors will help surgeons personalize and stratify phantom limb pain and help patients with risk counseling. In particular, informed clinical decisions regarding these modifiable factors can be considered when needed. A secondary objective of our study was to identify the incidence of phantom limb pain in Chinese patients with malignant tumor amputations.
Data Collection
The following items and information were collected from hospital records and telephone follow-up: age, sex, marriage, race, living environment (urban or rural), education level, smoking habits, drinking preferences, frequency of amputation (single or multiple amputations), left and right side of amputation, site of amputation (upper or lower limb), level of amputation (knee, elbow proximal or distal), American Society of Anesthesiologists (ASA) rating, and anesthetic method Pathological diagnosis of BSTT (yes or no), pre-amputation pain (yes or no), pre-operative chemotherapy (yes or no), phantom limb sensation (PLS), and phantom limb pain (yes or no). Postoperative wound growth in malignant tumors is slow due to systemic depletion and the effects of postoperative chemotherapy drugs. In this study, prosthetic limb was generally delayed and their information was not collected. If the respondent does not understand them, the interviewer can explain the question. The diagnostic criteria for phantom limb pain is that after amputation surgery, the patient subjectively feels that the limb that has been removed still exists, accompanied by pain of different degrees and nature. The distal limb is mostly in the position of flexion and convulsion, mostly in the distal stump, and the pain sensations are like electric shock, jumping pain, stabbing pain, drilling pain, crushing pain, burning pain, or twisting pain. Most of the pain is paroxysmal, but also persistent pain may occur. The definition of phantom limb sensation is that after amputation, the patient feels that the amputated limb still exists, and can clearly feel the activity of the amputated limb under the control of the brain, and even can feel its length, size, and temperature changes. Proximal amputation includes amputation of the elbow joint, amputation above the elbow, amputation of the knee joint, and amputation above the knee joint. Distal amputation is the amputation of the distal parts of the elbow and knee joints. All trial data extraction was done by experienced anesthesiologists, who were not aware of the study hypothesis and were not involved in the patient’s medical treatment process. They extracted the data, and if the results were significantly different, it was necessary to discuss the accuracy of the input data. By using standard data collection forms, information bias is minimized.
Statistical Analysis
The incidence of phantom limb pain after amputation has been reported to be about 50 to 80%. If 5 to 7 variables were evaluated in the regression model, the minimum sample size was about 50 to 70 phantom limb pain patients, so as to avoid violating the principle of about 10 outcome events for each independent variable in the regression model (the occurrence of PLP) and give full play to the power of the binary logistic regression model.28 We used SPSS software version 26 (IBM Corporation, New York, USA) for data analysis and ROC curves. Normality was tested for continuous variables using the Shapiro–Wilk test. Measurement data with normal distribution were presented as mean ± standard deviation, and measurement data with skewness distribution were present-ed as median and interquartile range. Categorical data were expressed as frequencies and proportions. Univariate regression analysis was used to obtain P values and unadjusted odds ratios (OR). Parameters with P value < 0.05 in the univariate regression analysis were used to conduct stepwise multivariate logistic regression analysis by forward method to determine the risk factors of PLP after amputation and establish a risk model. In addition, Hosmer and Lemeshow goodness-of-fit tests was implemented to verify the fitness of the logistic regression model. The logistic regression based prediction model was used to obtain the receiver operating characteristic (ROC) curve, and the area under the curve (AUC) was used to further evaluate the accuracy of the prediction model in evaluating phantom limb pain.
Results
Demographic and Clinical Characteristics of the Participants
All 2935 records were preselected (Figure 1). A total of 168 patients with bone and soft tissue tumors were included in the study. There were 98 males (58.3%) and 70 females (41.7%). The median (interquartile range) age was 48 (29–58) years, and the age range was 9–75 years. The amputation sites were upper limbs in 27 cases and lower limbs in 141 cases, including 140 cases of proximal amputation and 28 cases of distal amputation. In this study, 97 patients reported phantom limb pain and 71 patients had no phantom limb pain. In this study, 215 cases of bone and soft tissue tumors were investigated in detail, and 14 common pathological types of amputation were screened in detail. Osteosarcoma, soft tissue sarcoma, and chondrosarcoma are the most common causes of amputation for bone and soft tissue tumors. Adamantinoma, primitive neuroectodermal tumor, multiple myeloma, and dysplasia of bone and fibrous outcome are rare causes (Table 1).
 |
Table 1 Pathological Diagnosis of the Subjects |
Univariate Analysis of Two Groups of Patients
As can be seen from Table 2 and Table 3, the univariate analysis showed the significant difference between patients with and without phantom limb pain in terms of age (OR,1.021; 95% CI, 1.003 to 1.038; P= 0.019), ASA grade (II, OR,2.292; 95% CI,1.128 to 4.660; P=0.022), preoperative pain (OR,4.727; 95% CI,2.383 to 9.376; P < 0.001), number of amputations (OR,3.657; 95% CI, 1.825 to 7.328; P< 0.001), postoperative phantom limb sensation (OR,4.728; 95% CI2.091 to 10.690; P < 0.001), amputation level (OR,5.400; 95% CI,2.146 to 13.586; P < 0.001). When combined with previous literature and clinical experience,10,14 upper and lower limb amputation were forced into the model, although not significantly (OR,1.897; 95% CI,0.827 to 4.354). Because other variables were not statistically significant in univariate analysis (P>0.05), their influence on logistic multivariate regression analysis and prediction model construction could be ignored.
 |
Table 2 Comparison of Between Patients with and without Phantom Limb Pain by Univariate Analysis |
 |
Table 3 Level and Site of Amputation and Phantom Limb Pain by Univariate Analysis |
Independent Risk Factors for Phantom Limb Pain by Logistic Regression Analyses
Figure 2 shows the variables included in the multivariate analysis model. Multivariate analysis showed that age (OR,1.054; 95% CI,1.027 to 1.080), preoperative pain (OR,5.773; 95% CI,2.362 to 14.104), number of surgeries (OR,3.425; 95% CI,1.505 to 7.795), amputation site (OR,5.848; 95% CI,1.837 to 18.620), amputation level (OR,8.031; 95% CI,2.491 to 25.888) were independent risk factors for phantom limb pain after amputation for bone and soft tissue tumors. The detailed results are shown in Table 4.
 |
Table 4 Multivariate Analysis Identifying Factors Independently Associated with Phantom Limb Pain |
 |
Figure 2 Forest plot showing the effect of each variable against phantom limb pain in multivariate analysis. |
Construction of Phantom Limb Pain Prediction Model
According to the results of multivariate logistic analysis, a prediction model was established. ROC curves (Figure 3) were used to further evaluate the accuracy of all predictors. As shown in the figure, the area under the curve of the logistic regression model was 0.834 (95% CI: 0.773–0.896, p < 0.001). The cut-off value, sensitivity and specificity were 0.4732, 0.887, 0.662, respectively. The Hosmer-Lemeshow test showed that the prediction model had an acceptable goodness of fit (χ2 = 8.006, p = 0.433, Nagelkerke R2 = 0.427).
 |
Figure 3 Receiver operating characteristic curve (ROC) obtained by the risk model based on logistic regression. |
Discussion
Despite the extensive literature on the incidence and treatment of neuropathic pain, there is still a lack of studies detailing neuropathic pain after amputation for BSTT, especially in Chinese ethnicity. We explored neuropathic pain after amputation to identify independent risk factors and develop predictive models for phantom limb pain in amputated patients with BSTT. We first performed a univariate analysis of amputation patients with bone and soft tissue tumors. The post-screening variables were then included in a binary logistic regression model to develop a risk model and identify independent risk factors for PLP. The risk factors of PLP in patients with bone and soft tissue tumors were age, preoperative pain, number of operations, lower limbs (compared with upper limbs), and proximal amputation. Understanding the risk factors for phantom limb pain will help optimize patient care and provide necessary information for orthopedic surgeons and patient outcomes.
Pain brings not only mental pain but also an indelible huge impact on patients’ work, study, and life.29 Yin et al showed that daily activities were affected in 7.8% of patients, and social interactions were lost in 29.0% of patients. There were 17.3% and 18.9% patients with depression and loss of interests, 25.7% patients with sleep disorders, and even 16.1% patients with PLP wanted to die. 78.9% of the patients did not find effective treatment.21 In the disease code issued by the World Health Organization, there is a category of the disease called “painful disability”, which is defined as the social impairment caused by severe pain in some able-bodied people. Similar problems can occur with phantom limb pain, where prolonged pain often makes it difficult for these people to resume their original social functions, even if these functions are not affected by their limb. Thus, studies of risk factors for this disease are needed to guide future treatment.
One aim of this study was to investigate the prevalence of PLP in a Chinese population sample. Limakatso et al published a systematic review and meta-analysis of the incidence and risk factors of phantom limb pain in patients with amputation. Pooled across studies, the prevalence was estimated to be 64% but highly heterogeneous.8,13 PLP is a very common postoperative adverse event in patients with amputation. Most studies reported a prevalence of PLP of 50% to 80%,10 and our study showed a three-month postoperative prevalence of 57.7% in the population with amputation due to tumor. Yin et al from Sichuan University in China showed that 29% of amputees with mixed causes (injury 60.5%, chronic disease 21.1%, cancer 18.4%) developed phantom limb pain.21 However, the causes of amputation in all patients in this study were bone and soft tissue tumors, which is different from other studies where vascular diseases and trauma were the main causes of amputation. The incidence of PLP in patients with malignant amputations in the study by Jiang et al was 54.3%, but they did not exclude toe or finger amputations, and the inclusion criteria were all adult populations.13 Proximal amputation and lower limb amputation accounted for a large proportion of the amputation patients included in this study, which may increase the incidence of phantom limb pain. By reviewing the literature, it can be found that the prevalence of PLP reported in literatures varies greatly.30 Racial differences, different study methods, inclusion and exclusion criteria, follow-up period, and setting in which samples were collected may have contributed to the differences in incidence.31 When interpreting the results of different studies, close attention should be paid to the study design details of the literature. Possible reasons for the discrepancy between our study and the literature may be: subjects of this study are Chinese, while most of the previous literature were mainly white populations or black populations, and multiple biological, social, and psychological mechanisms may contribute to the difference in incidence.31–33 Second, our study was retrospective and therefore relied on complete medical records and patient or family memory. Some patients may forget about mild PLP after amputation. Finally, it is well known that there is a peak in the incidence of BSTT in adolescents. Unlike previous studies, we included adolescent patients with tumor amputation, and central reorganization and plasticity in adolescents may differ from that in older adults.
Although the conclusions regarding the relationship between preoperative pain and PLP are inconsistent, the higher risk of PLP in patients with amputation who have preamputation pain is the conclusion of most of the literature.5,8,15,34 In our study, 66.1% of PLP patients had pain before surgery due to malignant tumor invasion or pathological fracture. The incidence of phantom limb pain in patients with preoperative pain was 5.773 times higher than that in patients without preoperative pain. Noguchi et al used multivariate binary logistic regression analysis and concluded that a history of diabetes and preoperative pain that could not be controlled by analgesics were independent factors associated with the occurrence of phantom limb pain.15 Yin et al and Jiang et al reported that preoperative pain was a risk factor for PLP.13,21 There is a close relationship between phantom limb pain and chronic pain before operation and acute pain with poor control after operation. Ahmed et al found that 36.67% of adult tumor amputees had a distressing experience of preoperative pain.22 Interestingly, several other studies have also shown conflicting results.35–37 Preoperative pain was not closely related to the occurrence of phantom limb pain after amputation. Kooijman et al reported that there might be a lack of association between preoperative pain and phantom pain in upper limb amputation.36 Some results may be biased due to retrospective studies. Therefore, large sample multi-center prospective cohort studies should be conducted in the future.
We found an alarming 8.03-fold increase in the risk of postoperative PLP in patients with proximal amputation, and it is important to note that for statistical purposes, we did not distinguish between proximal upper and proximal lower limbs. There are several explanations for this “seemingly surprising” result. Firstly, this is more likely to reflect greater surgical trauma, with pelvic amputation or shoulder disconnection representing greater surgical trauma and postoperative recovery time, leading to an increased risk of postoperative pain and infection. Secondly, it may be due to the thicker, more concentrated nerve fibers in the proximal limb. Third, proximal amputation may indicate a larger projected area of the affected cortex, but experimental studies are needed to confirm the exact pathophysiological mechanism. Previous studies have shown that proximal amputees are more likely to develop PLP than distal amputees.8,36,38,39 Similar to the classification method used in this study, Ahmed et al divided the level of amputation into proximal and distal by elbow and knee.22 In their study, proximal amputations occurred above the knee (43%), above the elbow (17%), and distal amputations occurred below the knee (34%) and below the elbow (7%). The prevalence of PLP was higher at the proximal amputation level than at the distal amputation level. Dijkstra et al stated that lower limb amputation is the most important risk factor for PLP,40 while other studies showed that upper limb amputation is a risk factor.10,41 In this study, patients with lower limb amputation had a significantly higher incidence of PLP after surgery than those with upper limb amputation. The reasons for this phenomenon may be speculated in the following aspects. First of all, compared with the lower limbs, the physical advantages of the upper limbs are easy to be seen by the eyes, resulting in the fixed consciousness that the limbs no longer exist, and easy to produce visual compensation. Secondly, the hand movement is flexible, and the compensation of contralateral motion reaches unity faster. Third, the reason for this phenomenon may be the particularity of bone and soft tissue tumor populations. The incidence of malignant bone and soft tissue tumors was higher in the lower extremity than in the upper extremity, and the lower extremity was more likely to occur in the pelvis and femur. Tumors of the pelvis and proximal femur require special surgical methods, and the common surgical methods are hemipelvic resection and hip disarticulation, which are extremely traumatic, have a lot of blood loss and seriously affect the patient’s return to society (Figure 4). Prolonged bed rest and chemotherapy resulted in extremely slow recovery of all body functions after surgery. Fourth, lower limbs are more important than upper limbs for patients to return to social life and work. Those lacking lower limbs are more likely to be in a longer period of psychological pain and negative emotions.
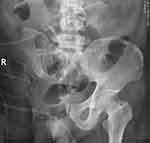 |
Figure 4 Radiographic image after hemipelvic resection. |
We observed a 3.4-fold increased risk of PLP in patients with BSTT who had undergone multiple surgeries. Jiang et al found that the number of amputations, proximal amputation, and preoperative pain may be factors associated with the strength of PLP.13 One possibility to explain this discrepancy is that PLP is directly proportional to the intensity of catastrophizing inputs involved in the perioperative period in patients with amputation. The more intense and painful the amputation experience, the more likely PLP is.
This study found that age increased the risk of PLP after amputation for bone and soft tissue tumors. This age effect confirms the finding by Gallagher et al that older age is associated with a higher prevalence of pain after amputation.42,43 It has been shown that amputees with congenital limb defects or childhood amputations usually have low or even nonexistent morbidity.44 The possible explanation is that central plasticity is relatively strong in children and adolescents, and due to lack of use, central reorganization processes may take over brain areas with limb deficits, resulting in a reduced incidence of PLS and PLP. However, Dr. Abdullah M. Kaki et al did not find any association with age in their study,45 and diabetic complications are the main cause of lower limb amputation in their study. Jonathan Lans, M.D., Ph.D.et al found the opposite results in the adult population,14 with a higher incidence of neuropathic pain among younger patients, which may be explained by a decrease in the regenerative potential with aging, since nerves tend to regenerate more rapidly in younger patients. The heterogeneity of results in different studies may be due to the fact that scholars have not stratified according to age, such as children, adolescents, young adults, middle-aged people and the elderly. Due to the rapid development of the treatment of BSTT, the comprehensive treatment based on limb salvage is becoming more and more dominant, so the data of tumor amputation is very precious and limited. We did not perform age subgroup analyses due to tumor sample size issues. In future studies, large sample data should be adequately collected for age subgroup analysis.
We developed a predictive model to identify risk factors for phantom limb pain after surgery for bone and soft tissue tumors. The prediction model showed a strong predictive ability. The Hosmer-Lemeshow test was used to evaluate the goodness of fit of the prediction model, and the results showed that the goodness of fit was within the acceptable range. The clinical application of this prediction model is helpful to identify individuals at high risk of phantom limb pain after surgery, help medical staff to choose a more appropriate treatment mode, and improve patient outcomes.
Despite the multifaceted adjustment for confounding factors and the relatively large sample size of bone and soft tissue tumors, some limitations of this study should be noted. First, its inherent limitation is the retrospective design, which may affect the accuracy of data collection. Patient or family memory may be biased due to the lower survival rate of amputees with malignant tumors. In addition, pain experience is highly subjective, and self-reported phantom limb pain cannot be objectively verified. Third, this was a single-center study in a tertiary referral facility, and the situation may be different in primary care units, so it is not representative of the average. Fourthly, due to the particularity of the study sample, the difference of time node and data collection environment, this study only carried out risk factor analysis from a limited number of aspects, failing to include all risk factors, such as whether to wear prosthetic limbs, rehabilitation training, stump pain, etc. It is hoped that there will be a larger sample size of data for all-round research in the future.
Conclusions
Based on previous literature, this is one of the few attempts to provide epidemiological evidence for amputees with BSTT. In this study, we investigated the risk factors for the development of phantom limb pain after amputation for bone and soft tissue tumors and developed a predictive model. Five factors were identified as independently associated with phantom limb pain: preoperative pain, proximal amputation, lower limb amputation (compared with upper limb amputation), number of surgeries, and age. These factors will help orthopedic surgeons and anesthesiologists to individualize and stratify the risk of complications and predict that active intervention can be achieved. In particular, more informed clinical decisions can be made to target these modifiable factors when needed, ultimately improving outcomes for amputees. Future studies with prospective and multicenter designs are warranted to verify our findings.
Data Sharing Statement
The data sets used and analyzed during the current study are available from the corresponding authors upon reasonable request.
Acknowledgments
All authors are thanked for their participation and dedication. Thanks to Zekun Li and Huanhuan Li for their help in the data collection process. We thank Cunhui Zhang for help in statistical analysis.
Funding
This research was supported by the Medical Science Research Project of Hebei Province, China in 2023 (20230753) and the provincial medical excellent talents project funded by the government in 2022 (30320222704).
Disclosure
The authors declare that there is no conflict of interest in the publication of this article.
References
1. Finger S, Hustwit MP. Five early accounts of phantom limb in context: paré, descartes, lemos, bell, and mitchell. Neurosurgery. 2003;52(3):675–686. doi:10.1227/01.NEU.0000048478.42020.97
2. Splavski B, Rotim K, Boop FA, Gienapp AJ, Arnautović KI. Ambroise paré: his contribution to the future advancement of neurosurgery and the hardships of his times affecting his life and brilliant career. World Neurosurg. 2020;134:233–239. doi:10.1016/j.wneu.2019.10.187
3. Park MT, Mignucci-Jiménez G, Houlihan LM, Preul MC. Management of injuries on the 16th-century battlefield: ambroise paré’s contributions to neurosurgery and functional recovery. Neurosurg Focus. 2022;53(3):E2. doi:10.3171/2022.6.FOCUS21710
4. Nathanson M. Phantom limbs as reported by s. Weir mitchell. Neurology. 1988;38(3):504–505. doi:10.1212/wnl.38.3.504
5. Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurology. 2002;1(3):182–189. doi:10.1016/S1474-4422(02)00074-1
6. Culp CJ, Abdi S. Current understanding of phantom pain and its treatment. Pain Physician. 2022;25(7):E941–E957.
7. Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anaesth. 2001;87(1):107–116. doi:10.1093/bja/87.1.107
8. Limakatso K, Bedwell GJ, Madden VJ, Parker R, Eshraghi A. The prevalence and risk factors for phantom limb pain in people with amputations: a systematic review and meta-analysis. PLoS One. 2020;15(10):e240431. doi:10.1371/journal.pone.0240431
9. Nikolajsen L, Jensen TS. Phantom limb pain. Curr Rev Pain. 2000;4(2):166–170. doi:10.1007/s11916-000-0052-0
10. Kuffler DP. Origins of phantom limb pain. Mol Neurobiol. 2018;55(1):60–69. doi:10.1007/s12035-017-0717-x
11. Sahu A, Sagar R, Sarkar S, Sagar S. Psychological effects of amputation: a review of studies from India. Ind Psychiatry J. 2016;25(1):4–10. doi:10.4103/0972-6748.196041
12. Fuchs X, Flor H, Bekrater-Bodmann R, Edita N, Navratilova E. Psychological factors associated with phantom limb pain: a review of recent findings. Pain Res Manag. 2018;2018:5080112–5080123. doi:10.1155/2018/5080123
13. Jiang S, Zheng K, Wang W, Pei Y, Qiu E, Zhu G. Phantom limb pain and sensations in Chinese malignant tumor amputees: a retrospective epidemiological study. Neuropsychiatr Dis Treat. 2021;17:1579–1587. doi:10.2147/NDT.S299771
14. Lans J, Groot OQ, Hazewinkel MHJ, et al. Factors related to neuropathic pain following lower extremity amputation. Plast Reconstr Surg. 2022;150(2):446–455. doi:10.1097/PRS.0000000000009334
15. Noguchi S, Saito J, Nakai K, Kitayama M, Hirota K. Factors affecting phantom limb pain in patients undergoing amputation: retrospective study. J Anesth. 2019;33(2):216–220. doi:10.1007/s00540-018-2599-0
16. Neumüller J, Lang-Illievich K, Brenna CTA, Klivinyi C, Bornemann-Cimenti H. Calcitonin in the treatment of phantom limb pain: a systematic review. CNS Drugs. 2023;37(6):513–521. doi:10.1007/s40263-023-01010-x
17. Iberite F, Muheim J, Akouissi O, et al. Restoration of natural thermal sensation in upper-limb amputees. Science. 2023;380:6646):731–735. doi:10.1126/science.adf6121
18. Schone HR, Baker CI, Katz J, et al. Making sense of phantom limb pain. J Neurol Neurosurg Psychiatry. 2022;93(8):833–843. doi:10.1136/jnnp-2021-328428
19. Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168–2176. doi:10.1172/JCI94003
20. Chen G, Lai W, Hu Z, et al. The dragon strikes: lessons from the wenchuan earthquake. Anesth Analg. 2010;110(3):908–915. doi:10.1213/ANE.0b013e3181cbc62c
21. Yin Y, Zhang L, Xiao H, et al. The pre-amputation pain and the postoperative deafferentation are the risk factors of phantom limb pain: a clinical survey in a sample of Chinese population. BMC Anesthesiol. 2017;17(1). doi:10.1186/s12871-017-0359-6
22. Ahmed A, Bhatnagar S, Mishra S, Khurana D, Joshi S, Ahmad S. Prevalence of phantom limb pain, stump pain, and phantom limb sensation among the amputated cancer patients in India: a prospective, observational study. Indian J Palliat Care. 2017;23(1):24–35. doi:10.4103/0973-1075.197944
23. Döring K, Trost C, Hofer C, et al. How common are chronic residual limb pain, phantom pain, and back pain more than 20 years after lower limb amputation for malignant tumors? Clin Orthop Relat Res. 2021;479(9):2036–2044. doi:10.1097/CORR.0000000000001725
24. Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive cns plasticity? Nat Rev Neurosci. 2006;7(11):873–881. doi:10.1038/nrn1991
25. Bao B, Sun Y, Lin J, et al. Altered cortical thickness and structural covariance networks in upper limb amputees: a graph theoretical analysis. CNS Neurosci Ther. 2023;29:2901–2911. doi:10.1111/cns.14226
26. Bao B, Zhu H, Wei H, et al. Altered intra- and inter-network brain functional connectivity in upper-limb amputees revealed through independent component analysis. Neural Regen Res. 2022;17(12):2725. doi:10.4103/1673-5374.339496
27. Makin TR, Flor H. Brain (re)organisation following amputation: implications for phantom limb pain. Neuroimage. 2020;218:116943. doi:10.1016/j.neuroimage.2020.116943
28. Biccard BM, Gopalan PD, Miller M, et al. Patient care and clinical outcomes for patients with covid-19 infection admitted to african high-care or intensive care units (acccos): a multicentre, prospective, observational cohort study. Lancet. 2021;397(10288):1885–1894. doi:10.1016/S0140-6736(21)00441-4
29. Makin TR. Phantom limb pain: thinking outside the (mirror) box. Brain. 2021;144(7):1929–1932. doi:10.1093/brain/awab139
30. Stankevicius A, Wallwork SB, Summers SJ, Hordacre B, Stanton TR. Prevalence and incidence of phantom limb pain, phantom limb sensations and telescoping in amputees: a systematic rapid review. Eur J Pain. 2021;25(1):23–38. doi:10.1002/ejp.1657
31. Rahim-Williams FB, Riley JR, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in african americans and hispanics. Pain. 2007;129(1–2):177–184. doi:10.1016/j.pain.2006.12.016
32. Kim HJ, Yang GS, Greenspan JD, et al. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain. 2017;158(2):194–211. doi:10.1097/j.pain.0000000000000731
33. Güereca YM, Kell PA, Kuhn BL, et al. The relationship between experienced discrimination and pronociceptive processes in native americans: results from the Oklahoma study of native American pain risk. J Pain. 2022;23(6):1006–1024. doi:10.1016/j.jpain.2021.12.010
34. Larbig W, Andoh J, Huse E, et al. Pre- and postoperative predictors of phantom limb pain. Neurosci Lett. 2019;702:44–50. doi:10.1016/j.neulet.2018.11.044
35. Wall R, Novotny-Joseph P, Macnamara TE. Does preamputation pain influence phantom limb pain in cancer patients? South Med J. 1985;78(1):34–36. doi:10.1097/00007611-198501000-00009
36. Kooijman CM, Dijkstra PU, Geertzen JHB, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87(1):33–41. doi:10.1016/S0304-3959(00)00264-5
37. Richardson C, Crawford K, Milnes K, Bouch E, Kulkarni J. A clinical evaluation of postamputation phenomena including phantom limb pain after lower limb amputation in dysvascular patients. Pain Manag Nurs. 2015;16(4):561–569. doi:10.1016/j.pmn.2014.10.006
38. Mioton LM, Dumanian GA, Fracol ME, et al. Benchmarking residual limb pain and phantom limb pain in amputees through a patient-reported outcomes survey. Plast Reconst Surg Global Open. 2020;8(7):e2977. doi:10.1097/GOX.0000000000002977
39. Lans J, Hoftiezer Y, Lozano-Calderón SA, Heng M, Valerio IL, Eberlin KR. Risk factors for neuropathic pain following major upper extremity amputation. J Reconstr Microsurg. 2021;37(05):413–420. doi:10.1055/s-0040-1718547
40. Dijkstra PU, Geertzen JHB, Stewart R, van der Schans CP. Phantom pain and risk factors: a multivariate analysis. J Pain Symptom Manage. 2002;24(6):578–585. doi:10.1016/S0885-3924(02)00538-9
41. Davidson JH, Khor KE, Jones LE. A cross-sectional study of post-amputation pain in upper and lower limb amputees, experience of a tertiary referral amputee clinic. Disabil Rehabil. 2010;32(22):1855–1862. doi:10.3109/09638281003734441
42. Gallagher DAMM. Phantom limb pain and residual limb pain following lower limb amputation: a descriptive analysis. Disabil Rehabil. 2001;23(12):522–530. doi:10.1080/09638280010029859
43. Münger M, Pinto CB, Pacheco Barrios K, et al. Protective and risk factors for phantom limb pain and residual limb pain severity. Pain Pract. 2020;20(6):578–587. doi:10.1111/papr.12881
44. Diers M, Fuchs X, Bekrater-Bodmann R, Flor H. Prevalence of phantom phenomena in congenital and early-life amputees. J Pain. 2023;24(3):502–508. doi:10.1016/j.jpain.2022.10.010
45. Almehman DA, Faden AS, Aldahlawi BM, Bafail MS, Alkhatieb MT, Kaki AM. Post-amputation pain among lower limb amputees in a tertiary care hospital in jeddah, Saudi Arabia. Saudi Med J. 2022;43(2):187–196. doi:10.15537/smj.2022.43.2.20210609
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
