Back to Journals » Journal of Inflammation Research » Volume 16
Research Progress of Cordyceps sinensis and Its Fermented Mycelium Products on Ameliorating Renal Fibrosis by Reducing Epithelial-to-Mesenchymal Transition
Authors Zhang Y , Li K, Zhang C, Liao H , Li R
Received 20 March 2023
Accepted for publication 21 June 2023
Published 7 July 2023 Volume 2023:16 Pages 2817—2830
DOI https://doi.org/10.2147/JIR.S413374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Yaling Zhang,1,2 Kaiyun Li,1 Chao Zhang,1 Hui Liao,3 Rongshan Li1
1Department of Nephrology, Fifth Hospital of Shanxi Medical University (Shanxi Provincial People’s Hospital), Taiyuan, People’s Republic of China; 2Department of Nephrology, Taiyuan Central Hospital, Taiyuan, People’s Republic of China; 3Department of Pharmacy, Fifth Hospital of Shanxi Medical University (Shanxi Provincial People’s Hospital), Taiyuan, People’s Republic of China
Correspondence: Rongshan Li, Department of Nephrology, Fifth Hospital of Shanxi Medical University (Shanxi Provincial People’s Hospital), No. 29 Shuangtasi Street, Taiyuan, Shanxi Province, 030012, People’s Republic of China, Email [email protected]
Abstract: Renal fibrosis is a hallmark and common outcome of various chronic kidney diseases (CKDs) and manifests pathologically as accumulation and deposition of extracellular matrix (ECM) in the kidney. Epithelial-to-mesenchymal transition (EMT) has been shown to be an important mechanism involved in renal fibrosis. Cordyceps sinensis, a traditional Chinese medicine, has long been used for the treatment of renal fibrosis. As research on the mycelium of C. sinensis progressed, a variety of medicines developed from fermented mycelium were used to treat CKD. However, their efficacies and mechanisms have not been fully summarized. In this review, five medicines developed from fermented mycelium of C. sinensis are presented. The pharmacodynamic effects of C. sinensis on different animal models of renal fibrosis are summarized. The in vitro studies and related mechanisms of C. sinensis on renal cells are detailed. Finally, the application and efficacy of these five commercial medicines that meet national standards in different types of CKD are summarized. From this review, it can be concluded that C. sinensis can alleviate various causes of renal fibrosis to some extent, and its mechanism is related to TGF-β 1 dependent signaling, inhibition of inflammation, and improvement of renal function. Further research on rigorously designed, large-sample, clinically randomized controlled trial studies and detailed mechanisms should be conducted.
Keywords: renal fibrosis, Cordyceps sinensis, fermented mycelium products, epithelial-to-mesenchymal transition
Introduction
Renal fibrosis is the hallmark and common outcome of various chronic kidney diseases (CKD), which manifests pathologically as excessive accumulation and deposition of extracellular matrix (ECM) in the kidney.1 The complicated process of renal fibrosis consists of four overlapping phases: inflammatory cell infiltration, myofibroblast activation, ECM production, tubular atrophy and microvascular rarefaction.2 Renal fibrosis affects almost every part of the kidney tissue and can manifest as glomerulosclerosis, tubulointerstitial fibrosis and arteriosclerosis, leading to abnormalities in clinical indicators such as creatinine, urea nitrogen and cystatin, and eventually to the development of inevitable renal failure.3,4
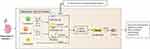
Almost all cell types in the kidney are involved in renal fibrosis, including fibroblasts, epithelial cells, endothelial cells, inflammatory cells, etc., and there is a dynamic cross-talk and interplay among them.5–7 Briefly, inflammatory cells are recruited to the renal interstitial region by the damaged kidney, producing large amounts of pro-inflammatory and pro-fibrotic cytokines, which in turn promote fibroblast activation and mesenchymal transdifferentiation, including epithelial-to-mesenchymal transition (EMT) and endothelial–mesenchymal transition (EndoMT).8,9 Beyond the above, macrophages can also generate macrophage–mesenchymal transition (MMT) per se.10 The end result of the above processes is increased expression of alpha smooth muscle actin (α-SMA) and decreased expression of epithelial cell markers such as E-cadherin, which eventually manifests as fibrosis pathologically and reduced renal function (Figure 1).
EMT is a central process in renal interstitial fibrosis, which was confirmed by two landmark studies in 2015.11,12 In numerous CKD animal models, inhibition of the EMT programme in transcript levels has been proved to reduce interstitial fibrosis, suggesting that improving EMT is crucial for the treatment of renal fibrosis.11–13 Multiple signaling pathways are involved in the progression of EMT, among which the intensively studied ones include classical transforming growth factor-β1 (TGF-β1)-Smad signaling, Wnt/β-catenin signaling, mitogen-activated protein kinase (MAPK) signaling, NF-ĸB signaling, etc.14–17 TGF-β1 signaling is the most studied of the above pathways.
The main therapeutic agents currently available to reverse or inhibit renal fibrosis are angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARBs), but the use of these agents is limited by their efficacies and side effects.18 Traditional Chinese medicine (TCM) has a long history in the treatment of renal fibrosis, of which C. sinensis is one of the most studied drugs.19–22 C. sinensis is a complex of larval corpse and fungal daughter formed by C. sinensis infecting larvae of Hepialidae.23 With the intensive research on C. sinensis, its fermented mycelium has been widely used for the treatment of CKD. A large number of clinical and animal studies have proved that the mycelium can alleviate renal fibrosis; however, its effects on inhibiting EMT are currently limited to being anti-inflammatory and antioxidant.22
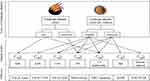
Till now, a variety of medicines developed from fermented mycelium of C. sinensis have been widely used in the treatment of CKD (Figure 2). It is time to comprehensively evaluate and compare the effectiveness of these medicines in alleviating renal fibrosis and their mechanisms, especially around the EMT process. In this paper, we review C. sinensis’s pharmacologic action in reducing renal fibrosis by antagonizing EMT based on different pathological mechanisms in various animal models (Figure 3). Then, we focus on the role of C. sinensis in the interaction between renal parenchymal cells and inflammatory cells (Figure 4). We summarize the mechanism of C. sinensis inhibiting EMT (Figure 5). In addition, the clinical effectiveness of these drugs in the treatment of different renal diseases has attracted our interest based on their ameliorative effect on EMT (Table 1).
 |
Table 1 Clinical Research into Artificially Cultured Cordyceps Sinensis in CKD Treatment |
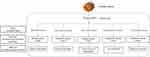 |
Figure 2 Development of isolated fungi from Cordyceps sinensis and medicines from fermented fungi. |
Medicines Developed from Fermented Mycelium of C. sinensis
The industrialization of fermented mycelium of C. sinensis originated from the research of natural C. sinensis. C. sinensis is a well-known medicinal mushroom in TCM and is a rare naturally occurring entomopathogenic fungus.41 According to the theory of TCM, C. sinensis can tonify the lung and the kidney. Its extracts have effects of nephroprotection, hepatoprotection, neuroprotection, and protection against ischemia/reperfusion-induced injury, as well as anti-inflammatory and anti-oxidant activities.42 Research on the isolation and culture of C. sinensis began in the late 1970s.43 Till now, 35 Cordyceps species have been reported in the literature to have medicinal properties or to isolate bioactive compounds.44
Of these 35 species, five have been developed for clinical application as drugs with national standards: Xinganbao capsules developed from Gliocladium roseum, Ningxinbao capsules from Cephalosporium sinensis, Zhiling capsules from Mortierella hepiali in 1985, Jinshuibao capsules developed from Paecilomyces hepialid in 1987, and Bailing capsules from Hirsutella sinensis in 1988.43 Except for Ningxinbao capsules, the instructions of the other four medicines mention that they can be used in the clinical treatment of CKD. Our current review on the research of C. sinensis and these medicines on ameliorating renal fibrosis will start from in vivo animal studies.
In vivo Animal Studies
As shown in Figure 3, studies on the effect of C. sinensis and its fermented mycelium products on renal fibrosis include multiple animal models, i.e. unilateral ureteral obstruction (UUO), diabetic nephropathy (DN), 5/6 nephrectomy etc. Among these animal models, UUO rat model is mostly popular for studies on renal interstitial fibrosis till now.45,46 The diabetes-based DN kidney fibrosis model is another well-studied model, followed by the 5/6 nephrectomy model.
Unlike acute kidney injury (AKI), where the inflammatory response is predominant, the pathological mechanisms of renal fibrosis in the above animal models are distinctive. The kidneys of animals modeled with UUO tend to show increased abundance of pro-fibrotic factors, activation of fibrotic signaling pathways targeting TGF-β, ECM deposition, and increased renal EMT. Renal pathological changes in DN include glomerular basement membrane thickening, glomerular thylakoid dilatation, etc. Glomerular thickening and sclerosis are associated with renal fibrosis, which is mainly associated with the TGF-β1 signaling pathway.
Although different renal fibrosis models have their own characteristics, TGF-β1 plays a crucial role in regulating the renal fibrosis, especially in the EMT process. Next, we will discuss the effects of C. sinensis on different animal models, centering on the TGF-β1 mediated EMT process.
On the UUO Model
Regulation EMT Process Mainly Mediated by TGF-β1 Signaling
The model of UUO in the rodent is widely used in studying the molecular mechanism and treatment efficacy in renal interstitial fibrosis, which simulates progressive renal fibrosis in humans and can present as three processes at the cellular level: inflammatory cell infiltration, tubular cell apoptosis and necrosis, and phenotypic transition of resident renal cells, i.e. EMT and EndoMT.45 Plasma uremia toxins are not generally increased in the UUO model, because of compensatory changes in the contralateral kidney.45 Inhibition of EMT in UUO mice has been shown to reduce renal interstitial fibrosis.47
In Figure 3, we can see the original and extracted solutions of artificially cultured C. sinensis, which include some active compounds such as cordycepin and adenosine, all studied using the UUO model, with intervention times ranging from 1 to 4 weeks.48,49 The results consistently show that C. sinensis reduces the expression of α-SMA and inhibits the production of collagens and fibronectin, resulting in alleviate renal interstitial fibrosis in UUO, mainly through the TGF-β1-Smad signaling pathway.48,49 The studies mentioned above focus on C. sinensis inhibiting EMT and thereby alleviating renal interstitial fibrosis of UUO, which has also been verified in vitro studies.
Inhibition of Inflammation: Mediation of the Macrophage Phenotype
Studies show that macrophage phenotype plays an important role in the progression of renal fibrosis, and the “classically” activated (M1) macrophage can promote renal inflammation, whereas “alternatively” activated (M2) macrophage has the effect of resolving inflammation and repairing injury.50 However, related studies have shown that the effect of C. sinensis on M1/M2 polarization during renal injury appears to be complex.
Zheng et al proved that N6-(2-Hydroxyethyl) adenosine, an active ingredient purified from C. sinensis, has a strong effect on suppression of inflammation via modulating the NF-κB signaling pathway and rebalancing the M1/M2 macrophage ratio.49 In this study, N6-(2-Hydroxyethyl) adenosine inhibits the accumulation of M1 in ligated kidneys in a dose-dependent manner, while its effects on M2 are complex, manifesting as regulation of IL-10, a marker of M2, which increases first and then decreases and tend to be normal ultimately. Taken together, the effects of N6-(2-Hydroxyethyl) adenosine on the macrophage phenotype is more inclined to rebalance the M1/M2 ratio to limit inflammation. According to related research, M2 macrophages can be divided into M2a, M2b, M2c.51,52 Further studies of N6-(2-Hydroxyethyl) adenosine on different M2 subtypes should be distinguished.
As we described before, macrophages themselves can undergo MMT. The MMT process occurring predominantly within M2 has been demonstrated in UUO mice, but more validation is lacking. And the study showed that MMT cells were a major source of collagen-producing fibroblasts in the UUO kidney, accounting for more than 60% of α-SMA+ myofibroblasts.10 Whether C. sinensis reduces renal fibrosis by regulating macrophage phenotypes or reducing MMT deserves further research.
On the DN Model
Regulation EMT Process Mediated by Multiple Signaling
The kidney of the DN model manifests as glomerulosclerosis and renal interstitial fibrosis histopathologically, exhibiting proliferation of mesangial cells, glomerular hypertrophy, thickening of renal tubule basement membranes, and infiltration of inflammatory cells.53,54 Yu et al showed that an extract of C. sinensis could reduce deposition of TGF-β1 in glomerular and tubulointerstitium accompanied with elevating deposition of collagen.55 Additionally, a nucleoside/nucleobase-rich extract from C. sinensis could significantly attenuate histopathological changes of renal tissue in the DN mice, and inhibit EMT to reduce ECM deposition by regulating the p38/ERK signaling pathways.56 Yang et al demonstrated that C. sinensis polysaccharides can inhibit renal tubular EMT via blocking the TGF-β1/Smad signaling pathway.57
Inhibition of Inflammation in the DN Model
As mentioned above, inflammatory cell infiltration can be observed in the DN model during the course of renal fibrosis. Yang et al showed that C. sinensis polysaccharides suppress the inflammation response via blocking toll-like receptor 4 (TLR4)/NF-κB to inhibit renal fibrosis.57
Another study revealed that Ophiocordyceps sinensis antagonized inflammation in DN rats and damaged podocyte by inhibiting the activation of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome and improving renal function, but lacks direct indicators of renal fibrosis.58 Given the involvement of inflammatory processes in renal fibrosis, C. sinensis targeting inflammatory processes in the treatment of renal fibrosis should be a promising research idea, and the associated anti-inflammatory mechanisms need to be further validated.
Improvement of Renal Function and Metabolic Syndrome
Unlike other animal models of CKD with pathological changes of renal fibrosis, the DN model involved in this review is the result of the combined intervention of a high-fat diet and streptozotocin, which is characterized by both renal dysfunction and metabolic syndrome such as obesity, hyperglycemia, and hyperlipidemia.59 Studies have confirmed that the effect of C. sinensis in improving renal dysfunction, such as reducing serum creatinine (Scr), blood urea nitrogen (BUN) and albuminuria levels, is closely related to its effect on improving DN metabolic syndrome. This may also be one of the bases for the improvement of renal fibrosis by C. sinensis.55–57,60
On the 5/6 Nephrectomy Models
Regulation of the EMT Process Mainly Mediated by TGF-β1 Signaling
Pan et al suggested that C. sinensis exerts its antifibrotic effect on the 5/6 nephrectomy model depending on inhibition of the TGF-β1-Smad pathway, and can downregulate Smad3 and Smad2 phosphorylation and decrease the TGF-β type I receptor (TβRI) and TGF-β type II receptor (TβRII).61 A study published in 2008 showed that Bailing capsules had the same effect as natural herbs in reducing renal fibrosis in a 5/6 nephrectomy model by modulating connective tissue growth factor (CTGF).62 Zhu et al further demonstrated that C. sinensis extracts could inhibit renal fibrosis by mediating the TGF-β1-CTGF pathway.63 Guo et al demonstrated that C. sinensis could improve renal fibrosis in 5/6 nephrectomized rats by alleviating mitochondrial damage, and the exact mechanism needs to be further investigated.64
Improvement of Renal Function
The 5/6 nephrectomy animal model of progressive renal failure is generated by removing the right kidney and the left upper and lower poles (2/3) of rodents and is characterized by observable elevations in Scr, BUN and proteinuria, and renal damage as marked tubulointerstitial fibrosis. The 5/6 nephrectomized rats were treated with artificially cultured C. sinensis starting at 7 or 14 days postoperatively and showed significant improvement in renal function compared to the non-treated group after 4 to 12 weeks of dosing, including a significant decrease in BUN and Scr.61,63–65
On the Aristolochic Acid Nephropathy (AAN) Models
Regulation of the EMT Process Mediated by TGF-β1 Signaling
AAN is a disease of progressive interstitial renal fibrosis caused by the use of TCM containing aristolochic acid. Animal models of AAN established by the instillation of aristolochic acid can have increased urinary protein excretion and decreased creatinine clearance due to kidney damage.66
Studies have confirmed the protective effect of C. sinensis on renal interstitial fibrosis in the AAN model, but the mechanism is not well understood. Chai et al demonstrated that C. sinensis could attenuate EMT in AAN models by decreasing TGF-β1 expression.67 Xu et al demonstrated that the antagonistic effect of C. sinensis on EMT may be related to its inhibitory effect on Snail expression, a downstream target of TGF-β1.66
Improvement of Renal Function
The AAN animal models are characterized by decreased glomerular filtration rate (GFR), and studies have demonstrated that artificially cultured C. sinensis significantly increases the level of GFR and decreases urinary protein.66–68
Aristolochic acid-induced kidney injury suggests that herbal medicine is a double-edged sword. While attention should be paid to herbal medicine to protect the kidney, attention should also be paid to herbal medicine-induced kidney injury. From the safety point of view, it is clear that C. sinensis has an ameliorating effect on renal fibrosis, and overdose of C. sinensis cannot further worsen renal fibrosis. Therefore, we believe that the clinical application of C. sinensis and its fermented products is safe and reliable.
Other Animal Models
Renal Protection of the AKI Model
The mechanisms of cisplatin-induced AKI are related to oxidative stress, inflammation, hypoxia, and apoptosis. Deng et al demonstrated that aqueous and ethanolic extracts of C. sinensis significantly attenuated renal histological changes, Scr and BUN production, and renal nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 levels in a cisplatin-induced AKI model.69 In addition, the ethanol extract prevented cisplatin-induced kidney injury by inhibiting TLR4 expression and NF-κB activation, as well as by significantly increasing the production of antioxidant enzymes.69
Renal Protection of the Adenine-Induced CKD Model
In rats with 2% adenine-induced renal tubular interstitial fibrosis, C. sinensis slowed early renal interstitial fibrosis by downregulating TGF-β1 expression and upregulating hepatocyte growth factor (HGF) expression.70
Renal Protection of the Spontaneously Hypertensive Rat (SHR) Model
Hypertensive kidney injury is a complication of hypertension, and tubulointerstitial fibrosis is proved to be the main pathogenesis. The SHR model with intraperitoneal resveratrol injection exhibited collagen deposition, an increased albumin/creatinine ratio, and increased levels of renal injury molecules-1 and β2-MG.71 Cai et al demonstrated that the treatment of SHRs with C. sinensis delayed the deposition of renal ECM markers and improved renal function by inhibiting autophagic stress, which has been demonstrated in in vitro studies.72
In vitro Studies
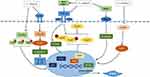
Almost all renal resident cells involved in renal fibrosis are illustrated in Figure 4, among which tubular epithelial cells and fibroblasts attracted more interesting attention. In vitro studies about C. sinensis treating of renal fibrosis referred to macrophage phenotype but not aimed at MMT. In addition, pericyte, endothelial cells could also regenerate and produce ECM after injury, thereby potentially contributing to glomerular fibrosis.5 Podocyte injury, which has been induced by diabetes, hypertension, etc., can develop into progressive glomerular injury and glomerulosclerosis.5 However, there is no relevant research on whether C. sinensis has a protective effect on them.
On Renal Tubular Epithelial Cells
The use of TGF-β1 in renal tubular epithelial cells is a common experimental tool to mimic the in vitro EMT process. In addition, high concentrations of glucose, aristolochic acid, albumin and ACE II can be used to induce the conversion of renal tubular epithelial cells to EMT.
C. sinensis polysaccharide inhibited TGF-β1-induced progression of renal tubular epithelial rat cells (NRK-52E cell line) to EMT in vitro through the classical TGF-β1-Smad signaling pathway.48,57 C. sinensis also inhibited EMT by downregulating Snail expression in proximal renal tubular epithelial cells.66
Studies have shown that the autophagic pathway is positively correlated with antifibrotic effects. C. sinensis reduced Ang-II-induced ECM protein expression in NRK-52E cells by inhibiting the silent information regulator T1 (SIRT1) signaling pathway.72 Nucleoside/nucleotide-rich extracts of C. sinensis inhibit the EMT process and ECM accumulation in human renal cortex proximal tubule epithelial cells (HK-2), which were induced by high glucose through modulation of MAPK signaling pathway.56
On Fibroblasts
Fibroblast activation plays an important role in renal interstitial fibrosis. C. sinensis suppresses fibroblast activation by regulating TGF-β1-Smad signaling and TGF-β1-CTGF signaling.49,73,74 Zhu et al reported that ergosterol peroxide, a major bioactive compound isolated from C. sinensis, attenuated TGF-β1-induced proliferation of renal fibroblasts and reduced ECM production via the MAPK pathway. The cell line in this study was a normal rat kidney fibroblast cell line (NRK-49F).74
On Macrophages
The initiation of inflammation and the high production of inflammatory factors play an important role in the process of renal fibrosis. C. sinensis exerts an inhibitory effect on fibrosis by reducing lipopolysaccharide-induced inflammatory cytokine production by RAW264.7 macrophages, which in turn modulates NF-ĸB signaling.49,69
During renal fibrosis, there are complex interactions between kidney-recruited inflammatory cells and renal intrinsic cells. They can both undergo mesenchymal transformation into myofibroblasts and secrete ECM.6 Currently, renal tubular epithelial cells and renal mesenchymal fibroblasts are more frequently reported, while other cells involved in mesenchymal transformation, such as macrophages, pericytes, renal vascular endothelial cells and fibroblasts of other origins, are relatively less studied, which may be a direction in the future.
Among these cells, macrophages are involved in the process of renal fibrosis in several ways. First, macrophages play a major role in the inflammatory process at the initiation of fibrosis; second, macrophage activation produces a large number of inflammatory factors that play an important role in the induction of other cellular transdifferentiation; and third, macrophages can be involved in the MMT process induced by their own production of inflammatory factors. The role of macrophages in fibrosis is complicated by the fact that they have different phenotypes and can transform according to the microenvironment in which they are located. It is currently believed that different phenotypes of macrophages can play different roles in promoting/inhibiting fibrosis. The study showed that Cordyceps can inhibit the conversion of macrophages to M1 phenotype, and its effect on M2 remains to be further investigated. In addition, recent studies have shown that lymphocytes, basophils and other inflammatory cells are also involved in the process of renal fibrosis, and there is still a lack of research on the effect of C. sinensis on them.
Mechanism Studies
Many molecular pathways are involved in the progression of EMT in renal fibrosis and, among them, TGF-β1 is a core profibrotic cytokine.75,76 Studies on the treatment of renal fibrosis by C. sinensis mainly focused on TGF-β1-dependent signaling. The pharmacodynamic effects of C. sinensis in the treatment of renal fibrosis are also mediated through other signaling pathways, such as MAPK signaling, SIRT1 signaling, and NF-κB signaling.49,56,71
TGF-β1 Dependent Signaling
TGF-β1 can exert a profibrotic effect by modulating its downstream molecules though Smads-dependent or non-independent signaling. Among them, TGF-β1-Smad signaling is the most classical signaling pathway in renal fibrosis, which plays a key role by regulating Smads protein phosphorylation.77 The activated TGF-β1 binds to TβRII and recruits TβRI, and then phosphorylates downstream Smad2 and Smad3, which then form an oligomeric complex with Smad4. The complex is translocated into the nucleus to regulate the transcription of target genes. Unlike Smad2, 3, and 4, Smad7 is a repressive Smad that exerts a negative effect on TGF-β1/Smad signaling.14
In 5/6 nephrectomy rats, artificially cultured C. sinensis attenuated renal fibrosis via downregulating TGF-β1, TGFβRI, TGFβRII, Smad2, Smad3 and upregulating Smad7.61 Cordycepin, an effective component of C. sinensis, has been proved to alleviated renal interstitial fibrosis in vivo and in vitro on UUO mice by suppressing the expression of p-Smad2/3 did not mention TGF-β1.48 In vitro studies have demonstrated that C. sinensis polysaccharide can inhibit TGF-β1-induced activation of fibroblasts by blocking nuclear translocation and accumulation of activated Smad2/3 proteins, and upregulating the expression of anti-fibrotic HGF.57 Further in vivo studies should be confirmed.
Bone morphogenetic protein 7 (BMP-7) is a natural TGF-β1 antagonist that acts by inhibiting TGF-β1/Smad3.78 In streptozotocin-induced diabetic mice, C. sinensis alone or in combination with telmisartan inhibited the expression of TGF-β and α-SMA by upregulating BMP-7.79
CTGF is another downstream target molecule of TGF-β1.80,81 Studies using various animal models of renal fibrosis have demonstrated that C. sinensis can downregulate CTGF levels, but the exact mechanism of how TGF-β1 and CTGF interact with each other is unknown.63,68,82 Artificial fermented C. sinensis treatment in UUO, DN, and AAN rats inhibited the overexpression of TGF-β1 and CTGF, and promoted the plasminogen activator inhibitor-1 (PAI-1) factor that antagonizes ECM degradation.68,82,83 Two kinds of C. sinensis extract, total extract and acetic ether extract, decrease the depositions of ECM proteins in 5/6 nephrectomy rats and are accompanied by reduced expression of TGF-β1 and CTGF.63
Likewise, TGF-β1 can inhibit Snail directly or through downregulating Smads to reduce the production of ECM thus alleviating renal fibrosis. It has been proved that C. sinensis could downregulate TGF-β1 and Snail expression in renal tissue, antagonize EMT and renal interstitial fibrosis, and improve renal function in AAN rats.66,67 Moreover, C. sinensis inhibits TGF-β1-Snail signaling to alleviate renal fibrosis in DN rats.84
Wnt/β-Catenin Signaling
Wnt/β-catenin is an evolutionarily conserved and complex developmental signaling pathway that plays an important role in the regulation of renal fibrosis.15 It is relatively silent in normal kidneys but activated after kidney injury. After kidney damage, Wnt can bind to membrane receptors and inhibit the degradation of β-catenin, thus keeping the latter in a state of continuous activation and exerting an inhibitory effect on kidney fibrosis by activating the transcription of downstream target genes.15 Conversely, blocking the Wnt/β-catenin pathway by inhibiting Wnt can promote the development and progression of renal fibrosis.85
Only one study has shown that artificially fermented C. sinensis can reduce fibronectin expression in DN rats by inhibiting DKK1, the upstream of Wnt, while maintaining stable expression of β-catenin, but there is no mention of the effect of C. sinensis on Wnt.86
MAPK Signaling
MAPK signaling, which concludes ERK, p38 and JNK signaling, can regulate cellular activities by phosphorylate specific serine and threonine of target protein substrates and its activation is a key modulator in the EMT process.87 Dong et al proved that the extracts of C. sinensis inhibit EMT and ECM accumulation by blocking MAPK signaling in DN rats.56 This study demonstrated that the high glucose-induced renal tubular epithelial cells and the streptozotocin-induced diabetic mice were all accompanied by the activation of p38, ERK, and JNK signaling pathways. C. sinensis treatment significantly suppressed the phosphorylation of p38 and ERK MAPK, while JNK phosphorylation was not affected in vivo or in vitro. It is well known that the cross-talk between MAPKs and TGF-β signaling plays an important role in renal fibrosis. Another in vitro study proved that ergosterol peroxide, purified from C. sinensis, blocks TGF-β1-induced fibroblast-to-myofibroblast transdifferentiaton in a rat kidney fibroblast cell line (NRK-49F) via suppressing phosphorylation of the ERK1/2, p38, and JNK pathways.74 The above-mentioned study also demonstrated that ergosterol peroxide reduced TGF-β1-induced CTGF expression in fibroblast cells. However, how C. sinensis exerts its antifibrotic effects through the MAPK signaling, ie whether TGF-β1 must be dependent, warrants further investigation.
NF-κB Signaling
NF-κB signaling pathways are known to be a major contributor to renal fibrosis.88 In a UUO mouse model, N6-(2-Hydroxyethyl) adenosine exerted anti-fibrotic and anti-inflammation effects by suppression of the NF-κB signaling, meanwhile inhibiting TGF-β1/Smad signaling.49 A water extract of C. sinensis greatly inhibits the expression of TLR4 and cisplatin-induced NF-κB activation.69 Another study also demonstrated that C. sinensis polysaccharides, by blocking TLR4/NF-κB signaling, could inhibit the inflammatory response and regulate the dysregulation of intestinal microbiota, thus exerting beneficial effects on renal tubular interstitial fibrosis in DN rats.57
SIRT1 Signaling
Physiological autophagy is beneficial for maintaining cellular homeostasis, but whether autophagy can improve fibrosis has not been well understood. FOXO3a is an important downstream molecule of SIRT1. Cai et al found that C. sinensis reduced the accumulation of ECM in the kidney by upregulating the expression of SIRT1/FOXO3a, thereby ameliorating renal fibrosis in hypertensive rats.72 Huang et al proved that C. sinensis alleviates SHR renal interstitial fibrosis via the SIRT1/p53 pathway, while reducing α-SMA expression in the kidney.71 Deng et al demonstrated that C. sinensis not only significantly inhibits inflammation but also has a significant role in increasing the production of SIRT1 and p-AMP-activated protein kinases in the renal tissues.69
NLRP3/Caspase-1/IL1β/IL-18 Signaling
The NOD-like receptor family pyrin domain containing 3 (NLRP3) can been activated by TLRs/NF-κB, which then promotes the downstream Caspase-1 and proinflammatory factors IL-1β and IL-18.89 Multiple studies have shown that the NLRP3 inflammasome and its downstream pyroptosis and inflammation play an important role in the development of renal fibrosis.90,91 It has been proved that the expression of NLRP3 and its downstream pyroptosis, which includes caspase-1, IL-β and IL-18, upregulate in DN rats and downregulate after being treated with Ophiocordyceps sinensis.58
Clinical Studies
Medicines developed from artificial C. sinensis mycelium have been widely used in the treatment of CKD. According to TCM theory, these medicines have an effect on tonifying the kidney and it benefits from its essence. Clinical studies showed that the combination of these medicines with western medicines is more effective than the group of western medicines alone in improving patients’ GFR.24,26,28 The downside is that the quality of these clinical studies needs to be further improved.
There are more clinical reports on the combination of Jinshuibao capsules, Bailing capsules/tablets and Zhiling capsules with ACEI/ARBs on DN treatment. These studies show that these combinations are superior to ACEI/ARBs alone in improving renal function and reducing proteinuria production in patients.24,25,28,34 Studies have shown that these medicines have the effect of reducing serum inflammatory cytokines, such as C-reactive protein (CRP), IL-6, TNF-α, and others.27,32,33 Bailing capsule can reduce collagen-IV and laminin levels in DN patients, which are the main components of ECM.29 The clinical data of DN patients using Ningxinbao capsule and Xinganbao capsule are mainly focused on the early stage of DN, and clinical application is relatively limited.38,39
These medicines are also used in the treatment of chronic glomerulonephritis and chronic renal failure, exerting a renoprotective effect through anti-inflammatory effects and reduction of ECM such as collagen and laminin production.30,31,36,40 A study showed that Zhiling capsules reduced tissue-type plasminogen activator (t-PA) and PAI-1, thereby improving renal function in patients with chronic glomerulonephritis.35 The combination of Zhiling capsules and valsartan for the treatment of CKD can be clinically effective by improving the immune function of patients through the regulation of T lymphocytes, which are also involved in EMT.37
The downside is that these clinical trials used only tests of renal function, such as estimated GFR, or proteinuria (especially the urine protein/creatinine ratio) as observed endpoints. Some specific biomarkers of the fibrosis process, which could be used as primary endpoints for patients to get better, were not addressed in any of these clinical studies. In addition, renal biopsy is the gold standard for the detection of renal fibrosis, and most clinical outcomes are not currently supported by renal biopsy data.
Renal fibrosis is a common pathway for the progression of all renal diseases, and the renoprotective effect of Cordyceps has been recognized by clinicians and confirmed by in vivo and ex vivo studies; therefore, we believe that C. sinensis has an anti-nephritic fibrosis effect, but more data are needed to support it.
Conclusion and Perspectives
In conclusion, we believe that C. sinensis has an anti-renal fibrosis effect to a certain extent. Studies using various animal models have demonstrated that C. sinensis and its active ingredients improve renal fibrosis by inhibiting EMT and reducing ECM via multiple signaling pathways. Among them, inhibition of TGF-β1 production and anti-inflammatory effects are the main mechanisms of C. sinensis, with a few studies focusing on the downstream and upstream molecules of TGF-β1. Research into infiltration of inflammatory cells and interaction between inflammatory cells and intrinsic cells of C. sinensis have not been detailed enough. Although current clinical data support the clinical role of C. sinensis, more supporting data are needed on its anti-nephrogenic fibrosis effect due to the limitations of renal biopsy.
Acknowledgments
This study was supported by the Demonstration Project on Reformation and Quality Development of Public Hospitals (SCP-2023-8), the Local Science and Technology Development Funds Projects Guided by Central Government (No. YDZJSX2021C027), the Medical Key Research Projects of Shanxi Province (No. 2020XM02), and the Basic Research Program of Shanxi Province (No. 202103021224370).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80(1):309–326.
2. Yuan Q, Liu Y. Recent advances on understanding of the cellular and molecular mechanism of renal fibrosis. J Anhui Univ. 2018;42(5):115–124.
3. Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv Chronic Kidney Dis. 2017;24(2):117–129.
4. Yan H, Xu J, Xu Z, Yang B, Luo P, He Q. Defining therapeutic targets for renal fibrosis: exploiting the biology of pathogenesis. Biomed Pharmacother. 2021;143:112115.
5. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–2306.
6. Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87(2):297–307.
7. Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–107.
8. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36.
9. Zhang Y, Jin D, Kang X, et al. Signaling pathways involved in diabetic renal fibrosis. Front Cell Dev Biol. 2021;9:696542.
10. Wang S, Meng XM, Ng YY, et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 2016;7(8):8809–8822.
11. Grande MT, Sánchez-Laorden B, López-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21(9):989–997.
12. Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21(9):998–1009.
13. Sheng L, Zhuang S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front Physiol. 2020;11:569322.
14. Meng XM, Tang PM, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82.
15. Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl. 2014;4(1):84–90.
16. Campbell MT, Hile KL, Zhang H, et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2011;168(1):e61–e69.
17. Chen KH, Hsu HH, Yang HY, et al. Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway. Int J Biochem Cell Biol. 2016;74:135–144.
18. Zhang F, Liu H, Liu D, et al. Effects of RAAS inhibitors in patients with kidney disease. Curr Hypertens Rep. 2017;19(9):72.
19. Wang DT, Huang RH, Cheng X, Zhang ZH, Yang YJ, Lin X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-β/Smad and NF-κB signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol. 2015;26(1):4–12.
20. Zhang HF, Wang YL, Gao C, et al. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol Sin. 2018;39(12):1855–1864.
21. Zhou S, Ai Z, Li W, et al. Deciphering the pharmacological mechanisms of taohe-chengqi decoction extract against renal fibrosis through integrating network pharmacology and experimental validation in vitro and in vivo. Front Pharmacol. 2020;11:425.
22. Tan W, Wang Y, Dai H, et al. Potential therapeutic strategies for renal fibrosis: cordyceps and related products. Front Pharmacol. 2022;13:932172.
23. Zhang HW, Lin ZX, Tung YS, et al. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014;2(12):D8353.
24. Li Y, Xu G. Clinical Efficacy and Safety of Jinshuibao Combined With ACEI/ARB in the Treatment of Diabetic Kidney Disease: a Meta-Analysis of Randomized Controlled Trials. J Ren Nutr. 2020;30(2):92–100.
25. Lu Q, Li C, Chen W, Shi Z, Zhan R, He R. Clinical efficacy of jinshuibao capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:6806943.
26. Zhang M, Gao X, Zhang L. Efficacy of Jinshuibao capsule combined with prednisone in early aristolochic acid nephropathy. Capital Med. 2009;16(16):46–47.
27. Huang J, Li J, Liu T. Effect of combined therapy with hypha Cordyceps and ginkgo leaf tablet on micro-inflammation in patients undergoing maintenance hemodialysis. Chin J Integrated Traditional Western Med. 2008;28(6):502–504.
28. Yu W, Duan S, Yu Z. The effect of Bailing capsules combined with losartan to treat diabetic glomerulosclerosis and the combination’s effect on blood and urine biochemistry. Am J Transl Res. 2021;13(6):6873–6880.
29. Wang Y, Zhang Y, Shi L. Influence of bailing capsules and alprostadil on inflammatory factors, liver and renal function in patients with diabetic nephropathy. Western J Traditional Chine Med. 2022;35(06):115–117.
30. Liu Z, Gao B, Li X. Efficacy of the Bailing capsules plus enalapril maleate on chronic glomerulonephritis. Clin J Chine Med. 2022;14(14):115–118.
31. Zhang L. Analysis of the effect of Baling capsule combined with cyclophosphamide in treating chronic glomerulonephritis. Practical Integrated Chine Western Med. 2021;21(06):77–78.
32. Hu X, Cui B, Gao Q, Wang Z, Liu Y. Meta-analysis of Bailing Capsules in improvement of microinflammation and nutritional status among maintenance hemodialysis patients. China J Chine Materia Med. 2022;47(9):2547–2555.
33. Zhi Y, Wei Z, Cao Y. Effect of Bailing Capsules combined with a comprehensive intervention on the renal fibrosis and renal function in patients with chronic renal failure. J Changchun Univ Chine Med. 2022;38(02):213–216.
34. Wang W, Qi J. Clinical study of Zhiling Capsule combined with telmisartan in treatment of early diabetic nephropathy. Drugs Clin. 2018;33(06):1494–1497.
35. Wu H, Tu B. Clinical study on Zhiling Capsules combined with benazepril in treatment of chronic glomerulonephritis. Drugs Clin. 2019;34(06):1789–1792.
36. Jin Y, Zou D, Liu S. Clinical study of zhiling combined with compound α -ketoacid for chronic renal failure. Drugs Clin. 2022;37(04):813–817.
37. Xu X, Jin L. Curative effect of Zhiling capsule combined Valsartan on chronic nephropathy and its influence on renal function. Chine J General Practice. 2016;14(12):2051–2054.
38. Shi Y, Wei Z, Qiao S. The effect of Ning Xinbao for early kidney injury in diabetes mellitus. Shandong Med. 2004;44(35):10–11.
39. Zhang G, Wang W. Clinical observation of treatment of diabetic nephropathy with Xinganbao capsule. Med J Chine Peoples Health. 2015;27(10):80–101.
40. Chang X, Wang J, Wang N. The curative effect of xinganbao combined with losartan in 23 cases of IgA nephropathy. Chine J Integrated Traditional Chine Western Med Nephropathy. 2012;13(04):349–350.
41. Ashraf SA, Elkhalifa A, Siddiqui AJ, et al. Cordycepin for health and wellbeing: a potent bioactive metabolite of an entomopathogenic cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules. 2020;25(12):2735.
42. Liu W, Gao Y, Zhou Y, Yu F, Li X, Zhang N. Mechanism of cordyceps sinensis and its extracts in the treatment of diabetic kidney disease: a review. Front Pharmacol. 2022;13:881835.
43. Zhang H, Li Y, Mi J, et al. GC-MS profiling of volatile components in different fermentation products of cordyceps sinensis mycelia. Molecules. 2017;22(10):54.
44. Olatunji OJ, Tang J, Tola A, Auberon F, Oluwaniyi O, Ouyang Z. The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316.
45. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75(11):1145–1152.
46. Martínez-Klimova E, Aparicio-Trejo OE, Tapia E, Pedraza-Chaverri J. Unilateral ureteral obstruction as a model to investigate fibrosis-attenuating treatments. Biomolecules. 2019;9(4):141.
47. Du F, Li S, Wang T, et al. Cordyceps sinensis attenuates renal fibrosis and suppresses BAG3 induction in obstructed rat kidney. Am J Transl Res. 2015;7(5):932–940.
48. Gu L, Bie R, Tu Y. Mechanisms of cordycepin on improving renal interstitial fibrosis via regulating eIF2α/TGF-β/Smad signaling pathway. China J Chine Materia Med. 2014;39(21):4096–4101.
49. Zheng R, Zhu R, Li X, et al. N6-(2-Hydroxyethyl) Adenosine From Cordyceps cicadae Ameliorates Renal Interstitial Fibrosis and Prevents Inflammation via TGF-β1/Smad and NF-κB Signaling Pathway. Front Physiol. 2018;9:1229.
50. Hu X, Xu Y, Zhang Z, et al. TSC1 affects the process of renal ischemia-reperfusion injury by controlling macrophage polarization. Front Immunol. 2021;12:637335.
51. Ross EA, Devitt A, Johnson JR. Macrophages: the Good, the Bad, and the Gluttony. Front Immunol. 2021;12:708186.
52. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461.
53. Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FR. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57(6):1439–1445.
54. Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27(2):195–207.
55. Yu SH, Dubey NK, Li WS, et al. Cordyceps militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS One. 2016;11(11):e166342.
56. Dong Z, Sun Y, Wei G, Li S, Zhao Z. A nucleoside/nucleobase-rich extract from cordyceps sinensis inhibits the epithelial-mesenchymal transition and protects against renal fibrosis in diabetic nephropathy. Molecules. 2019;24(22):4119.
57. Yang J, Dong H, Wang Y, et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442–456.
58. Wang C, Hou XX, Rui HL, et al. Artificially cultivated Ophiocordyceps sinensis alleviates diabetic nephropathy and its podocyte injury via inhibiting P2X7R expression and NLRP3 inflammasome activation. J Diabetes Res. 2018;1390418.
59. Yan LJ. The nicotinamide/streptozotocin rodent model of type 2 diabetes: renal pathophysiology and redox imbalance features. Biomolecules. 2022;12(9):141.
60. Cao T, Xu R, Xu Y, Liu Y, Qi D, Wan Q. The protective effect of Cordycepin on diabetic nephropathy through autophagy induction in vivo and in vitro. Int Urol Nephrol. 2019;51(10):1883–1892.
61. Pan MM, Zhang MH, Ni HF, et al. Inhibition of TGF-β1/Smad signal pathway is involved in the effect of Cordyceps sinensis against renal fibrosis in 5/6 nephrectomy rats. Food Chem Toxicol. 2013;58:487–494.
62. Fei Z, Tang D. Effect of Bailing Capsule on renal connective tissue growth factor expression in a remnant kidney model. Chine J Biomed. 2008;3(2):105–109.
63. Zhu R, Chen YP, Deng YY, et al. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J Zhejiang Univ Sci B. 2011;12(12):1024–1033.
64. Guo S, Zhong F, Zhou Q, et al. Renal protective effect of Cordyceps sinensis on 5 /6 nephrectomy-induced renal fibrosis in rats. J Shanghai Jiaotong Univ. 2012;32(01):1–8.
65. Zhang MH, Pan MM, Ni HF, et al. Effect of Cordyceps sinensis powder on renal oxidative stress and mitochondria functions in 5/6 nephrectomized rats. Chin J Integrated Traditional Western Med. 2015;35(4):443–449.
66. Xu XY, Chai JJ, Chen YP, et al. Hirsutella sinensis Attenuates Aristolochic Acid-Induced Renal Tubular Epithelial-Mesenchymal Transition by Inhibiting TGF-β1 and Snail Expression. PLoS One. 2016;11(2):e149242.
67. Chai JJ, Chen YP, Rui HL. Effects of Hirsutella sinensis on TGF-beta1 and Snail expressions and transdifferentiation of tubular epithelial-myofibroblast in renal tissue of rats with chronic aristolochic acid nephropathy. Chin J Integrated Traditional Western Med. 2009;29(4):325–329.
68. Zhu YF, Chen YP, Rui HL, Dong HR, Hu Z. Protective effects of Hirsutella sinensis on renal interstitial fibrosis: experiment with rat model of chronic aristolochic acid nephropathy. Chin Med J. 2007;87(38):2667–2671.
69. Deng JS, Jiang WP, Chen CC, et al. Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-κB/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid Med Cell Longev. 2020;2020:7912763.
70. Li N, Chen X, Yang D, Zhao D, Bi L. Prevention and treatment of renal interstitial fibrosis by Bailing capsule in rats. Chine Med Herald. 2006;3(30):16–18.
71. Huang YS, Wang X, Feng Z, et al. Cordyceps cicadae Prevents Renal Tubular Epithelial Cell Apoptosis by Regulating the SIRT1/p53 Pathway in Hypertensive Renal Injury. Evid Based Complement Alternat Med. 2020;2020:7202519.
72. Cai Y, Feng Z, Jia Q, et al. Cordyceps cicadae Ameliorates Renal Hypertensive Injury and Fibrosis Through the Regulation of SIRT1-Mediated Autophagy. Front Pharmacol. 2021;12:801094.
73. Li L, He D, Yang J, Wang X. Cordycepin inhibits renal interstitial myofibroblast activation probably by inducing hepatocyte growth factor expression. J Pharmacol Sci. 2011;117(4):286–294.
74. Zhu R, Zheng R, Deng Y, Chen Y, Zhang S. Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-β1-induced activation of kidney fibroblasts. Phytomedicine. 2014;21(3):372–378.
75. Gifford CC, Tang J, Costello A, et al. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin Sci (Lond). 2021;135(2):275–303.
76. Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76.
77. Ma TT, Meng XM. TGF-β/Smad and Renal Fibrosis. Adv Exp Med Biol. 2019;1165:347–364.
78. Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond). 2013;124(4):243–254.
79. Duan Y, Yang S, Chen B. Regulating effects of Cordyceps sinensis capsule and telmisartan tablets on renal tubular epithelial-mesenchymal transition in in diabetic nephropathy rats. Chin J Clin Pharmacol. 2016;32(23):2170–2173.
80. Nguyen TQ, Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm Res. 2008;25(10):2416–2426.
81. Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res. 2015;165(4):512–530.
82. Zhu B, Chen C. Effect of artificial cordyceps sinensis on the transformed growth factor-β1 and connective tissue growth factor expression in interstitial fibrotic rats. Clin Rational Drug Use. 2011;4(33):11–12.
83. Zhang L, Yan Y. Protective effect of Cordyceps sinensis on diabetic nephropathy in a rat model. Jiangxi Med J. 2014;49(11):1188–1192.
84. Chen J, Yao W, Zhu S, Zhu H, Ye L. Cordyceps sinensis attenuates renal epithelial-mesenchymal-transition in diabetic mice vial TGF-β/Snail signal pathway. Clin J Med Officers. 2017;45(2):140–144.
85. Li SS, Sun Q, Hua MR, et al. Targeting the Wnt/β-Catenin Signaling Pathway as a Potential Therapeutic Strategy in Renal Tubulointerstitial Fibrosis. Front Pharmacol. 2021;12:719880.
86. Du Y, Liu Y, Lu S, Liu Y, Qi W, Fu Y. Effects of Cordyceps sinensis on expressions of DKK1 and β-catenin in diabetic rats. J Shandong Univ. 2012;50(06):26–30.
87. Li Z, Liu X, Wang B, et al. Pirfenidone suppresses MAPK signalling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology. 2017;22(8):589–597.
88. Lan H. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7(7):1056–1067.
89. Xue YS, Enosi Tuipulotu D, Tan WH, et al. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–1052.
90. Wang MZ, Wang J, Cao DW, et al. Fucoidan alleviates renal fibrosis in diabetic kidney disease via inhibition of NLRP3 inflammasome-mediated podocyte pyroptosis. Front Pharmacol. 2022;790937.
91. Ram C, Gairola S, Syed AM, et al. Biochanin A alleviates unilateral ureteral obstruction-induced renal interstitial fibrosis and inflammation by inhibiting the TGF-β1/Smad2/3 and NF-kB/NLRP3 signaling axis in mice. Life Sci. 2022;298:120527.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.