Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Ribociclib Plus Letrozole in Italian Male Patients with Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Case Studies of Phase 3b CompLEEment-1 Trial
Authors Caputo R , Fabi A, Romagnoli E, Baldini E, Grasso D, Fenderico N , Michelotti A
Received 1 June 2022
Accepted for publication 9 August 2022
Published 18 October 2022 Volume 2022:14 Pages 351—362
DOI https://doi.org/10.2147/BCTT.S376902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Roberta Caputo,1 Alessandra Fabi,2,3 Emanuela Romagnoli,4 Editta Baldini,5 Donatella Grasso,6 Nicola Fenderico,6 Andrea Michelotti7
1Dipartimento di Senologia, Istituto Nazionale Tumori Fondazione Pascale, IRCCS, Naples, Italy; 2Dipartimento di Oncologia Medica, Istituto Nazionale Tumori Regina Elena - Istituti Fisioterapici Ospitalieri, Rome, Italy; 3Precision Medicine in Breast Cancer, Department of Woman and Child Health and Public Health, Scientific Directorate, Fondazione Policlinico Universitario Agostino Gemelli, IRCCS, Roma, Italy; 4U.O.C. Oncologia, Presidio Ospedaliero di Macerata, Macerata, Italy; 5Director of the U.O.C. Medical Oncology, San Luca Hospital via Guglielmo Lippi Francesconi, Lucca, Italy; 6Oncology, Novartis Farma SpA, Origgio, Italy; 7UO Oncologia Medica 1, Azienda Ospedaliero Universitaria Pisana, Ospedale S. Chiara, Pisa, Italy
Correspondence: Roberta Caputo, Dipartimento di Senologia, Istituto Nazionale Tumori Fondazione Pascale, IRCCS, Via Mariano Semmola, 53, Napoli, 80131, Italy, Tel +39 3339714308, Fax +39 0815903726, Email [email protected]
Abstract: Male breast cancer (BC) is rare, globally constituting only 0.5– 1% of all patients with BC. In Italy, more than 2000 new male BC cases were registered between 2000 and 2014. The survival rate was lower in males than in females. Delayed diagnosis may be the reason for poorer outcome observed in male BC patients compared with female patients. Due to lack of substantial evidence and low availability of published data on male BC, the current treatment recommendations are based on evidence derived from trials on female patients. In Italy, most of the male BC patients are estrogen and progesterone receptor–positive. Targeted therapy in combination with endocrine therapy provides a clinically meaningful outcome in patients with hormone receptor–positive (HR-positive), human epidermal growth factor receptor 2–negative (HER2-negative) advanced BC. CompLEEment-1 is a single-arm, open-label, multicenter, phase 3b trial investigating the safety and efficacy of a CDK4/6 inhibitor, ribociclib, in combination with letrozole in men and women. Herein, we report the results from a retrospective analysis of five Italian male patients who completed the core phase. In this case series, the combination of ribociclib and letrozole was well tolerated and appeared to be effective in the male cohort with HR-positive, HER2-negative advanced BC in Italy. CompLEEment-1 trial representative of a real-world setting would add value by supporting the existing efficacy and safety profile of ribociclib in combination with letrozole in male patients with HR-positive, HER2-negative advanced BC.
ClinicalTrials.gov Registration Number: NCT02941926.
Keywords: CDK4/6 inhibitor, targeted therapy, male breast cancer, progression-free survival, case report
Introduction
Male breast cancer (BC) is rare, globally constituting only 0.5–1% of all patients with BC with increasing incidence rates from 8.5 thousand in 1990 to 23.1 thousand in 2017.1,2 In Italy, over 2000 new male BC cases were registered between 2000 and 2014,3 most patients being estrogen receptor–positive (ER-positive; 96.4%) and progesterone receptor–positive (PR-positive; 82.5%). Nearly half of the estimated male patients had high levels of cell proliferation marker Ki-67 and 14.7% had evidence of human epidermal growth factor receptor-2 (HER2) amplification.3 Lower 5-year survival rate in males3 could be attributed to delayed diagnosis, leading to more advanced tumor stages, and associated comorbidities given that male patients are often elderly at the time of diagnosis.1,4
With lack of published data on male patients, the current treatment guidelines are derived from studies conducted on female BC patients.1 Targeted therapy in combination with endocrine therapy (ET) have shown to provide a clinically meaningful outcome in these patients.5 Ribociclib, a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i), in combination with ET is approved for first- or second-line treatment of HR-positive, HER2-negative advanced BC6–9 based on the data from phase 3 MONALEESA trials that consistently demonstrated survival benefit compared to ET monotherapy.10–12
CompLEEment-1 (NCT02941926) is a single-arm, open-label, multicenter, phase 3b trial investigating the safety and efficacy of ribociclib combined with letrozole in a diverse patient cohort representative of real‑world clinical practice, including men, pre-, peri-, or postmenopausal women who have not received prior ET for advanced disease. The trial was conducted in accordance with the Good Clinical Practice guidelines and complies with the Declaration of Helsinki. Herein, we report the results from a retrospective analysis of the five Italian male patients who completed the core phase (cut-off date: November 8, 2019).
Case Presentation
Case Report 1
A male patient aged 48 years who was a habitual smoker of about 10 cigarettes/day for 20 years with a familial history of BC was diagnosed with a nodule of about 3 cm in the periareolar region of the right breast in October 2011. At the time of screening, the patient had no reported comorbidities. Genetic testing revealed the absence of BRCA1 or BRCA2 mutations. He underwent radical mastectomy of the right breast with axillary lymph node dissection, and subsequent histological examination showed infiltrating ductal carcinoma with poor cellular differentiation. Immunohistochemistry showed positive staining for ER (70%), PR (20%), Ki-67 (50%), and receptor tyrosine-protein kinase c-erbB2 (0). Final pathological tumor node metastasis stadium was pT2 (29 mm) pN1a (1+/16) M0. Later, the patient received adjuvant chemotherapy with epirubicin and cyclophosphamide for four cycles every 3 weeks, followed by hormonal therapy with tamoxifen 20 mg/day. In June 2016, a computed tomography (CT) scan revealed multiple lung and bone metastases. From June 23, 2016 to July 7, 2016, the patient underwent radiotherapy (RT) on the vertebral spine (3 Gy/die for 10 days on D12-L4) and sacrum (3 Gy/die for 10 days on L5-sacrum) for analgesic purposes. In July 2016, he received a frontline chemotherapy with bevacizumab in combination with paclitaxel for advanced BC. In September 2017, CT scan showed bone progression, new liver metastases, and a suspected right occipital metastasis. Consequently, patient underwent palliative RT in a single fraction (7 Gy single fraction) on some metamers of the lumbar spine (L2-L4) and on the fourth right rib. In October 2017, magnetic resonance imaging (MRI) revealed a well-enhanced focal solid lesion in the right occipital area (12 mm). From October 30, 2017 to November 6, 2017, the patient had undergone stereotaxic RT on the right occipital lesion (30 Gy in five fractions). By the end of November 2017, he was advised to initiate second-line chemotherapy with vinorelbine and capecitabine.
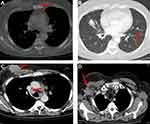
However, to find the best treatment choice in a complex patient with brain, lung, liver, and bone metastases, the patient was enrolled into the CompLEEment-1 study program in December 2017. He had no visceral crisis and was asymptomatic due to brain metastasis, which was clinically stable for 4 weeks. Furthermore, the patient was not taking corticosteroid and antiepileptic therapy. He had Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 and reported bone pains controlled with low-dose opioid therapy. In late November 2017, a baseline CT scan showed metastatic disease to right occipital area, lung, liver, bones (12th thoracic vertebral body, 1st lumbar vertebral body, 3rd and 4th lumbar vertebral body, sacrum, the 3rd, 4th, and 5th right ribs, right humerus, and sternum handlebar). A basal bone scan was performed according to the protocol. Baseline laboratory parameters: cancer antigen (CA) 15–3 was 285.1 U/mL (0.0–30.0 U/mL) (Figure S1), carcinoembryonic antigen (CEA) of 24.1 ng/mL (0.0–5.0 ng/mL) (Figure S2), WBC count of 8.10 K/µL, hemoglobin of 14.9 g/dL, platelet count of 249 K/µL, and complete metabolic panel was normal. QTcF was 425 ms and resting heart rate was 86 bpm at screening electrocardiogram. The patient received ribociclib 600 mg/day (3 weeks on/1 week off) combined with letrozole 2.5 mg/day continuous and concomitant luteinizing hormone–releasing hormone (LHRH) agonist goserelin 3.6 mg once/month subcutaneously starting from December 2017. Additionally, the patient was given denosumab 120 mg subcutaneously every month to prevent skeletal-related events. First re-evaluation was carried out at the end of February 2018 after 12 weeks of therapy. The CT scan showed partial pulmonary (lung metastasis to right upper lobe 13 × 11 vs 16 × 12 mm) and hepatic response (metastasis to eighth hepatic segment 15 × 13 vs 19 mm) (Figure 1A and B, Figure 2A and B) and stable bone and brain disease. In addition, tumor markers had halved (CA 15–3 were 130.5 vs 285.1 U/mL and CEA were 6.1 vs 24.1 ng/mL). No hematological toxicity was reported, and metabolic panel continued to be normal. No QTcF prolongation was observed. The patient no longer reported bone pain, and his ECOG PS was 0. By August 2018, after 10 cycles of therapy, the revaluation CT scan showed further reduction of lung (lung metastasis to the right upper lobe 11 × 6 vs 16 × 12 mm) and liver metastases (metastasis to the eighth hepatic segment 12 × 9 vs 19 mm and to the fifth hepatic segment 13 vs 15 mm) (Figure 3A and B, Figure 4A and B). The same CT scan showed an increase in size of the right occipital metastasis (22 vs 5 mm) (Figure 5A and B). The tumor markers showed further reduction (CA 15–3: 64.0 U/mL; CEA: 3.2 ng/mL). The patient reported mild headache for few days.
 |
Figure 1 (A) Red arrows show lung metastases at baseline and (B) after 12 weeks of therapy with ribociclib. |
 |
Figure 2 (A) Red arrows show liver metastases at baseline and (B) after 12 weeks of therapy with ribociclib. |
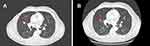 |
Figure 3 (A) Red arrows show lung metastases at baseline and (B) after 10 cycles of therapy with ribociclib. |
 |
Figure 4 (A) Red arrows show liver metastases at baseline and (B) after 10 cycles of therapy with ribociclib. |
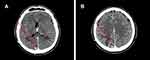 |
Figure 5 (A) Red arrows show brain metastases at baseline and (B) after 10 cycles of therapy with ribociclib. |
In September 2018, the brain MRI confirmed an increase in size of the right occipital lesion due to a large central colliquative necrosis suggestive of radionecrosis, requiring close monitoring per radiotherapist’s discretion. Therefore, the patient continued CompLEEment-1 trial. In November 2019, after 2 years of therapy without dose reduction, the revaluation CT scan showed liver response and stable disease in another site. The laboratory parameters continued to be normal. CA 15–3 continued to decline (49.4 U/mL) and CEA was normal. Brain metastasis was monitored with MRI. In July 2020 after 31 months of therapy, CT scan showed complete hepatic response and stable disease in other sites (Figure 6A and B). In February 2021, after 3 years and 2 months of therapy, CT scan showed hepatic progression of disease (Figure 7A and B). Tumor markers were on the rise: CA 15–3 was 116.4 U/mL and CEA was 20.8 ng/mL. Progression-free survival (PFS) was 49.0 months and improvement in quality of life (QOL) was observed. No new adverse event (AE) was reported. No dose reduction was needed. However, the patient discontinued the combination treatment of ribociclib and letrozole due to the hepatic and biohumoral progression of the disease, and in March 2021, the patient started therapy with eribulin.
 |
Figure 6 (A) Hepatic complete response and (B) stable brain disease after 31 months of therapy with ribociclib. |
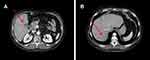 |
Figure 7 (A) and (B) Red arrows show liver metastases after 49 months of therapy with ribociclib. |
Case Report 2
A male patient aged 66 years was initially diagnosed for early BC in December 2011 at the age of 54 years. He underwent right mastectomy with sentinel lymph node biopsy in January 2011. The patient had an infiltrating ductal carcinoma of 20 mm and no BRCA1 or 2 mutations. Immunohistochemistry showed ER (95%), PR (20%), Ki-67 (30%), HER2 (1 positive); PT1c N0sn M0. The patient received tamoxifen for 5 years (2011–2016). In September 2017, multiple lung and liver metastases were detected in the abdomen and chest CT scan. The patient underwent a lung biopsy, and the histological examination revealed secondary lesion compatibility with breast primitiveness (ER [95%]; PR [20%]; Ki-67 [30%]; HER2 [1 positive]).
In December 2017, the patient was enrolled in the CompLEEment-1 study. After a month treatment with ribociclib and letrozole, the patient reported a grade 2 treatment-related neutropenia. No dose modification was necessary, and the event resolved spontaneously by January 2018. A similar AE was observed in March 2018 and neither dose modification nor delay was applied, and the patient recovered by May 2018. No serious AEs were observed. The best response to treatment was stable disease according to RECIST 1.1 criteria. The last follow-up visit was on November 8, 2021. The patient continued to have stable disease with good general condition (ECOG PS 0). The treatment is ongoing and the next follow-up visit is programmed in April 2022.
Case Report 3
A male patient aged 73 years, nonsmoker, with no relevant medical history except acute diverticulitis and G1 uricemia was treated with medical therapy at the age of 67 and 51 years, respectively. The patient was initially diagnosed with ulcerated nodule at the right inframammary sulcus and multiple axillary lymph nodes in May 2017. CT scan showed right breast swelling (40 × 51 mm) with infiltration of the right nipple. A highly suspicious metastatic lesion was observed in some right axillary and mediastinal lymphadenopathies, lung, and bone (sternum and right hemisome of D8) (Figure 8A–D). In May 2017, breast biopsy revealed dermal infiltration of poorly differentiated carcinoma of mammary origin. Immunohistochemistry showed ER (98%), PR (0), Ki-67 (20%), HER2 (0), and axillary lymph node aspiration malignant neoplasm.
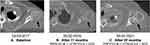
In July 2017, the patient was enrolled in CompLEEment-1 trial. In October 2017, partial response was achieved at first tumor evaluation after three treatment courses. Ribociclib was well tolerated, and no grade >2 toxicity was observed. The most frequent toxicities were thrombocytopenia, hot flashes, arthralgia, and grade 1 myalgia. As per the CT scan evaluation (April 2020), partial response in breast was observed, lymph nodes were maintained, skeletal disease was stable, and the pulmonary nodule was hardly recognizable (Figure 9A–D). In April 2020, the patient developed severe cutaneous rash to the iodinated contrast medium. Disease re-evaluations with positron emission tomography fluorodeoxyglucose (PET FDG) evaluations (August 2020 and January 2021) showed a low hypermetabolism in the breast localization (SUV max 3.2) and no pathological uptake in the lung, lymph node, and skeletal sites (Figure 10). Treatment with ribociclib in combination with letrozole is ongoing, with good tolerability.
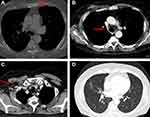 |
Figure 9 CT scan evaluation after treatment. (A) Red arrows in the figures show sternum lytic metastasis, (B) mediastinal lymph node, (C) axillary lymph node, and (D) lung metastasis. |
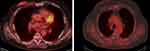 |
Figure 10 PET FDG evaluation. |
Case Report 4
A male patient aged 60 years suffered from left shoulder pain at the age of 56 years (September 2016) and was diagnosed with BC. After 3 months of empirical treatments, X-ray and MRI recorded the presence of a lithic area at the left humerus. In January 2017, bone biopsy revealed a grade 3 metastatic lesion from an adenocarcinoma, with the expression of neuroendocrine markers and focal prostate-specific antigen (PSA). Involvement of several bone sites, such as left humerus (SUV max 8.9), C7 vertebra (SUV max 5.4), left scapula (SUV max 8.2), right iliac wing (SUV max 9.3), and left sterno-clavicular, was evident in 18F-FDG PET systemic staging. Therefore, there was a non-specific focal uptake at the prostate and in the ascending colon. The evaluations aimed at finding a colon carcinoma via colonoscopy were negative, whereas the prostatic ultrasound showed a left hypoecogenic area with an irregular capsula, even if the blood serum PSA was in range. In February 2017, a subsequent prostatic biopsy excluded the presence of a prostatic neoplasm. The patient underwent a total body CT scan in March 2017, which confirmed the left humerus lesions, and revealed a left retro areolar lesion of 13 mm diameter, left supraclavicular nodes of 10 mm diameter, aortic pulmonary nodes of 18 mm diameter, and nodules in adrenal glands (right of 12 mm diameter and left of 17 mm diameter) (Figure 11A). Due to this suspicious lesion at the left mammary gland and the increase of CA 15–3 marker (72.3 vs normal value up to 35), the patient underwent an ultrasound and X-ray examination. In mammary biopsy (match), a diagnosis of grade 2/3 ductal infiltrating carcinoma was made, and the molecular pattern linked to a luminal subtype (ER [90%], PR [20%], HER2 [0]). For bone metastasis, the patient received RT in the left humerus (DT 45 Gy), and he started initial bisphosphonates, and subsequently denosumab 120 mg every 28 days.
In March 2017, the patient enrolled into the CompLEEment-1 program. The patient also began therapy for bone pain (ibuprofen + prednisone). ECOG PS was 1. After 11 months of treatment, CT scan showed disappearance of the known lymph nodes, stability of adrenal gland lesions, and an important response of the left humerus lytic metastasis (Figure 11B). After 25 months, the humerus lesion showed healing (Figure 11C) and stabilization of the other lesions. Patient stopped analgesic drugs (ECOG PS was 0) and is continuing combined ribociclib and letrozole for a total of 52 months until now and is in good clinical condition, maintaining partial response of humerus lesion and a stable disease of the other metastasis without any side effects. The treatment is ongoing, and restaging was planned in January 2022 with a stability of bone disease.
Case Report 5
A male patient aged 67 years with no past medical history had initial palpated mass in right breast in June 2017. The biopsy revealed infiltrating carcinoma, ER (95%), PR (90%), Ki-67 (60%), HER2 (1-positive not overexpressed/negative) (Figure S3). CT scan of the chest abdomen pelvis showed numerous bilateral pulmonary nodules suspicious for metastatic disease, right axillary lymphadenomegaly diameter of 1.7 cm, lymphadenomegaly in the right hilum of 1.5 cm, and small nodular formation in the right breast periareolar of 1.7 cm. Bone scan confirmed a minute osteolytic alteration middle arch VIII right breast (Figure 12A–D). Physical examination showed skin nodules with ulceration of the right breast. The patient was staged as cT4cN1aM1 stage IV.
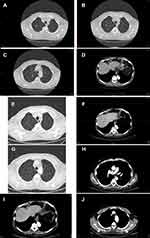 |
Figure 12 (A–D and G–J) CT scan of bone, lymph node, lung; (E and F) follow-up CT scan lung and lymph node. |
The patient started ribociclib 600 mg/day (3 weeks on/1 week off) plus letrozole 2.5 mg/day continuous. Baseline laboratory parameters were: CA 15–3, 12.3 U/mL (0.030.0 U/mL); CEA, 1.8 U/mL (<3 U/mL); WBC, 3.5 K/μL; hemoglobin, 16.6 g/dL; platelet, 168 K/μL (150,450 K/μL); and absolute neutrophil 2.01 K/μL. Complete metabolic panel was normal. Tolerability was good with grade 1 fatigue, grade 1 hot flashes, and grade 3 neutropenia at 15th day of treatment with normal complete metabolic panel. Second cycle of therapy was initiated in the same regimen.
Follow-up CT scan in October 2017 showed reduction of all pulmonary and lymph nodal metastases (Figure 12E and F). The patient showed persistent grade 2 neutropenia at the third cycle, and ribociclib dose was reduced to 400 mg; further reduction of ribociclib dosage was performed at the fourth and fifth cycle (at 200 mg). CT scan showed stable disease until the last examination in October 2021 (Figure 12G–J).
Discussion
Due to lack of substantial evidence and published data on male BC, the current treatment guidelines are derived from studies on female patients.1 The etiology is poorly understood, partly due to its relative scarcity. Several risk factors are identified in male BC, including genetic and hormonal abnormalities. By histological subtype, the invasive ductal carcinomas that present with atypical features are dominant in male patients, followed by the papillary carcinomas.13 Similar to our series, evidence suggests 42% of the male BC cases diagnosed in TNM stage 3 or 4,14 with median age at the time of diagnosis above 60 years.15
A close relation between the BRCA1 and BRCA2 gene mutation and male BC was observed in patients with BC.16 However, in these case series, none of the patients had BRCA mutations. Conditions reported to be associated with the occurrence of breast neoplasms in male patients are cirrhosis,17 obesity, testicular trauma, radiation therapy exposure, and use of exogenous estrogen.18 Estrogen and progesterone receptors were delineated to play a role in male BC, which are present in approximately 90% and 81% of the male BC cases, respectively.13 Overexpression of HER2 is related to worst prognosis in patients.19
Male patients with endocrine responsive disease often receive tamoxifen as the optimal adjuvant therapy. The response rate and overall survival (OS) by adjuvant chemotherapy is less studied.16 Some studies have demonstrated an improved efficacy compared with adjuvant anthracycline-based therapies.14 To date, chemotherapy with subsequent RT is considered as the standard of care for male patients with ductal carcinomas. However, disease progression was evident post chemotherapy or RT.
Targeted therapies in combination with ET demonstrate promising clinical outcomes in patients with HR-positive, HER2-negative advanced BC.5 In the phase 3 MONALEESA trials, ribociclib in combination with ET has consistently showed a clinically meaningful survival benefit compared to ET alone.10 A significant improvement in OS benefit was observed in postmenopausal patients with HR-positive, HER2-negative advanced BC in ribociclib and ET arm vs ET arm in both MONALEESA-2 and −3 trials (63.9 vs 51.4 mo; HR, 0.76 and 53.7 vs 41.5 mo; HR, 0.73, respectively).20,21 In the MONALEESA-7 trial, ribociclib in combination with a nonsteroidal aromatase inhibitor demonstrated significant OS benefit in premenopausal women with advanced disease.22
The phase 3b, CompLEEment-1 trial aimed to explore safety, tolerability, and clinical efficacy of ribociclib in combination with letrozole in patients with HR-positive, HER2-negative advanced BC. A primary analysis of CompLEEment-1 study based on 3246 patients demonstrated favorable safety profile, consistent with phase 3 MONALEESA-2 trial data.23 The efficacy and safety results from the subgroup analysis of male patients in CompLEEment-1 trial supported the use of ribociclib in combination with letrozole in HR-positive, HER2-negative advanced BC in a close to real-world setting.24
In this case series, except for one patient (case 1), none had a medical history of BC. All five patients presented with high ER, PR, and Ki-67 expression, and HER2-negative status. All had prior chemotherapy and subsequent RT or surgery. However, disease progression led these heavily pretreated patients to enroll into the CompLEEment-1 program.
Ribociclib in combination with letrozole given in the patient (case 1) with central nervous system metastasis who had received RT showed clinically improved PFS, QOL, and similar safety profile. Although dose reduction was not needed, this patient discontinued the treatment due to hepatic and biohumoral progression. Another patient with lung and liver metastases (case 2) had stable disease with excellent general condition. No new safety signal was observed and grade 2 neutropenia being the only AE, resolved spontaneously without requiring any dose modification. The patient is continuing the combination treatment of ribociclib and letrozole. Partial response was observed in an elderly man (case 3) after three cycles of treatment. Ribociclib was well tolerated with consistent safety profile. Another 60-year-old male patient (case 4) with a grade 3 metastatic bone lesion showed healing and stabilization of the lesions after 2 years of treatment. A long-term clinical benefit was observed in this male patient with luminal advanced BC treated with ribociclib as frontline therapy. A 67-year-old man (case 5) with bone metastasis exhibited a normal metabolic panel after receiving ribociclib in combination with letrozole. Clinical response was maintained even after the dose reduction that followed persistent grade 2 neutropenia.
In this case series of Italian male patients with advanced BC, the combination of ribociclib and letrozole was well tolerated and ribociclib appeared to be effective. CompLEEment-1 trial representative of a real-world setting would add value by supporting the existing efficacy and safety profile of ribociclib in combination with letrozole in male patients with HR-positive, HER2-negative advanced BC.
Abbreviations
ABC, advanced breast cancer; AE, adverse event; BRCA1/2, breast cancer gene1/2; CA, cancer antigen; CDK4/6i, cyclin-dependent kinase 4 or 6 inhibitor; CEA, carcinoembryonic antigen; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; ER-positive, estrogen receptor–positive; ET, endocrine therapy; FDG, fluorodeoxyglucose; HER2-negative, human epidermal growth factor receptor-2-negative; HR-positive, hormone receptor–positive; Ki-67, cell proliferation marker; LH-RH, luteinizing hormone-releasing hormone; MRI, magnetic resonance imaging; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PR-positive, progesterone receptor–positive; PSA, prostate-specific antigen; QOL, quality of life; QTcF, QT prolongation with Fredericia correction; RECIST, Response Evaluation Criteria in Solid Tumors; RT, radiotherapy; TNM, tumor, nodes, metastases; vs, versus; WBC, white blood cells.
Data Sharing Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval
The trial was conducted in accordance with the Good Clinical Practice guidelines and complies with the Declaration of Helsinki. The protocol and informed consent form were reviewed and approved by a properly constituted Institutional Review Board/Independent Ethics Committee/Research Ethics Board before study commencement. The names of the approving ethics committees are listed in Table S1.
Consent to Participate and Publish
Informed consent for participation and publication was obtained from each participant in this research prior to study commencement.
Acknowledgments
The CompLEEment-1 trial (ClinicalTrials.gov identifier NCT02941926). Financial support for medical editorial assistance was provided by Novartis Farma SpA, Italy. The authors thank the patients who have enrolled in this study and their families, as well as all the participating investigators and their site teams. The authors also thank Arundhati Halder, PhD, of Novartis Healthcare Pvt Ltd (Hyderabad, India) for providing medical editorial assistance in accordance with the Good Publication Practice (GPP3) guidelines for the preparation of this manuscript.
Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Disclosure
Grasso D is an employee of Novartis Farma and holds stocks/stock options from Novartis; has received grants, payments, support for attending meetings/travel from Novartis; occupies leadership/fiduciary role in other board, society, committee or advocacy group, paid or unpaid, and participation on a data safety monitoring board/advisory board in Novartis; and has received equipment, materials, drugs, medical writing, gifts, or other services from Novartis. Fenderico N is an employee of Novartis Farma, has received grants, payments, support for attending meetings/travel from Novartis, and has participated on a data safety monitoring board/advisory board in Novartis. Caputo R reports Grant, financial fees, speaker bureau that would give the appearance of potentially influencing. The authors report no other conflicts of interest in this work.
References
1. Yalaza M, Inan A, Bozer M. Male breast cancer. J Breast Health. 2016;12(1):1–8. doi:10.5152/tjbh.2015.2711
2. Chen Z, Xu L, Shi W, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990-2017. Breast Cancer Res Treat. 2020;180(2):481–490. doi:10.1007/s10549-020-05561-1
3. Mangone L, Ferrari F, Mancuso P, et al. Epidemiology and biological characteristics of male breast cancer in Italy. Breast Cancer. 2020;27(4):724–731. doi:10.1007/s12282-020-01068-1
4. Zanna I, Silvestri V, Palli D, et al. Smoking and FGFR2 rs2981582 variant independently modulate male breast cancer survival: a population-based study in Tuscany, Italy. Breast. 2018;40:85–91. doi:10.1016/j.breast.2018.04.017
5. Bottcher TM, Cold S, Jensen AB. Treatment of advanced HR+/HER2- breast cancer with new targeted agents in combination with endocrine therapy: a review of efficacy and tolerability based on available randomized trials on everolimus, ribociclib, palbociclib and abemaciclib. Acta Oncol. 2019;58(2):147–153. doi:10.1080/0284186X.2018.1532603
6. National Comprehensive Cancer Network. Clinical practice guidelines in oncology - breast cancer (version 1.2022); 2022. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419.
7. Gennari A, Andre F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi:10.1016/j.annonc.2021.09.019
8. Shah AN, Metzger O, Bartlett CH, Liu Y, Huang X, Cristofanilli M. Hormone receptor-positive/human epidermal growth receptor 2-negative metastatic breast cancer in young women: emerging data in the era of molecularly targeted agents. Oncologist. 2020;25(6):e900–e908. doi:10.1634/theoncologist.2019-0729
9. Rossi V, Berchialla P, Giannarelli D, et al. Should all patients with HR-positive HER2-negative metastatic breast cancer receive CDK 4/6 inhibitor as first-line based therapy? A network meta-analysis of data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT trials. Cancers. 2019;11(11):1661. doi:10.3390/cancers11111661
10. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a Phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi:10.1093/annonc/mdy155
11. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316.
12. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi:10.1056/NEJMoa1911149
13. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51–57. doi:10.1002/cncr.20312
14. Madeira M, Mattar A, Passos RJ, et al. A case report of male breast cancer in a very young patient: what is changing? World J Surg Oncol. 2011;9(1):16. doi:10.1186/1477-7819-9-16
15. Cutuli B. Strategies in treating male breast cancer. Expert Opin Pharmacother. 2007;8(2):193–202. doi:10.1517/14656566.8.2.193
16. Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi:10.1200/JCO.2002.20.6.1480
17. Misra SP, Misra V, Dwivedi M. Cancer of the breast in a male cirrhotic: is there an association between the two? Am J Gastroenterol. 1996;91(2):380–382.
18. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995;82(3):341. doi:10.1002/bjs.1800820319
19. Bruce DM, Heys SD, Payne S, Miller ID, Eremin O. Male breast cancer: clinico-pathological features, immunocytochemical characteristics and prognosis. Eur J Surg Oncol. 1996;22(1):42–46. doi:10.1016/S0748-7983(96)91418-3
20. Hortobagyi GN, Burris HA, Yap YS, et al. Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32(suppl 5):S1283–S1346.
21. Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi:10.1016/j.annonc.2021.05.353
22. Lu YS, Im SA, Colleoni M, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851–859. doi:10.1158/1078-0432.CCR-21-3032
23. De Laurentiis M, Borstnar S, Campone M, et al. Full population results from the core phase of CompLEEment-1, a phase 3b study of ribociclib plus letrozole as first-line therapy for advanced breast cancer in an expanded population. Breast Cancer Res Treat. 2021;189(3):689–699. doi:10.1007/s10549-021-06334-0
24. Campone M, De Laurentiis M, Zamagni C, et al. Ribociclib plus letrozole in male patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: subgroup analysis of the phase IIIb CompLEEment-1 trial. Breast Cancer Res Treat;2022. 25. doi:10.1007/s10549-022-06543-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.