Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Research Progress on Molecular Subtyping and Modern Treatment of Triple-Negative Breast Cancer
Authors Tong L, Yu X , Wang S , Chen L, Wu Y
Received 15 June 2023
Accepted for publication 15 August 2023
Published 24 August 2023 Volume 2023:15 Pages 647—658
DOI https://doi.org/10.2147/BCTT.S426121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Ling Tong,1,2,* Xiangling Yu,1,* Shan Wang,1,2 Ling Chen,2 Yibo Wu1
1Human Reproductive and Genetic Center, Affiliated Hospital of Jiangnan University, Wuxi, People’s Republic of China; 2Department of Breast Surgery, Affiliated Hospital of Jiangnan University, Wuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Chen, Department of Breast Surgery, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China, Email [email protected] Yibo Wu, Human Reproductive and Genetic Center, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China, Email [email protected]
Abstract: Breast cancer has become the most common malignant tumor worldwide. Triple-negative breast cancer (TNBC) is a type of breast cancer that is negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Compared with other molecular subtypes of breast cancer, TNBC is the most aggressive and highly heterogeneous. TNBC is insensitive to endocrine and anti-HER2 therapy, and chemotherapy is currently the main systemic treatment. With the continuous development of detection techniques and deepening research on TNBC molecular subtypes, drugs targeting immune checkpoints and different targets have emerged, such as atezolizumab, pembrolizumab, poly (ADP-ribose) polymerase (PARP) inhibitors, trophoblast cell-surface antigen 2 (TROP-2), and antibody-drug conjugates. These therapies provide new hope for TNBC treatment. Based on the analysis and classification of TNBC, this article summarizes the immunotherapy, targeted therapy, and new treatment combinations, providing references for the precise treatment of TNBC in the future.
Keywords: triple-negative breast cancer, immunotherapy, targeted therapy, precision therapy
Introduction
Breast cancer is currently the most common malignant tumor in women worldwide. According to data from the International Agency for Research on Cancer, there were 2.1 million new cases of breast cancer and nearly 700,000 deaths due to breast cancer globally in 2020.1 Breast cancer can be classified into hormonal receptor-positive (HR), HER2-positive, and triple-negative breast cancer (TNBC) based on molecular markers. TNBC, which accounts for 15–20% of all breast cancers, is a type of malignant tumor with a poor prognosis. It is characterized by negative expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), and its high degree of biological heterogeneity and invasiveness, make it the most aggressive and heterogeneous type of breast cancer.2,3 Due to its unique biological behavior, it is insensitive to endocrine therapy and traditional HER2-targeted therapy, and chemotherapy is still considered the main systemic treatment for TNBC. However, there is a significant portion of patients who develop resistance to chemotherapy in clinical practice.4 Therefore, identifying therapeutic targets (Such as EGFR, P13k/AKT/Mtor pathway, etc.) and more effective treatment modalities for TNBC is a clinical challenge and priority that needs to be urgently addressed.
With the vigorous development of bioinformatics analysis technology and omics research, cancer research is gradually developing in the direction of multi-omics and refinement. In recent years, a variety of potential therapeutic targets have been discovered from the aspects of genomics, metabolomics, and proteomics, and many of the research results have certain clinical transformation value. Therefore, it is a future research direction to formulate corresponding treatment plans according to the unique and complex molecular characteristics of tumors of each patient, to achieve precise treatment of diseases, and to improve treatment efficacy and survival outcomes. This article will summarize the new progress in the treatment of TNBC, and provide a basis for establishing a precise treatment plan for TNBC in the future.
Molecular Subtyping of TNBC
TNBC is not a singular tumor but a highly heterogeneous mixed-type tumor, with significant differences in tumor biology and drug sensitivity among different patients. With the development of detection technologies, researchers can analyze the biological characteristics of tumors from multiple dimensions such as gene sequences, protein expression, and metabolism. By identifying specific biological features, they can discover crucial therapeutic targets. Therefore, identifying the molecular subtyping of TNBC will provide a foundation for further finding critical therapeutic targets, developing targeted drugs, and establishing precise treatment strategies.
Based on different molecular features, multiple methods have been developed to subtype TNBC. In 2011, Lehmann et al conducted clustering analysis on gene expression profile data of TNBC patients and divided TNBC into six subtypes, namely basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), luminal androgen receptor (LAR), mesenchymal (M), and mesenchymal stem-like (MSL).5 In 2019, Jiang et al used a transcriptome-wide analysis of Chinese TNBC samples and categorized TNBC into four subtypes - BLIS, IM, LAR, and MES. They also revealed the critical molecular targets of each subtype and proposed precise treatment strategies based on molecular subtyping6 (Figure 1).
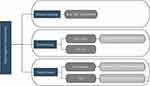 |
Figure 1 Modern treatment framework for triple-negative breast cancer patients. Abbreviations: PARP, Poly ADP-ribose polymerase; ADC, Antibody-drug conjugate. |
Immunotherapy
The immune checkpoint receptors, programmed cell death protein 1(PD-1)/ programmed cell death ligand 1 (PD-L1), are upregulated on activated T cells and are induced in the tumor microenvironment by TNF-γ. Tumor cells over-express the ligand for PD-1 and PD-L1, which inhibits the activation of T cells and cytotoxic T cells through the PD-1/PD-L1 axis, shortening the survival time of T cells while weakening their ability to fight against tumor cells, ultimately leading to cancer immune escape.7 Preclinical studies have also found that blocking the PD-1/PD-L1 pathway can increase macrophage phagocytosis, thereby slowing tumor growth and extending survival time in mouse models8 (Figure 2).
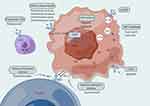 |
Figure 2 Illustrative diagram of drug treatment mechanisms for triple-negative breast cancer. |
PD-L1 Monotherapy for Advanced TNBC
TNBC is more responsive to immune checkpoint blockade therapy than other subtypes of breast cancer due to its higher level of immunogenicity, tumor-infiltrating lymphocytes (TILs), and PD-L1 expression. Atezolizumab, a PD-L1 inhibitor, is an Fc-humanized monoclonal IgG1 antibody that selectively binds to and inhibits the interaction of PD-1 and B7.1 receptors with PD-L1, leading to increased T cell sensitivity to tumors. In a Phase I study of a clinical trial, the safety and antitumor activity of atezolizumab were evaluated in metastatic TNBC patients, showing good clinical outcomes and potential benefits. Atezolizumab is now a first-line immunotherapy drug for survival benefits in metastatic TNBC patients.9 Although monotherapy is effective, most TNBC patients have a poor response to single-agent immunotherapy; therefore, adding chemotherapy drugs can directly stimulate T and B cell responses, improve the tumor immune microenvironment, and enhance tumor immunity through phagocytosis, exerting a multi-effect regulatory role.10,11
PD-L1 in Combination with Chemotherapy is Used for Advanced TNBC
According to the results of the Phase III IMpassion130 trial, the combination of atezolizumab with albumin-bound paclitaxel demonstrated significant improvement in progression-free survival (PFS) when compared to the placebo plus albumin-bound paclitaxel group. Additionally, there was a significant benefit in overall survival (OS) within the PD-L1 positive subgroup.12,13 These results suggest that atezolizumab in combination with albumin-bound paclitaxel is an important first-line treatment for patients with metastatic or locally advanced TNBC. In the phase III IMpassion131 trial, patients were randomized to receive atezolizumab plus paclitaxel or placebo plus paclitaxel. Adding atezolizumab to paclitaxel did not improve PFS in the PD-L1 positive population. The median PFS for the atezolizumab plus paclitaxel group and the placebo plus paclitaxel group were 6 months and 5.7 months, respectively. The final results showed no difference in OS between the two groups. It is speculated that the reason for this could be the use of steroids, which may have weakened the effect of the immune checkpoint inhibitor, as patients in IMpassion131 received more steroids than those in IMpassion130.14
PD-L1 in Combination with (Neo) Adjuvant Chemotherapy is Used for Early-Stage TNBC
In the phase III IMpassion031 trial, untreated stage II–III TNBC patients were randomized to receive chemotherapy in combination with atezolizumab or a placebo. Chemotherapy consisted of albumin-bound paclitaxel. The pathological complete response rate for the placebo group was only 41.4%, while the atezolizumab group was 57.8%, indicating that adding atezolizumab to neoadjuvant chemotherapy significantly improved the patient’s pathological complete response (PCR) rate and safety.15 Another animal experiment demonstrated that as neoadjuvant therapy, paclitaxel chemotherapy plus PD-L1 inhibitor significantly improved the OS of mice compared to paclitaxel chemotherapy plus anti-VEGF-A antibody.16 In addition, a Phase II GepaarNUEVO randomized trial compared the efficacy of neoadjuvant chemotherapy plus durvalumab with placebo in untreated early-stage TNBC patients. According to the latest results, there was no significant difference between the durvalumab group and the placebo group in the primary endpoint of PCR. However, significant differences were observed in the secondary endpoints of disease-free survival (DFS) and OS, with 3-year OS rates of 95.2% and 83.5% for the chemoimmunotherapy group and chemotherapy group alone, respectively. The researchers concluded that adding durvalumab to neoadjuvant chemotherapy significantly improved the long-term outcomes of TNBC patients.17
Ongoing studies include a trial (NCT03281954) investigating whether neoadjuvant chemotherapy in combination with atezolizumab is superior to chemotherapy plus placebo given before breast cancer surgery, but the joint primary endpoints of PCR and event-free survival (EFS) are yet to be reported further.18 An open-label phase IIIb global study (NCT04148911) is observing the efficacy of atezolizumab plus albumin-bound paclitaxel in participants with unresectable locally advanced or metastatic PD-L1 positive TNBC. Another study (NCT03371017) is evaluating the efficacy and safety of atezolizumab in combination with chemotherapy compared to placebo plus chemotherapy in patients with unresectable recurrent TNBC.
Programmed Death-1 (PD-1) Monotherapy is Effective in Treating Advanced TNBC
Pembrolizumab, a PD-1 inhibitor, was first proposed for TNBC treatment in 2014. The KEYNOTE-012 trial confirmed the safety and antitumor activity of pembrolizumab monotherapy in advanced TNBC patients.19 In the phase II KEYNOTE-086 trial, subjects were divided into cohort A and cohort B. Cohort A included previously treated patients with metastatic TNBC, while cohort B included treatment-naive patients with metastatic TNBC. All patients in cohort B were PD-L1 positive, while 61.8% of patients in cohort A were PD-L1 positive. The results indicated that pembrolizumab as the first-line treatment for PD-L1 positive metastatic TNBC had significant efficacy.20 However, the phase III KEYNOTE-119 trial, which recruited 622 patients with metastatic TNBC, showed that the OS of the pembrolizumab group was 10.7 months, while the chemotherapy group was 10.2 months. Improvement in OS was observed with increasing PD-L1 enrichment in tumors classified as PD-L1 positive. Overall, the pembrolizumab group did not significantly improve the OS of previously treated metastatic TNBC patients compared to chemotherapy.21 This difference may be due to the development of drug and immune resistance in patients previously treated with systemic therapy.
Efficacy of PD-1 in Combination with Chemotherapy in Treating Advanced TNBC
In a phase III first-line study (KEYNOTE-355) targeting patients with metastatic TNBC, untreated patients with locally recurrent or metastatic TNBC were randomly assigned to receive chemotherapy plus placebo or pembrolizumab plus chemotherapy. The results showed that compared to chemotherapy plus placebo, the pembrolizumab plus chemotherapy group significantly improved PFS in patients with a higher expression of PD-1. The median PFS for pembrolizumab plus chemotherapy was 9.7 months, while the median PFS for chemotherapy plus placebo was 5.6 months. Pembrolizumab plus chemotherapy significantly improved PFS in patients with CPS≥10 metastatic triple-negative breast cancer compared to the placebo chemotherapy group.22
PD-1 Combined with Neoadjuvant Therapy for Early-Stage TNBC
The latest development in the treatment of early-stage TNBC is the addition of immune checkpoint inhibitors to neoadjuvant chemotherapy, which has shown significant advantages in terms of EFS and PCR. The results of a systematic review indicate that regardless of PD-L1 status, compared to placebo, pembrolizumab has better efficacy in early-stage TNBC patients.23 A Phase 1b open-multi-cohort KEYNOTE-173 study showed that neoadjuvant chemotherapy combined with pembrolizumab treatment in high-risk, early-stage TNBC patients resulted in lower toxicity and effective anti-tumor activity.24 In addition, in the KEYNOTE-522 study, previously untreated stage II or III TNBC patients who received neoadjuvant therapy were randomly assigned in a 2:1 ratio to receive standardized chemotherapy drugs. Midterm analysis showed that in early-stage triple-negative breast cancer patients, compared to those who only received chemotherapy, the addition of pembrolizumab to chemotherapy significantly increased the PCR rate, and pembrolizumab combined with chemotherapy was superior to chemotherapy alone. Furthermore, patients who continued to use pembrolizumab after surgery had significantly prolonged EFS.25 Based on the positive results of the KEYNOTE-522 study, the FDA approved the combination of pembrolizumab and chemotherapy as neoadjuvant therapy and continued use of pembrolizumab as adjuvant therapy in patients with high-risk early-stage TNBC after surgery.
Ongoing PD-1 studies include a multicenter phase II trial (NCT05068141) aimed at evaluating the efficacy and safety of PD-1 in combination with albumin-bound paclitaxel in advanced TNBC patients; a randomized phase II trial (NCT05809895) studying whether the combination of tislelizumab and chemotherapy can improve PFS in breast cancer patients; and another trial (NCT05447702) evaluating the efficacy and safety of camrelizumab in combination with apatinib and chemotherapy as neoadjuvant therapy for TNBC patients.
In summary, current studies suggest that compared to single-agent chemotherapy, the use of combination chemotherapy and immunotherapy in treating TNBC patients can significantly improve anti-tumor effects. Furthermore, early combination therapy, which involves the addition of immunotherapy to neoadjuvant chemotherapy, may have better and more long-lasting anti-tumor effects.
Immunotherapy Combined with Radiotherapy
Radiotherapy has a dual effect of inducing DNA damage-mediated tumor cell death and immune modulation, which can make the tumor microenvironment more inflammatory and promote immune modulation. A study evaluated the efficacy and safety of the combination of pembrolizumab and radiotherapy in metastatic TNBC patients. The results showed that the overall response rate (ORR) of patients in the pembrolizumab combined with the radiotherapy group was 17.6%, and it was found that the combination of the two was safe and showed encouraging results in patients with poor prognosis and metastatic TNBC.26
The combination of immunotherapy and radiotherapy is promising in the treatment of TNBC and provides a new treatment direction for advanced TNBC patients. Ongoing studies on radiotherapy combined with immunotherapy include a phase II study (NCT04690855) aimed at evaluating the efficacy and safety of talazoparib, radiotherapy, and atezolizumab in PD-L1-positive metastatic TNBC patients; a phase II clinical trial (NCT03483012) that observes whether the combination of atezolizumab and stereotactic radiosurgery can enhance the immune response of TNBC patients to cancer, as well as the efficacy and safety of treatment; and a phase II study (NCT04837209) that evaluates the safety and effectiveness of the combination of durvalumab, niraparib, and radiotherapy in metastatic TNBC.
Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4)
CTLA-4 is a member of the immunoglobulin-related receptor family that competitively binds to CD80 dimers and CD86 monomers on antigen-presenting cells (APCs) in order to inhibit the co-stimulatory process of T cells, and can also inhibit autoimmune responses by suppressing TH17 (T helper subsets) differentiation and activating treg cells.27 Currently, clinical CTLA-4 antagonists include ipilimumab and tremelimumab. One trial showed that combination therapy with ipilimumab and nivolumab had good efficacy in treating TNBC patients, with patients benefiting from it.28 In vitro, studies have shown that ipilimumab affects the regulation of the tumor microenvironment and increases immune responses by releasing IL-2.29 Another clinical study included recurrent malignant tumor patients who were observed to have enhanced CTLA-4 expression and increased levels of activated T lymphocytes after a single infusion of ipilimumab.30
DNA Damage Repair and Targeted Therapy with PARP Inhibition
Poly (ADP-Ribose) Polymerase Inhibitors (PARPi) as Monotherapy and in Combination with Chemotherapy for TNBC
Poly ADP-ribose polymerase (PARP) is a superfamily of 18 proteins that control genetic stability and DNA repair by participating in the DNA base excision repair pathway. PARP-1 and PARP-2 proteins are induced by DNA breakage and are involved in the DNA repair process. Therefore, PARP inhibitors are important therapeutic drugs for BRCA-mutated TNBC. In a randomized phase III OLYMPIA trial (NCT02032823) evaluating olaparib in patients with germline BRCA-mutated and high-risk HER2-negative primary breast cancer who have completed local treatment or neoadjuvant or adjuvant chemotherapy, the primary outcome being PFS. In the TNBC subset, the median PFS in the olaparib group was significantly longer than that in the standard treatment group, with a 3-year disease-free survival of 85.9% in the olaparib group and 77.1% in the placebo group.31 In the phase III randomized controlled OlympiAD trial (NCT02000622), olaparib monotherapy was compared with standard treatment in patients with germline BRCA-mutated and HER2-negative metastatic breast cancer. Patients were randomly assigned to receive olaparib tablets or TPC (capecitabine, eribulin, or vinorelbine) therapy. The median OS in the olaparib group was 19.3 months, compared to 17.1 months in the TPC group. Although there was no significant improvement in OS in the olaparib group compared with TPC, there may be a meaningful benefit in terms of OS for patients who have not received chemotherapy for metastatic disease.32 While PARP inhibitors have shown good efficacy in TNBC, the issue of some TNBC cases being insensitive to PARP and acquired treatment resistance in BRCA-mutated cancer remains a problem in long-term studies. Therefore, PARP inhibitors have been studied in combination with platinum agents or other homologous recombination sensitizers to sensitize cancer cells. An ongoing phase II BROCADE3 trial (NCT02163694) is evaluating the comparison between veliparib in combination with carboplatin and paclitaxel versus placebo in combination with platinum and paclitaxel in breast cancer patients with BRCA1 or BRCA2 and HER2-negative metastatic or locally advanced unresectable breast cancer. The median PFS in the veliparib group was 12.5 months, compared to 6.14 months in the control group. Further analysis showed that the PCR rate in the veliparib plus carboplatin and paclitaxel group and the control group were 51% and 26%, respectively, indicating a better efficacy in the veliparib group.33 In the GeparOLA trial (NCT02789332), the efficacy and safety of paclitaxel combined with olaparib in TNBC were studied. Patients were randomly assigned to receive either paclitaxel plus olaparib (PO group) or paclitaxel plus carboplatin (PCb group), followed by the use of epirubicin/cyclophosphamide. Subgroup analysis showed that the PCR rate was higher in the PO group than in the PCb group.34
Clinical Studies of PARPi in Combination with Immunotherapy for TNBC
PARP inhibitors increase T-cell activity and stimulate antigen-presenting cells, making them an effective combination target for inducing an immune response. Preclinical models have shown that the combination therapy of PARP inhibitors and anti-PD-L1 drugs significantly improves treatment outcomes compared to the use of either drug alone.35 The phase III TOPACIO/KEYNOTE-162 trial evaluated the efficacy of the PARP inhibitor niraparib in combination with pembrolizumab for the treatment of metastatic TNBC. It was found that patients with BRCA1/2 mutations (ORR 47%) and those with a PD-L1 CPS≥1 score responded better (ORR 32%) than other groups. In the combination therapy, 4% of patients experienced grade 3 immune-related adverse events, and no higher-level immune toxicity was observed. The results indicate that niraparib in combination with pembrolizumab offers promising anti-tumor activity in patients with advanced or metastatic TNBC, with a higher response rate observed in patients with BRCA mutations.36 Additionally, the feasibility of combining anti-PD-L1 drugs with PARP inhibitors for breast cancer was also confirmed in the MEDIOLA trial, where the use of durvalumab in combination with olaparib in BRCA-mutated breast cancer patients resulted in an ORR of 63%, an mPFS of 8.2 months, and a MOS of 20.5 months.37
Ongoing studies: Several randomized trials are underway to further evaluate the efficacy of PARP inhibitors in combination with ICI and chemotherapy in TNBC, including the phase II/III MK-7339-009/KEYLYNK-009 trial evaluating maintenance therapy (NCT04191135); a phase II randomized trial evaluating the efficacy of olaparib in combination with atezolizumab in BRCA-mutated patients (NCT02849496); another phase II/III study (NCT04191135) exploring the therapeutic effect of combining olaparib with pembrolizumab and chemotherapy in advanced or metastatic TNBC patients; a study (NCT03801369) evaluating the efficacy of durvalumab plus olaparib in the treatment of advanced TNBC; and a trial (NCT02163694) evaluating the efficacy of veliparib in combination with carboplatin and paclitaxel in patients with advanced breast cancer and BRCA mutation.
PI3K/AKT/mTOR Pathway
The PI3K/AKT/mTOR signaling pathway is implicated in cancer development and treatment resistance. In TNBC, the PI3K gene is the second most commonly detected mutation after TP53, and PI3K mutations are frequently observed in residual TNBC. Targeting the relevant targets in the PI3K/AKT/mTOR signal transduction pathway could become a new treatment strategy for TNBC. Therefore, two promising phase II trials were designed to target the PI3K pathway, which is altered in about 30% of TNBC and has preclinical evidence of sensitivity to PI3K inhibitors. The PAKT trial showed that adding an AKT inhibitor (capivasertib) to first-line paclitaxel treatment in TNBC significantly prolonged PFS and OS. The benefit of PIK3CA/AKT1/PTEN alterations was even more pronounced in tumor patients.38 The LOTUS trial compared first-line treatment of metastatic triple-negative breast cancer using paclitaxel with or without ipatasertib.39 The phase II FAIRLANE study also evaluated the efficacy of ipatasertib in combination with paclitaxel in early-stage TNBC and found that the ipatasertib group had a higher PCR rate in patients with mutations in the PI3K/AKT/mTOR signaling pathway than the placebo group.40 In summary, multiple preclinical studies have shown that PI3K/AKT/mTOR signaling pathway inhibitors are effective in patients carrying key mutations in the pathway. Despite the promising results, there are still some shortcomings in this field, such as drug-induced adverse effects and bypass activation, which require further targeted exploration.
Epidermal Growth Factor Receptor (EGFR)
EGFR has ligand-induced tyrosine kinase activity, playing an important role in the cell cycle by promoting tumor cell division, and proliferation, and stimulating primary tumor occurrence and metastasis. EGFR is overexpressed in about 70–78% of basal-like TNBC samples, making it an effective therapeutic target, especially for BL2 subtype tumors with enhanced EGFR gene expression.41 Gefitinib (EGFR inhibitor) can reduce cancer cell proliferation and enhance the effects of carboplatin and docetaxel, and the combination of gefitinib with other targeted drugs can inhibit tumor growth. In the phase II clinical trial of neoadjuvant chemotherapy with paclitaxel plus cyclophosphamide in ER-negative early-stage breast cancer patients, patients were given either gefitinib as an addition or a placebo, the results showed that the PCR rates were 17% and 12% for the gefitinib and placebo groups, respectively, with a statistically significant difference between TNBC and non-TNBC patients, suggesting that TNBC is more sensitive to gefitinib.42
Vascular Endothelial Growth Factor (VEGF)
The growth of endothelial cells in blood vessels combined with its corresponding receptor VEGF can promote tumor angiogenesis, increase blood vessel permeability, and stimulate tumor proliferation and metastasis. Studies have found that the expression levels of VEGF in TNBC patients (especially the BLIS subtype) are significantly higher than in non-TNBC patients, thus anti-angiogenic drugs such as apatinib and bevacizumab can effectively prevent tumor development. The Gepar Quinto trial found that the addition of bevacizumab to anthracycline-based and taxane-based neoadjuvant therapy for TNBC can significantly increase the patient’s PCR rate.43 However, due to a high rate of early discontinuation and long treatment duration, the clinical application of bevacizumab treatment has been impacted to a certain extent. Tumor angiogenesis is a complex process, and to achieve anti-angiogenic treatment for tumors, a series of molecular mechanisms and precise target studies are required, as well as further clinical trial evaluations of the efficacy and adverse reactions of anti-angiogenic treatment.
Ganglioside GD2
Breast cancer stem cells (BCSCs) are associated with tumor initiation, metastasis, and chemoresistance. Ganglioside GD2 is upregulated in primary TNBC tumors compared to normal breast tissue and can identify BCSC. Further research by Battula et al on targeted BCSC markers revealed that GD2 is a cell surface marker expressed in subsets of breast cancer cells and promotes tumorigenesis, suggesting that GD2 is a tumor-specific marker in TNBC patients.44 Currently, many studies are developing novel inhibitors targeting the GD2 biosynthetic pathway, with the most promising results being the production of antibodies targeted against GD2 biosynthesis. Dinutuximab is the first FDA-approved anti-GD2 antibody used as a first-line treatment for pediatric neuroblastoma patients. Recently, studies by Lyet et al have shown the potential use of detuximab in TNBC treatment.45 These promising results may pave the way for the development of a TNBC therapy.
HIF-1α is a Critical Target in the Regulation of TNBC
Recent studies have shown that NF-κB, RAS-RAF-MEK-ERK, PI3K/Akt/mTOR, and JAK-STAT signaling pathways regulate the expression of HIF-1α, which in turn drives TNBC cell proliferation, angiogenesis, and BCSC enrichment. HIF-1α regulates the transcription of multiple target genes, inducing glycolysis, angiogenesis, invasion and metastasis, tumor stems cell enrichment, and immune escape.46–48 Therefore, in TNBC, HIF-1α primarily induces tumor angiogenesis by regulating the expression of VEGF, hepatocyte growth factor, vascular cell adhesion molecule 1, and VEGFR receptors. However, although targeting HIF-1α brings new hope for TNBC patients, they still face challenges.49 Currently, no drug has been approved for TNBC, and drugs that regulate HIF-1α expression are mainly focused on preclinical studies, with their actual clinical effects, patient tolerability, and drug regimens still to be further evaluated and validated.
Antibody-Drug Conjugate (ADC)
ADC is a new type of anti-tumor drug formed by covalently linking cytotoxic drugs and monoclonal antibodies. Monoclonal antibodies bind to antigens on target cells (ie cancer cells) and deliver the chemotherapy drugs to the cancer cells. In addition to the powerful killing effect of traditional chemotherapy drugs, it has the tumor targeting of antibody drugs, which can significantly improve the effectiveness and safety of the drug. Sacituzumab govitecan (SG) is a targeted antibody against trop-2. SG chemically links a monoclonal antibody hRS7 IgG1κ targeting trop-2 with the effective payload SN-38 through the cleavable CL2A connector. In vivo, SG binds to trop-2 on the surface of tumor cells, enters by endocytosis, and the CL2A connector breaks to release SN-38 to inhibit the DNA and RNA synthesis of tumor cells and exert a powerful tumor-killing effect.
SG as Monotherapy and in Combination with PARPi for the Treatment of TNBC
In the ASCENT trial (NCT02574455), a randomized phase III study, we evaluated the efficacy of SG compared to physicians’ choice of single-agent chemotherapy in patients with recurrent or refractory metastatic TNBC. A total of 468 patients were randomly assigned to receive SG or chemotherapy. The median progression-free survival (PFS) was 5.6 months and the median overall survival was 12.1 months for the SG group, while the median PFS was 1.7 months and the median overall survival was 6.7 months for the chemotherapy group. The patient ORR was 35% in the SG group and only 5% in the chemotherapy group.50 This benefit is known to be correlated with the level of trop-2 expression in the tumor.51 Currently, the FDA has approved SG for use in adult patients with metastatic unresectable locally advanced TNBC who have received at least two prior therapies. Meanwhile, in preclinical models of TNBC, SG induced double-strand DNA breaks and enhanced the inhibitory effect of PARP inhibitors regardless of BRCA1/2 mutation status. These data provide a theoretical basis for an ongoing phase I/II study on the safety and efficacy of SG in combination with PARP inhibitors for the treatment of metastatic TNBC (NCT04039230).
Ongoing studies: The SASCIA trial (NCT04595565) and NeoSTAR trial (NCT04230109) aim to evaluate the role of SG in adjuvant therapy and neoadjuvant therapy, respectively. In the randomized phase II Saci-IOTNBC trial (NCT04468061), the efficacy of combination therapy with pembrolizumab and SG is being studied for PD-L1-negative TNBC.52
Datopotamab Deruxtecan (Dato-DXd, DS-1062a)
Dato-DXd is a novel trop2-targeting antibody-drug conjugate that has been evaluated for its anti-tumor activity and safety in preclinical models. It has demonstrated effective anti-tumor activity by efficiently delivering the drug to the tumor cells.53,54 In the TROPION-PanTumor phase I trial (NCT03401385), Dato-DXd was evaluated for ORR in advanced TNBC patients, which was found to be 34%.55
Ladiratuzumab Vedotin (SGN-LIV1A, LV)
LV is a new ADC that targets the zinc transporter protein LIV-1 with the microtubule inhibitor monomethyl auristatin E (MMAE) as the payload. LIV-1 is highly expressed in metastatic TNBC, and LV (NCT01969643) as a single agent has demonstrated good efficacy in treating metastatic TNBC, with an ORR of 32% and a median PFS of 11.3 weeks when used in combination with pembrolizumab.56 In addition, a preclinical study highlighted that ladiratuzumab vedotin can induce immunogenic cell death, alter the tumor microenvironment, and enhance the efficacy of immune therapy; a phase Ib/II trial (NCT03310957) is evaluating the safety and efficacy of SGN-LIV1A in combination with pembrolizumab for the treatment of late-stage TNBC, with an ORR of 54% reported and the trial ongoing.57,58
Glembatumumab-Vedotin
Glembatumumab-Vedotin is another ADC, targeting NMB, which is overexpressed on the surface of breast cancer cells. Glembatumumab is a monoclonal antibody targeting the extracellular domain of NMB glycoprotein, conjugated with monomethyl auristatin E.59 A study targeting recurrent breast cancer patients expressing NMB glycoprotein, including HER-2 positive tumors and TNBC, showed low objective response rates of approximately 6% and 7%, respectively.60 However, when analyzing only the TNBC group, a better response rate was detected, with the ADC response rate at 18% compared to 0% for conventional chemotherapy patients. These results can be attributed to the expression of NMB glycoprotein, which is much higher on the surface of TNBC cancer cells compared to HER-2-positive cells, suggesting CDX-011 can be used for TNBC treatment.
Conclusion and Future Perspective
TNBC is a subtype of breast cancer that is highly malignant, prone to recurrence and metastasis, and has a poor prognosis. It is insensitive to endocrine therapy and traditional anti-HER2 targeted therapy, and chemotherapy is the main treatment method. Although the use of chemotherapy in solid tumors is rapidly developing, it has not yet achieved good efficacy. With the development of omics and the in-depth study of TNBC molecular subtypes, targeted therapy and immunotherapy are changing the clinical practice of TNBC, and TNBC molecular subtype-guided treatment strategies are expected to contribute to precision treatment. Currently, immune checkpoint blockade with atezolizumab or pembrolizumab and ADC drug SG provide partial benefit to patients. However, the existing treatment options are still limited, and there are still many issues and challenges in improving the efficacy of TNBC, such as a comprehensive analysis of the TNBC ecosystem, the development of clinically accessible new targets, exploration of better drug combination strategies, and research on resistance mechanisms, etc. In the future, further understanding of TNBC heterogeneity is needed, including the unique characteristics of each patient and the conduct of precise clinical trials. More research is needed to improve the efficacy of existing drugs and overcome resistance, to further improve the prognosis of TNBC patients. We believe that with the further development of TNBC-targeted therapy strategies, TNBC patients will have the opportunity to achieve better clinical outcomes.
Acknowledgments
The authors express gratitude to all research staff who participated in the work.
Funding
This work was supported by the Top Talent Support Program for Young and Middle-aged people of Wuxi Health Committee (BJ2020047), Norman Bethune Medical Science Research Fund (71505970).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91–113. doi:10.1038/s41571-021-00565-2
3. Asleh K, Riaz N, Nielsen TO. Heterogeneity of triple negative breast cancer: current advances in subtyping and treatment implications. J Exp Clin Cancer Res. 2022;41(1):265. doi:10.1186/s13046-022-02476-1
4. Cho B, Han Y, Lian M, et al. Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA Oncol. 2021;7(7):1016–1023. doi:10.1001/jamaoncol.2021.1254
5. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi:10.1172/JCI45014
6. Hallett RM, Dvorkin-Gheva A, Bane A, Hassell JA. A gene signature for predicting outcome in patients with basal-like breast cancer. Sci Rep. 2012;2:227. doi:10.1038/srep00227
7. Chen X, Feng L, Huang Y, Wu Y, Xie N. Mechanisms and strategies to overcome PD-1/PD-L1 blockade resistance in triple-negative breast cancer. Cancers. 2022;15(1):104. doi:10.3390/cancers15010104
8. Yonemitsu K, Pan C, Fujiwara Y, et al. GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci Rep. 2022;12(1):12007. doi:10.1038/s41598-022-16080-y
9. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74–82. doi:10.1001/jamaoncol.2018.4224
10. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi:10.1016/j.immuni.2013.06.014
11. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi:10.1038/cdd.2013.67
12. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615
13. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi:10.1016/S1470-2045(19)30689-8
14. Miles D, Gligorov J, André F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004. doi:10.1016/j.annonc.2021.05.801
15. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–1100. doi:10.1016/S0140-6736(20)31953-X
16. Wu F, Xu P, Chow A, et al. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br J Cancer. 2019;120(2):196–206. doi:10.1038/s41416-018-0297-1
17. Loibl S, Schneeweiss A, Huober J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149–1158. doi:10.1016/j.annonc.2022.07.1940
18. Pérez-García J, Soberino J, Racca F, Gion M, Stradella A, Cortés J. Atezolizumab in the treatment of metastatic triple-negative breast cancer. Expert Opin Biol Ther. 2020;20(9):981–989. doi:10.1080/14712598.2020.1769063
19. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467. doi:10.1200/JCO.2015.64.8931
20. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–411. doi:10.1093/annonc/mdy518
21. Winer EP, Lipatov O, Im SA, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. doi:10.1016/S1470-2045(20)30754-3
22. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi:10.1016/S0140-6736(20)32531-9
23. Ali MA, Aiman W, Shah SS, Hussain M, Kashyap R. Efficacy and safety of pembrolizumab based therapies in triple-negative breast cancer: a systematic review of clinical trials. Crit Rev Oncol Hematol. 2021;157:103197. doi:10.1016/j.critrevonc.2020.103197
24. Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569–581. doi:10.1016/j.annonc.2020.01.072
25. Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–567. doi:10.1056/NEJMoa2112651
26. Ho AY, Barker CA, Arnold BB, et al. A Phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–860. doi:10.1002/cncr.32599
27. Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80:106221. doi:10.1016/j.intimp.2020.106221
28. Kooshkaki O, Derakhshani A, Hosseinkhani N, et al. Combination of ipilimumab and nivolumab in cancers: from clinical practice to ongoing clinical trials. Int J Mol Sci. 2020;21(12):4427. doi:10.3390/ijms21124427
29. Navarrete-Bernal M, Cervantes-Badillo MG, Martínez-Herrera JF, et al. Biological landscape of triple negative breast cancers expressing CTLA-4. Front Oncol. 2020;10:1206. doi:10.3389/fonc.2020.01206
30. Zhou J, Bashey A, Zhong R, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant. 2011;17(5):682–692. doi:10.1016/j.bbmt.2010.08.005
31. Tutt A, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi:10.1056/NEJMoa2105215
32. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi:10.1056/NEJMoa1706450
33. Diéras V, Han HS, Kaufman B, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(10):1269–1282. doi:10.1016/S1470-2045(20)30447-2
34. Fasching PA, Link T, Hauke J, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann Oncol. 2021;32(1):49–57. doi:10.1016/j.annonc.2020.10.471
35. Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi:10.1158/1078-0432.CCR-16-3215
36. Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(8):1132–1140. doi:10.1001/jamaoncol.2019.1029
37. Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155–1164. doi:10.1016/S1470-2045(20)30324-7
38. Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38(5):423–433. doi:10.1200/JCO.19.00368
39. Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360–1372. doi:10.1016/S1470-2045(17)30450-3
40. Oliveira M, Saura C, Nuciforo P, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol. 2019;30(8):1289–1297. doi:10.1093/annonc/mdz177
41. Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19(2):264–271. doi:10.1038/modpathol.3800528
42. Bernsdorf M, Ingvar C, Jörgensen L, et al. Effect of adding gefitinib to neoadjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat. 2011;126(2):463–470. doi:10.1007/s10549-011-1352-2
43. Fasching PA, Loibl S, Hu C, et al. BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of breast cancer: response and prognosis results in patients with triple-negative breast cancer from the geparquinto study. J Clin Oncol. 2018;36(22):2281–2287. doi:10.1200/JCO.2017.77.2285
44. Shao C, Anand V, Andreeff M, Battula VL. Ganglioside GD2: a novel therapeutic target in triple-negative breast cancer. Ann N Y Acad Sci. 2022;1508(1):35–53. doi:10.1111/nyas.14700
45. Ly S, Anand V, El-Dana F, et al. Anti-GD2 antibody dinutuximab inhibits triple-negative breast tumor growth by targeting GD2 + breast cancer stem-like cells. J Immunother Cancer. 2021;9(3):e001197. doi:10.1136/jitc-2020-001197
46. Changchun K, Pengchao H, Ke S, Ying W, Lei W. Interleukin-17 augments tumor necrosis factor α-mediated increase of hypoxia-inducible factor-1α and inhibits vasodilator-stimulated phosphoprotein expression to reduce the adhesion of breast cancer cells. Oncol Lett. 2017;13(5):3253–3260. doi:10.3892/ol.2017.5825
47. Shan M, Qin J, Jin F, et al. Autophagy suppresses isoprenaline-induced M2 macrophage polarization via the ROS/ERK and mTOR signaling pathway. Free Radic Biol Med. 2017;110:432–443. doi:10.1016/j.freeradbiomed.2017.05.021
48. Zhang T, Zhu X, Wu H, et al. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: combined administration of Polydatin and 2-Deoxy-d-glucose. J Cell Mol Med. 2019;23(5):3711–3723. doi:10.1111/jcmm.14276
49. Liu Q, Guan C, Liu C, Li H, Wu J, Sun C. Targeting hypoxia-inducible factor-1alpha: a new strategy for triple-negative breast cancer therapy. Bio Pharmaco. 2022;156:113861. doi:10.1016/j.biopha.2022.113861
50. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi:10.1056/NEJMoa2028485
51. Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32(9):1148–1156. doi:10.1016/j.annonc.2021.06.002
52. Howard FM, Pearson AT, Nanda R. Clinical trials of immunotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2022;195(1):1–15. doi:10.1007/s10549-022-06665-6
53. Okajima D, Yasuda S, Maejima T, et al. datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20(12):2329–2340. doi:10.1158/1535-7163.MCT-21-0206
54. Shastry M, Jacob S, Rugo HS, Hamilton E. Antibody-drug conjugates targeting TROP-2: clinical development in metastatic breast cancer. Breast. 2022;66:169–177. doi:10.1016/j.breast.2022.10.007
55. Rose S. “Very Compelling” results for ADC in TNBC trial. Cancer Discov. 2022;12(2):280–281. doi:10.1158/2159-8290.CD-NB2021-0407
56. Rizzo A, Cusmai A, Acquafredda S, Rinaldi L, Palmiotti G. Ladiratuzumab vedotin for metastatic triple negative cancer: preliminary results, key challenges, and clinical potential. Expert Opin Investig Drugs. 2022;31(6):495–498. doi:10.1080/13543784.2022.2042252
57. McGuinness JE, Kalinsky K. Antibody-drug conjugates in metastatic triple negative breast cancer: a spotlight on sacituzumab govitecan, ladiratuzumab vedotin, and trastuzumab deruxtecan. Expert Opin Biol Ther. 2021;21(7):903–913. doi:10.1080/14712598.2021.1840547
58. Saravanan R, Balasubramanian V, Swaroop Balamurugan SS, et al. Zinc transporter LIV1: a promising cell surface target for triple negative breast cancer. J Cell Physiol. 2022;237(11):4132–4156. doi:10.1002/jcp.30880
59. Vahdat LT, Schmid P, Forero-Torres A, et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): a randomized multicenter study. NPJ Breast Cancer. 2021;7(1):57. doi:10.1038/s41523-021-00244-6
60. Yardley DA, Weaver R, Melisko ME, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol. 2015;33(14):1609–1619. doi:10.1200/JCO.2014.56.2959
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
