Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Research Advances in Fusion Protein-Based Drugs for Diabetes Treatment
Authors Deng W , Zhao Z , Zou T, Kuang T , Wang J
Received 10 July 2023
Accepted for publication 22 December 2023
Published 23 January 2024 Volume 2024:17 Pages 343—362
DOI https://doi.org/10.2147/DMSO.S421527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Wenying Deng,1,* Zeyi Zhao,1,* Tao Zou,2 Tongdong Kuang,3,* Jing Wang1,*
1School of Basic Medical Sciences, University of South China, Hengyang, Hunan Province, 421001, People’s Republic of China; 2Department of Cardiovascular Medicine, First Affiliated Hospital of University of South China, Hengyang, Hunan Province, 421001, People’s Republic of China; 3Guangxi Key Laboratory of Diabetic Systems Medicine, Guilin Medical University, Guilin, Guangxi Province, 541199, People’s Republic of China
*These authors contributed equally to this workThese authors contributed equally to this study
Correspondence: Tongdong Kuang, Guangxi Key Laboratory of Diabetic Systems Medicine, Guilin Medical University, Guilin, 541199, People’s Republic of China, Email [email protected] Jing Wang, School of Basic Medical Sciences, University of South China, Hengyang, Hunan Province, 421001, People’s Republic of China, Tel +86 15200735915, Email [email protected]
Abstract: Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated blood glucose levels, resulting in multi-organ dysfunction and various complications. Fusion proteins can form multifunctional complexes by combining the target proteins with partner proteins. It has significant advantages in improving the performance of the target proteins, extending their biological half-life, and enhancing patient drug compliance. Fusion protein-based drugs have emerged as promising new drugs in diabetes therapeutics. However, there has not been a systematic review of fusion protein-based drugs for diabetes therapeutics. Hence, we conducted a comprehensive review of published literature on diabetic fusion protein-based drugs for diabetes, with a primary focus on immunoglobulin G (IgG) fragment crystallizable (Fc) region, albumin, and transferrin (TF). This review aims to provide a reference for the subsequent development and clinical application of fusion protein-based drugs in diabetes therapeutics.
Keywords: diabetes mellitus, fusion protein, Fc protein, glucagon-like peptide 1 receptor agonists, transferrin, albumin
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated glucose in the blood levels (hyperglycemia). Prolonged metabolic disorders can result in systemic damage to various organs including the kidneys, eyes, heart, blood vessels and nerves, leading to chronic progressive lesions, hypofunction, and organ failure.1–3 According to the 10th edition of the IDF diabetes atlas released by the International Diabetes Federation (IDF), the global number of people with DM is as high as 537 million, and its prevalence is increasing annually.4
Type 1 diabetes mellitus (T1DM) is characterized by the autoimmune-mediated destruction of pancreatic β-cells, resulting in an absolute lack of insulin secretion and is most commonly diagnosed in children and adolescents.4,5 Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and a relative lack of insulin secretion, which can cause by various etiologies. T2DM constitutes over 90% of clinical cases and is predominantly observed in middle-aged and elderly individuals.6
Currently, the management of diabetes mellitus is based on a globally accepted “five-pronged” approach, encompassing health education, diet control, physical exercise, self-monitoring, and pharmacological intervention. The global market value of diabetes therapeutics drug accounted to $118.1 billion in 2020, and it is projected to reach $317.9 billion by 2030, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2021 to 2030.7 Fusion protein-based drugs are favored in the market among various diabetes medications due to their extended half-life and stability, which also indicates an increasing market demand with the rise of chronic diseases.8 Genetic engineering technology allows for the development of fusion protein-based drugs that combine a functional target protein with a long half-life human-derived IgG Fc protein, albumin, or transferrin. Firstly, this fusion reduces the immunogenicity of the drug in vivo due to the human origin of the partner fusion protein. Secondly, the large relative molecular mass of the partner fusion protein, along with its unique ultra-long-acting mechanism, it reduces the kidney clearance of the target protein while preserving its original function, therefore extending the duration of drug effect.
Currently, the majority of papers primarily focus on a holistic approach to long-acting proteins, however, they failed to provide a detailed analysis of the target and clinical dosing of fusion protein-based drugs in the field of diabetes drug therapy. Hence, we conducted a systematic review of the fusion process and the targeted actions of various fusion protein-based drugs based on the latest research findings regarding fusion protein drugs used in the treatment of diabetes and its complications (Table 1). The present study aims to provide novel insights into the field of combination drug therapy for diabetes. Additionally, it aims to offer new research clues for the development and utilization of fusion protein-based drugs, as well as explore new possibilities for individualized diabetes treatment.
 |
Table 1 Related Fusion Protein Analogs for the Treatment of Diabetes |
Diabetes Mellitus
Diabetes is a prevalent chronic illness that affects individuals globally and is characterized by persistent hyperglycemia. It is a significant independent factor contributing to the development of cardiovascular diseases, which has the highest incidence and mortality rates worldwide.28 Diabetes mellitus can be classified into type 1 (T1DM) and type 2 (T2DM) based on the islet function. Islet function failure, with a low insulin release curve, corresponds to T1DM; while relatively intact islet function, characterized by a delayed peak insulin release curve, corresponds T2DM.
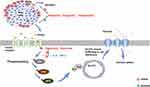
The regulatory mechanism of blood glucose is shown in Figure 1.29,30 After food intake, increased blood sugar level stimulates insulin secretion by islet β cells. Insulin then binds to the insulin receptor (IR) on the cell surface, promoting the IR phosphorylation. Phosphorylated IR further enhances the phosphorylation of the tyrosine structural domain on the insulin receptor substrate (IRS). This amplifies its active effect via the downstream phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt2) pathway and continues to phosphorylate downstream effector molecules, such as the Rab-GTPase-activating protein (Rab-GAP).31 Ultimately, this process allows Glucose Transporter 4 (GLUT-4) to transfer from the cytoplasm to the cellular membrane, regulating intracellular glucose uptake and utilization.32
Diabetes mellitus is a multifaceted disease with various etiologies. Different types of diabetes mellitus can be triggered by various factors. T1DM is an intricate autoimmune disease primarily associated with autoreactive memory T cells that fail to recognize their antigens, leading to immune tolerance and subsequent attack on islet β cells.33 A characteristic feature of patients with T1DM is the presence of T cells with a memory phenotype. These auto-reactive memory T cells are long-lived, highly responsive to antigenic stimuli, and play a significant role in the persistent deterioration of beta cell function in T1DM.33 Several immune interventions have shown some success in protecting beta cell function, such as Abatacept and Alefacept, which will be discussed below.34,35 These interventions have been found to deplete or modulate memory T cells. Well-tolerated specific immunotherapies can improve islet function in patients with T1D and are one of the promising strategies to slow down the process of β-cell destruction after the onset of T1D. A series of autoimmune antibodies for T1DM include: islet cell antibody, insulin autoantibody, glutamic acid decarboxylase antibody, islet antigen-2 antibody, and zinc transporter-8 antibody, which not only predict the likelihood of having T1DM, but also assist in diagnosing it with blood glucose values.36
T2DM is mainly caused by genetics, environmental factors (such as obesity, aging and a high-calorie diet) and inflammation, which promote each other and lead to insulin resistance.37,38 For example, chronic inflammation has been implicated in the pathogenesis of obesity-associated T2DM.39 And in turn obesity contributes to the development of chronic low-grade inflammation, as evidenced by elevated circulating levels of inflammatory cytokines.40 Inflammation plays a crucial role in the development of T2DM and is also medically controllable in T2DM.39,41 Inflammatory factors such as TNF-α, IL-6 and IL-1β often activate the NF-κB and JNK pathways through autocrine or paracrine signaling. This further promotes the formations of NLRP3 inflammatory vesicle and the releases of the cytokines and chemokines. Consequently, immune cells are recruited, leading to interference with insulin signaling in the peripheral tissues. These inflammatory factors can be utilized for predicting the risk of T2DM, and improving the corresponding inflammatory process is a common approach in the treatment of diabetes.
Fusion Protein
Since the synthesis of crystalline bovine insulin in China in 1965, the development of synthetic proteins has propelled mankind into an era of significant advancements in proteins drugs.42–45 The global protein therapeutics market was valued at $283.64 billion in 2020, and it is projected to reach $566.66 billion by 2030, with a compound annual growth rate (CAGR) of 7.1% from 2021 to 2030.46 Recombinant proteins are the major component of protein drugs, encompassing 197 recombinant proteins into the market from 2018 to 2022.47 The major types of recombinant proteins include monoclonal antibodies, hormones (e.g.insulin), cytokines, and fusion products. Fusion protein-based drugs are one of the long-acting drugs (with a half-life longer than 8 hours),48 which are produced by combining the target protein with another protein (Table 2). These fusion protein-based drugs exhibit extended half-life, minimal toxicity, high degree of specificity, excellent targeting capabilities, and remarkable stability, leading to their burgeoning popularity within the biopharmaceutical industry.
 |
Table 2 Types of Long-Acting Drugs |
Fusion protein-based drugs have three main fusion partners: the Fc fragment, albumin, and transferrin.48 The ultra-long-acting preparations of Fc fusion proteins and albumin fusion proteins depend primarily on endogenous proteins in the organism, namely the neonatal Fc receptor (FcRn). This receptor extends their biological half-life by preventing hepatic metabolic degradation through specific receptor-mediated cycling. FcRn is a non-covalent heterodimer composed of a 40 kDa α heavy chain and a 12 kDa β2m monomeric light chain. The α heavy chain consists of three extracellular regions (α1, α2, and α3), a transmembrane region, and a cytoplasmic tail of 44 amino acids.49 Although FcRn has a structure similar to that of the MHC I molecule, it possesses a blocked peptide-binding groove, preventing the presentation of peptide antigens to T cells and avoiding the elicitation of the body’s immune response.50 FcRn binds to Fc fragments or albumin through a pH-sensitive manner. Antibodies and albumin enter the cell by pinocytosis and bind to FcRn in the acidic endosome (pH=6.0). Subsequently, the FcRn-mediated antibody and FcRn-mediated albumin are released back into the extracellular environment and dissociate from FcRn at a pH of 7.4. This mechanism prevents the degradation of the antibodies and albumin by the acidic lysosomes, which enter the cell via pinocytosis, leading to the development of a long-lasting formulation. Studies have demonstrated that the fusion of FcRn with IgG Fc or albumin has been effective in delivering therapeutic drugs in the lung, oral cavity, genitalia, and uterus.51,52 It proves the promising development of Fc fusion protein in clinical applications.
In addition to combining functional proteins with Fc fragments and albumin, transferrin is also used as a bio-carrier for drug delivery, becoming a new fusion form. Transferrin enters the cell in a cytosolic form by binding to Transferrin receptor-1 (TFR1), and subsequently undergoes dissociation in the acidic environment of the endosome. It is then released extracellularly, thereby preventing the degradation of TF and TFR in the lysosome. This process facilitates recycling of transferrin upon release, effectively reusing Transferrin,53 thus improving TF half-life and efficacy. Apart from its application in the treatment of diabetes mellitus through the proinsulin-transferrin fusion protein (ProINS-TF), which will be mentioned later, transferrin can also be used to deliver proteins, drugs or DNA to target cells through diverse binding modalities, including biomolecules and nanoparticle platforms.54–57 The binding of transferrin to nanoparticle-coated insulin has demonstrated resistance to enzymatic hydrolysis and the ability to extend insulin efficacy for up to 10 hours.49
IgG Fc Fusion Protein
The Overview of IgG Fc Fusion Protein
The IgG Fc fusion protein is a genetically engineered recombinant protein that combines a functional protein with specific biological activities to the IgG Fc fragment. The immunoglobulins contain antigen-binding fragment (Fab) and Fc domain in vivo. The Fab region is composed of four structural domains: Variable Light domain (VL), Constant Light domain (CL), Variable Heavy domain (VH), and Constant Heavy domain-1 (CH-1), which are mainly responsible for antigen recognition. The Fc region, primarily responsible for immunomodulatory functions, encompasses two CH3 and two glycosylated CH2 structural domains located at the C-terminus of the antibody heavy chain. FcRn is mainly expressed in cytoplasm vesicles. Upon entry of the Fc fragment into the endosome through pinocytosis, FcRn interacts with specific amino acid residues at the CH2-CH3 interface under a pH environment of pH=6.51,52,66,67 Subsequently, FcRn-IgG detaches from the sorted nucleosomes as vesicles and tubules, fuses with the plasma membrane, and then releases IgG Fc fragments through exocytosis at pH=7.4.
Unbound IgG, which does not bind specifically to FcRn, remains within the vesicles of the sorted nucleosomes. It undergoes gradual acidification and maturation, eventually being disassembled by lysosomal enzymes.66,68 By virtue of the long-lasting mechanism of Fc binding to FcRn and immunity to lysosomal degradation in vivo, IgG exhibits a half-life up to 20 days.
In addition to the FcRn-mediated recycling mechanism,53 the remarkable extended half-life of Fc fusion proteins can be attributed to the following three factors: (1) Fc fusion protein greatly increases the spatial size of the protein and peptide drugs, surpassing the 20,000–40,000 Da threshold for glomerular filtration, thereby reducing the glomerular filtration rate of Fc protein.
Reducing the glomerular filtration rate of Fc protein. (2) The human-derived Fc fragment reduces the immunogenicity of the fusion protein, preventing elimination by the immune system. (3) The Fc fusion protein retains the hinge region of the natural linker IgG, which can prevent the interaction between two fused molecules and thus improve stability. The structure and function of the functional protein and the Fc fragment are relatively independent without interference, meanwhile, the Fc fragment is shielded from degradation by FcRn, which allows the fusion protein to have a longer effect.
The Selection of IgG Fc Fragment
The application of Fc fragments is not limited to IgG, studies have shown that IgA, IgE and IgM can also serve as effective alternatives to IgG for the therapeutic application. IgA or IgE recombinant proteins enhances antibody-dependent cell-mediated cytotoxicity (ADCC) and possess significant advantages in targeting and eliminating tumor cells. Moreover, utilizing the Fc fragment of IgE as a fusion protein promotes T cell proliferation.69–72 The Fc fragment of IgM exhibits anti-microbial activity by augmenting complement-dependent cytotoxicity (CDC) and can be employed as a subunit vaccine component to induce a robust and lasting immune response.73–75
Compared with other plasma proteins, IgG is currently the most advantageous plasma protein with natural long half-life protein not only taking up a large amount in serum and non-mucosal tissue, but also exhibiting a half-life from 2–4 weeks.76 IgG has classified into four categories based on disulfide bonds and amino acids composition in the hinge region. According to the descending order of serum concentration, it can be classified into IgG1 (60–70%), IgG2 (20–30%), IgG3 (5–8%) and IgG4 (1–3%).77 IgG1 peaks in plasma antibodies, and FcγRIII bonding mediates strong ADCC, CDC and ADCP (antibody-dependent cell-mediated phagocytosis). It is the most commonly used isoform of recombinant antibodies in antitumor therapy. IgG3 is the most functional subclass due to its high affinity for FcγR, activating complements and promoting FcγR-mediated function more effectively than any other immunoglobulin subclass.78,79 However, in virtue of its low affinity for FcRn, short half-life, and susceptibility to hydrolysis in the hinge region, its application is quite limited and no drugs of this subtype are available in clinical practice.79–81 The ADCC, ADCP and CDC of IgG2 and IgG4 are relatively weak and minimally harmful little damage to tissue cells. In the development of therapeutic fusion proteins, less cytotoxic Fc fragments of IgG2 and IgG4 are usually chosen as fusion vectors to minimize potential side effects.
Glucagon-Like Peptide 1 Receptor Agonists
Glucagon-like peptide-1 (GLP-1) is a type of enteroglucagon that is primarily secreted by L cells located in the distal small intestine and colon. GLP-1 exerts multiple physiological effects by binding to G protein-coupled receptors widely distributed throughout the body, thereby lowering blood glucose levels and improving insulin resistance.82,83 In the pancreas, it increases glucose-dependent insulin secretion, inhibits glucagon secretion, and protects pancreatic β cells.84,85 In the gastrointestinal tract, it delays postprandial gastric emptying, thereby delaying intestinal glucose absorption and reducing body weight.84,86 In the liver, it decreases glucose output and increases glycogen storage.87 In the heart, it increases glucose utilization and reduces lipid metabolism. This results in lower blood pressure and higher cardiac function, which offers cardioprotection and reduces the risk of cardiovascular events in patients.88,89 In the brain, it promotes satiety and reduces appetite through central suppression.87 However, the plasma half-life of endogenous GLP-1 is only about 2 minutes due to specific cleavage at the N-terminal by dipeptidyl peptidase-IV (DPP-IV), and the inherent high renal clearance of small molecule peptides.
Currently, there are ten GLP-1 receptor agonists (GLP-1RAs) approved for marketing: Exenatide, Liraglutide, Dulaglutide, Lixisenatide, Albiglutide, Semaglutide (two dosage forms: subcutaneous injection and oral administration), Tirzepatide, Efpeglenatide, PEG-Loxenatide and Beinaglutide, all of which can be used in the treatment of type 2 diabetes.90–93 These GLP-1RAs have a similar physiological function to GLP-1, but with a considerably longer half-life. The main adverse effects in subjects treated with GLP-1 RAs were gastrointestinal effects such as nausea, vomiting and diarrhoea.94,95
Dulaglutide
Dulaglutide is a long-acting GLP-1RA developed by Eli Lilly and Company and was ratified by the FDA for marketing in 2014.
To extend its half-life, Lilly employed a fusion approach by linking the C-terminus of 2 GLP-1s (7–37) with the N-terminal end of the IgG4 Fc fragment via a small molecule peptide chain. An optimized splice sequence was introduced to link the the GLP-1 and the hinge region of IgG4 Fc, thus restoring the full potency of the functional protein GLP-1 through specific amino acid site modifications, rendering it impervious to DPP-IV enzymatic degradation.96 Additionally, this process eliminates potential T-cell epitopes to reduce immunogenicity, resulting in the development of Dulaglutide, a highly effective, long-acting GLP-1 receptor agonist (GLP-1RA) possessing a 90% homology with GLP-1.
A prominent distinguishing characteristic of Dulaglutide different from other classes of oral hypoglycemic drugs is that it can protect β cells and avoid progressive β cell function decline.97,98 Compared with the traditional drugs metformin, exenatide injection and sitagliptin phosphate99–101, Dulaglutide exhibits superior efficacy in managing hypoglycemia and weight control. Numerous AWARD studies have demonstrated the effectiveness of Dulaglutide in lowering blood glucose levels, sustaining weight loss, and diminishing the incidence of hypoglycemia when administered alongside insulin.102 The fusion protein increases the lifespan of GLP-1 in plasma, prolonging its half-life up to 5 days.9 Therefore, it necessitates only a once-weekly subcutaneous injection and requires no dose titration, markedly enhancing patient compliance and rendering it a valuable treatment option for T2DM. Common side effects of Dulaglutide are gastrointestinal reactions, but they can be significantly reduced with prolonged use.10–12
Efpeglenatide
Different from Liraglutide and Dulaglutide, which are commonly used in clinic based on human GLP-1 structure, Efpeglenatide is a long-acting glucagon-like peptide-1 receptor agonist developed by Hanmi based on exendin-4 derived from animal GLP-1. Exendin-4, isolated from the venom of the Heloderma suspectum, is a specific and competitive antagonist of GLP-1.103 The drug consists of a modified exendin-4 molecule conjugated with a glycosylated IgG4 Fc fragment. Individual amino acid-modified exendin was ligated to IgG4 Fc fragment via a 3.4 kDa mini-Polyethylene Glycol (mini-PEG) linker by long-lasting peptide/protein technology.13 The small size of the mini-PEG linker reduces the loss of intrinsic agonist activity. The FC fragment greatly extends the half-life, permitting a prolonged action and pharmacokinetic/pharmacodynamic profile that can reach 5.6 to 7.5 days in patients with T2D.104 In this way, Efpeglenatide can support flexible dosing frequencies (from weekly, every 2 weeks, and monthly), is well tolerated by patients, and weekly dosing has a significantly more lasting effect on fasting glucose, with much less pharmacokinetic fluctuation following weekly dosing compared to monthly dosing.104–106 Efpeglenatide versus liraglutide in the treatment of T2DM, Efpeglenatide is comparable to liraglutide in terms of glucose metabolism and safety.13,107 In a phase 3 clinical trial AMPLITUDE-M, once-weekly Efpeglenatide as monotherapy in patients with type 2 diabetes was superior in lowering HbA1c and was able to reduce body weight and fasting glucose compared to placebo.108 Efpeglenatide, in addition to lowering glucose, significantly reduces several risk factors for cardiovascular and renal disease, such as blood pressure, LDL levels, and albumin/creatinine ratios, resulting in a 27% reduction in the risk of major cardiovascular adverse events and a 32% reduction in the risk of composite renal outcome events.109,110 Surprising results suggest that Efpeglenatide can have a controlling effect on blood glucose in pre-diabetic patients, and that it may help to reduce the likelihood of diabetes in high-risk patients.111 The most common adverse reactions in all Efpeglenatide are gastrointestinal adverse reactions such as nausea and vomiting.13
Basal Insulin Fc
Insulin is a protein hormone secreted by pancreatic β-cells in the pancreas and is the only hormone in the body responsible for reducing blood sugar levels. There are three types of insulin used for therapeutic purposes: animal insulin, human insulin and insulin analogues. Basal Insulin Fc (BIF; insulin efsitora alfa) is an insulin analogue developed by Eli Lilly and Company, with a molecular weight of 64.1 kDa. BIF consists of a fusion of a novel single-chain insulin variant and a human IgG2 Fc domain, having an ultra- long half-life.112 The insulin variant consists of an A-chain analog and a B-chain analog. The B chain is connected to the A chain by a short linker (link1), and the interdomain linker (link2) connects the A chain to Fc.14 The structure is designed to minimise insulin autoconjugation at locally high concentrations produced by BIF in the homodimeric state and has a prolonged insulin half-life.
Like Icodec, which has entered Phase III clinical trials, BIF is capable of being injected only once a week. In Phase I clinical studies, BIF exhibited a pharmacokinetic half-life of approximately 17 days and maintained its glucose-lowering activity for more than 5 days, perfectly fulfilling the requirement for once-weekly subcutaneous administration. Compared to daily insulin glargine, the ratio of the highest (peak) to lowest (trough) plasma concentrations of the BIF drug was lower (≈1.14), suggesting lower between-day glucose variability and more stable glucose reduction.113 Patients with T2DM previously treated with basal insulin were able to switch to BIF using a loading dose and achieve stable glycemic control.114 In two 26-week trials in patients with T1DM and T2DM respectively, BIF was found to be equally effective as degludec in stabilizing glycemic control on a daily basis.115,116 Hypoglycemia was the most common treatment-related adverse effect.113 Ultra-long-acting fusion proteins, constructed in a similar manner to insulin fusing Fc domain, have also been used in dogs and cats with DM. These proteins can also be administered once a week and have a satisfactory hypoglycemic effect.117,118
Insulin, as the only effective drug for patients with advanced T2DM and T1DM, finally needs to get rid of the inconvenience of daily administration. The weekly insulin being developed will greatly increase patients’ compliance. Look forward to phase 3 clinical data (QWINT) of BIF to further determine its effectiveness and safety.
Targeted Anti-Inflammatory Drugs Therapy for Diabetes Mellitus
Etanercept
Tumor necrosis factor-α (TNF- α) is a trimeric protein cytokine that is predominately secreted by macrophages. TNF-α binds to both tumor necrosis factor receptor type 1 (TNFR-1) and type 2 (TNFR-2), exerting a pivotal role in inflammatory activation through multiple signaling pathways.119,120 TNF-α was the initial pro-inflammatory cytokine proposed to be associated with the development of insulin resistance.121 It induces serine phosphorylation of pancreatic IRS-1 through JNK and IKK β pathway, thus decreasing insulin tyrosine kinase activity, impeding insulin-mediated glucose uptake by skeletal muscle and adipocyte, and promoting apoptosis of the pancreas’s β cells, thereby aggravating insulin resistance.122–124
Etanercept, an anti-rheumatic drug approved by Pfizer in 1998, is a soluble dimeric fusion protein (TNFRII-IgG1Fc) which formed by fusing the extracellular ligand structural domain of the TNF-α receptor with the Fc segment of IgG1. It competes with TNF-α for linking to the cell surface receptor TNFR, thereby effectively blocking the downward transmission of TNF-α to activate the JNK and IKKβ pathways.125 Etanercept has a half-life of 3–5.5 days and is usually subcutaneously injected. In a rat model of type 2 diabetes, Etanercept improved insulin and blood glucose levels in rats with type 2 diabetes.126 Research has demonstrated that it was not only effective in treating autoimmune diseases in patients, but also significantly improved their insulin resistance.127–129 There is also a case study showing that patients with psoriatic arthritis no longer need traditional glucose-lowering medications for poorly controlled T2DM due to Etanercept treatment.129 In a small preliminary study treating children with new-onset T1DM, researchers found that treating paediatric patients with Etanercept lowered glycated haemoglobin and increased insulin production, suggesting preservation of beta-cell function.15 Furthermore, Etanercept can protect the kidneys and improve the progression of diabetic nephropathy by blocking TNF-α-induced cytotoxicity and regulating inflammation.130
More research is needed to address the safety and efficacy of Etanercept in T1DM and T2DM. Etanercept maybe is suitable for diabetic patients suffering from chronic autoimmune diseases such as rheumatoid arthritis, etc. It improves concomitant DM by reducing inflammation while fighting immune diseases. The common adverse effects in clinical practice include infections, painful redness and swelling at the injection site, subcutaneous nodules, and infections from inflammatory response suppression.16
Abatacept
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a suppressive immunomodulatory molecule structurally akin to CD28. It binds to B7 molecules but exhibits an expression advantage on Treg, effectively impeding the proliferation and infiltration of effector T cell.131–134 The CTLA-4 gene was one of the important susceptibility genes for T1DM, and its expression significantly reduced in T1DM patients.135–137
Abatacept is an immunomodulator that used to treat rheumatoid arthritis. It was marketed by Bristol-Myers Squibb in 2005, which combines the extracellular region of CTLA-4 (CD152) and the IgG1-Fc fragment into a soluble fusion protein through recombinant DNA technology.138 Abatacept inhibits T cell activation through binding to the B7 molecule on antigen-presenting cells (APCs) and preventing its interaction with CD28 on T cells.139 Additionally, It can also improve diabetic nephropathy by blocking systemic T-cell activation, thus providing prevention and protection.140
Fujii et al showed that Abatacept, as an immunomodulator, improves insulin resistance in mice by promoting the conversion of adipose tissue macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype.141 Ursini et al discovered that Abatacept improves insulin sensitivity in patients suffering from rheumatoid arthritis.142,143 Clinical researches have manifested that Abatacept has a significant protective effect on islet β-cells in patients with T1DM. It slows the rate of β-cell function decline and exhibits good tolerability.144 Abatacept can be administered subcutaneously and has a half-life of approximately 2 weeks. Common adverse events associated with Abatacept include upper airway infections and nausea, with the most serious ones being severe infections and malignant tumors.17,18
sgp130Fc/Olamkicept
Interleukin-6 (IL-6) is a multifunctional inflammatory factor that that triggers intracellular signaling by binding specifically to the interleukin-6 receptor (IL-6R), activating the JAK/STAT and ras/MAP kinase pathways.145,146 IL-6 conjugates with IL-6R through two distinct signaling pathways: (1) the classical signaling pathway, which protects cellular tissue and has anti-inflammatory effects by binding to membrane-associated IL-6R, stimulating intestinal regeneration, inhibiting epithelial cell apoptosis, reducing bacterial infection and inducing acute temporal responses in the liver, and potentially beneficial for glucose metabolism.147,148 (2) Trans signaling pathway, which exerts pro-inflammatory effects by binding with sIL-6R, recruiting monocytes, inhibiting T cell apoptosis, and suppressing differentiation of regulatory T cells.147 A significant dose-response correlation exists between IL-6 levels and the risk of T2DM.149 Furthermore, elevated circulating IL-6 levels are a predictor factor of T2DM. IL-6 can be combined with sIL-6R and gp130 to exert pro-inflammatory effects through the JAK/STAT/SOCS3 pathway or JAK/JNK pathway, affecting IRS phosphorylation and inducing insulin resistance.150
Stefan Rose-John et al constructed a fusion protein known as sgp130Fc/Olamkicept, created by combining the extracellular part of dissoluble glycoprotein 130 (gp130) to the IgG1-Fc fragment.151 This fusion protein is suitable for intravenous medication and has a half-life of 4.7 days, Additionally, it selectively inhibits the IL-6 trans-signaling pathway. The sgp130Fc exclusively inhibits IL-6 trans-transduction by binding to sIL-6R without affecting classical IL-6 signaling. Therefore, the sgp130 prevents the pro-inflammatory effects while preserving IL-6 anti-inflammatory and auto-stabilizing abilities, thus avoiding severe immunosuppression and fatal infections. A recent in vitro study showed that sgp130Fc significantly reduced oxidative stress in mouse retinal endothelial cells, thereby resisting persistent hyperglycemia that leads to increased ocular retinal oxidative stress and inflammation, and delaying the progression of diabetes mellitus.152 Atherosclerotic cardiovascular disease (ASCVD) is a prevalent macro-vascular diabetic complication. sgp130Fc is currently poised to enter phase 2 clinical studies in ASCVD with previous results indicating favorable patient tolerability.153–155 In a mouse model of atherosclerosis, sgp130Fc showed vast atherosclerotic plaque degradation and vessel wall inflammation with few side effects in high-risk ASCVD patients.155,156 sgp130Fc, as the sole available anti-inflammatory protein that specifically blocks IL-6 transduction signaling, it may be more effective in the treatment of T2DM combined with other diabetes drugs in new treatment strategies.
Alefacept
Alefacept, a fusion protein developed by Biogen Inc. for the treatment of psoriasis, consists of two extracellular regions of lymphocyte function-associated antigen 3 (LFA-3) conjugated to an IgG1 Fc.20 By binding to IgG1 Fc, Alefacept has an extended half-life of 12 days and is used as a once-weekly dosage in T1DM therapy. LFA-3 is primarily found in antigen-presenting cells (APCs) and serves as a ligand for lymphocyte function-associated antigen-2 (CD2). CD2 is a surface protein expressed on the majority of human T-cells, with particularly high expression on effector-memory T (Tem) cells. As mentioned above, the pathogenesis of T1DM is directly related to the destruction of β-cells by effector T-cells.157 Alefacept, as a blocker of LFA-3/CD2 interaction, specifically binds to CD2 and inhibits the interaction of CD2 with the ligand LFA-3, thus blocking the co-stimulation of T-cells by APCs and depleting effector-memory T-cells to ultimately reduce the destruction of β-cells.21,158,159
In a Phase 2 clinical trial enrolling 49 subjects with new-onset T1DM (within 100 days of diagnosis), investigators evaluated the treatment difference of once-weekly Alefacept compared to placebo.21 C-peptide responses were significantly preserved with Alefacept compared to placebo at 1 and 2 years.160 Levels of C-peptide secretion are significantly associated with glycemic control, glycemic variability and partial clinical remission. At 24 months, the exogenous insulin requirements were lower and the incidence of major hypoglycemic events was reduced by approximately 50% in patients who received Alefacept injections. Alefacept’s immunological intervention in T1DM reduced insulin requirements and major hypoglycemic events in new-onset T1DM.What’s more, it also preserved residual beta-cell function in patients with newly diagnosed T1DM. Alefacept has a good safety profile, with the major adverse events being hypoglycemia and infection.
There is a lack of research on the use of Alefacept in T1DM at this stage, and it would be valuable to investigate alternative treatments by administering additional courses of Alefacept at higher dosages.
Others
The following two drugs have not been thoroughly researched for their effectiveness in treating diabetes, therefore, only their structures will be briefly mentioned.
The inflammatory factor IL-1β reduces insulin secretion and improves insulin resistance.161
Conversely, IL-1 receptor antagonists improve insulin secretion from β cells and reduce systemic inflammatory marker expression.162 Rilonacept, an IL-1 receptor antagonist marketed in 2008, is a long-acting dimeric fusion protein that fuses extracellular portion of IL-1 receptor 1 (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) with the Fc portion of IgG1. Rilonacept binds both IL-1α and IL-1β, thereby antagonising their binding to the IL-1 receptor. It has a half-life of 6–8 days.163,164 A Phase 1 trial of Rilonacept in patients with T1DM showed that Rilonacept was well tolerated in patients with newly diagnosed T1DM, with adverse effects mainly being injection spot reactions, cardiopulmonary infections, upper airway infections and mild neurological symptoms.22
Furthermore, a novel insulin sensitizer named YIMINSU has been developed by utilizing the structure of the hydroxyl terminal in the extracellular domain of the insulin receptor, a polyglycine sequence and Fc fragment of human immunoglobulin IgG4 (INBP-PolyG-IgG4Fc). It can improve insulin resistance by activating the AMPK/PI3K/AKT signaling pathway to initiate downstream cascade signals.165,166
Summary
The Fc fusion protein, as a derivative field of antibody application, has a wide range of prospective applications due to its unique characteristics. Although IgG Fc fusion protein has a longer half-life and less rejection in humans compared to other traditional hypoglycemic segments, they are not suitable for oral administration or the treatment of T1DM, and currently cannot be recommended as a first-line hypoglycemic agent. We anticipate that structural optimization of Fc fragments and regulation of FcRn-IgG interactions will be of attention in the near future.51
Currently, there exists a smaller IgG fragment called the 27 kDa Monomeric IgG1 Fc (MFc), which is smaller than the Fc fragment. This MFc fragment exhibits faster diffusion than the common Fc fragment, leading to quicker attainment of blood concentrations and a faster onset of action. Moreover, MFc remains unaffected upon binding and possesses a considerably longer half-life, allowing it to achieve the same effect as the Fc fragment.167,168 MFc binds closely with FcγRI, without any direct cytotoxicity and even safer than the Fc fragments of IgG2 and IgG4. It is expected to play a significant role in the treatment of chronic inflammatory diseases and solid tumors as an incipient drug-delivery protein. The selection of appropriate IgG fragments as fusion carriers, along with sequence modification, PEGylation, and glycosylation modification of Fc fragments, can prolong the plasma half-life of fusion proteins and optimize their structures and functions, ultimately achieving optimal therapeutic effects.
Albumin-Like Fusion Protein-Based Drugs
The Overview of Albumin and Its Fusion Protein-Based Drugs
Human serum albumin (HSA) is the most abundant protein in plasma, with a molecular weight of approximately 66.5 kDa. It avoids being quickly degraded through a pH-dependent recirculation mechanism mediated by FcRn, resulting in a half-life of 19 days. HSA is a heart-like molecule that contains three homologous structural domains (DI, DII and DIII). DI and DIII are implicated in the interaction of albumin and FcRN, with DIII playing a key role in binding.169,170 The differences between FcRn-IgG Fc and FcRn-albumin are as follows: (i) albumin-binding site is located on the opposite side of the IgG-binding site, and albumin binds to FcRN in a 1:1 manner;169,171 (ii) albumin does not bind to the classical FcγR, thereby avoiding the immunomodulatory effects and cytotoxic responses induced by the Fc segment, which reduce the risk of unwanted immune activation. Albumin is used as a medication vector due to its minimal immunogenicity, highly bio-compatibility, and lowly toxicity.172 Similar to Fc fusion proteins, albumin has a wide range of fusion targets which increases the bioavailability and half-life of the target protein.173
Albumin fusion protein-based drugs are a novel type of recombinant protein drugs produced by genetically fusing the structural domain of albumin to a functional protein possessing specific biological activity. There are usually two types of albumin used for functional protein fusion: HSA and recombinant human serum albumin (RHSA). HSA is obtained through isolating and purifying blood donor plasma, but carries the risk of virus or prion contamination. Conversely, RHSA is produced via the secretion of the target protein using a yeast expression system, followed by purification to eliminate any contaminants derived from yeast.174–176 It has also been reported that cost-effective rice seeds can also successfully express RHSA.177,178 Albumin fusion proteins have a long drug half-life, high histocompatibility, and offer diverse ligand binding sites which can improve drug delivery and reinforce pharmacokinetic performance.
Albiglutide
Albiglutide, a GLP-1RA, was the first albumin fusion protein drug ratified by FDA in 2014. Albiglutide is constructed by fusing two tandem copies of anti-DPP-IV hydrolyzed GLP-1 analogue to the N-terminus of albumin.179,180 The main reason for the extended half-life of albiglutide (6–8 days) and the need for only one weekly subcutaneous injection is the replacement of the DPP-IV hydrolysis site from alanine to glutamate and its conjugation to a high molecular weight protein.
In contrast to other GLP-1RAs such as Exenatide and Liraglutide, Albiglutide not only effective controls blood glucose concentrations and mean glycated hemoglobin levels, but also exhibits a lower likeliness of gastrointestinal adverse effects. Furthermore, it has a remarkable advantage in reducing major cardiovascular adverse effects, including atherothrombosis and myocardial infarction.23,24,179,181–185 The combined effect of Albiglutide, compared to a placebo, was a 22% reduction in the risk of major cardiovascular disease related deaths. Additionally, Albiglutide exhibits good tolerability in T2DM patients with mild, moderate or severe renal insufficiency. It did not require dose adjustments for renal impairment, significantly reduces HbA1c expression in combined drugs, and is better tolerated by the stomach and intestines.186,187
Albulin
Albulin is a long-acting insulin analogue. It is a recombinant fusion protein consists of the A and B chains of insulin connected by a dodecapeptide junction and attached to the amino terminus structural domain of natural HSA. In vitro studies have shown that, except for a low affinity for insulin-related receptors, Albulin functions similarly to insulin, especially when it inhibits gluconeogenesis in hepatocytes and stimulates glucose uptake by adipocytes.25 Experimental data indicate that the terminal half-life of albulin in normoglycemic mice is 7 hours, while the anticipated elimination half-life of albulin in a 70 kg man is approximately 50 hours.26
Summary of Albumin-Like Fusion Protein-Based Drugs
Albumin, as a large molecular protein, exhibits good bio-compatibility and possesses long half-life in drug fusion. However, attaching bulky proteins to fusion targets will inevitably affect the biological activity of fusion targets. Although higher doses of fusion proteins in treatment may compensate for the loss of efficacy, they also significantly increase the incidence of adverse effects. Recent studies have found that the fusion of high-affinity structural domain (ABD) of HSA with GLP-1 protein can maintain the natural bioactivity of GLP-1 and increase its half-life by 1000-fold. This finding offers promising prospects for the development of T2DM treatment.188
Transferrin-Like Fusion Protein-Based Drugs
Overview of Transferrin
Human transferrin (HTF), a novel efficient carrier with a molecular weight of 78 kDa, is mainly synthesized in the liver. Transferrin has two types of receptors: TFR1 and TFR2. TFR1 is predominantly located on the surface of somatic cells and facilitates the TF cellular uptake, it is associated with the production of mature erythrocytes. TFR2 is primarily expressed in hepatocytes and erythroid precursor cells, it is associated with the maintenance of iron levels in the body. Through the TF/TFR1 system, transferrin can increase the half-life in serum to 8 days.53,189
The HTF fusion protein combines the structural domain of transferrin with the target peptide to form a hybrid molecule through genetic engineering techniques, giving the stable properties of transferrin to the peptide. Transferrin, as a drug delivery system, can deliver drugs in various forms.54 HTF has the following advantages on treating diabetes mellitus:
- It has the ability to regulate insulin resistance.190 Iron homeostasis is essential for maintaining the normal pancreatic β-cell function and glucose metabolism. Iron overload in the body can induce iron death through increasing oxidative stress, lipid peroxide production, and free radical level. Furthermore, It disrupts insulin signaling pathways and impairs autophagy, resulting in pancreatic cell death and insulin resistance. Consequently, this leads to hyperglycemia and elevated serum iron levels, aggravating the insulin resistance.191–193 In diabetic patients, intravenous apolipoprotein-transferrin (apo-TF) can reduce the level of free iron, mitigate oxidative damage, postpone the insulin resistance, and decrease the incidence of cardiovascular disease194.
- It possesses the capability to traverse the blood-brain barrier.195,196 TFR1 exhibits extensive distribution within the blood-brain barrier, and the transport of substances mediated by the transferrin receptor has emerged as a prominent therapeutic target in the central nervous system with neurodegenerative disease treatment potentials.
- Administered orally in medication.55,197 HTF directs the absorption and transportation of drugs through the rich TFR in intestinal epithelial cells,198 Additionally, HTF possesses robust anti-protease hydrolysis capabilities, rendering it an efficient carrier for oral proteins and peptide-based therapeutic agents intended for systemic treatment. It can effectively prolong the efficacy of GLP-1 by linking with GLP-1.199
- Providing recombinant production systems with high efficiency and lower prices.200 Cheap and reliable edible genetically modified tobacco systems can produce therapeutic proteins and bioactive peptides for direct oral administration.
- The utilization of affordable, edible genetically modified (GM) tobacco as a primary resource eliminates the need for protein purification and processing Hence, the costs of production are greatly reduced compared to traditional yeast or animal cell cultures.
ProINS-TF
ProINS-TF is a novel long-acting liver-targeted insulin precursor drug composed of the C-terminal of human proinsulin (ProINS) fused with the N-terminal of human serum TF.201 The TF-TFR endocytic circulating pathway facilitates the conversion and release of proinsulin-transferrin (ProINS-TF) in the immuno-reactive insulin-transferrin (irINS-TF), the active form with a relative molecular mass of 88.1 kDa and a clearance half-life of 7.29 h.27 In streptozotocin-induced T1DM mice, ProINS-TF showed a hypoglycemic effect for up to 40h.202 ProINS is an insulin precursor, and under physiological conditions in which blood proinsulin levels are low, its function similarly to insulin except for hepatic specificity. Pharmacokinetic researches have shown that ProINS has a longer distribution and elimination half-life compared to insulin. However, the conversion of ProINS to insulin in vivo is notably inefficient. ProINS needs to be increased more than tenfold to achieve the equivalent hypoglycemic pharmacological effect. Despite this dosage escalation, the biological utilization of ProINS remains at less than one percent compared to insulin.203–206
By combining ProINS with HTF, its elimination half-life is extended from 0.5 h to 7.29 h, with a sustained hypoglycemic effect of at least 12 h.27 ProINS-TF has a high liver targeting specificity.207 Studies have shown that TFR-mediated endocytosis in the liver can activate ProINS-TF into irINS-TF to phosphorylate Akt, which plays a role in regulating blood glucose.201,208
irINS-TF effectively resolved the slow onset of ProINS-TF, further improved the binding affinity and prolonged the activation time of IR, and could achieve the same or even better sustained glucose-lowering effect compared to natural insulin in the treatment of T2DM. Utilizing the transgenic rice expression system, it is possible to produce ProINS-TF with high activity and oral administration at a low cost and a large scale, which provides a material guarantee for future research.197
Numerous prior studies have explored for transferrin fusion proteins on the perspective of receptor-ligand binding specificity or targeted delivery,209,210 however, few studies have been dedicated to the extension of targeting half-life, let alone the study of fusion transferrin hypoglycemic agents. The successful construction of ProINS-TF provides a promising avenue for the future development of long-acting hypoglycemic drugs.
Conclusion
Using molecular biology techniques to integrate therapeutic protein or peptide sequences into transporter proteins with long half-lives, such as Fc fragment, albumin and transferrin, can extend the duration of short-acting functional substances such as GLP-1 and ProINS. This process effectively mitigates insulin resistance and regulates blood glucose, providing a reference for the development of new long-acting diabetes drugs.
From a therapeutic perspective, protein therapies may be safer than gene therapies because they do not involve random or permanent genetic alterations.
In contrast to protein and peptide drugs which require frequent long-term subcutaneous injections, fusion protein-based drug therapies are unique in the following prospects: on the one hand, fusion drugs based on natural transporter proteins require less frequently administration. On the other hand, transferrin fusion proteins are produced by plant expression systems, resulting in cost-effectiveness and oral properties. This dual advantage enables more convenient, manageable, and patient-compliant treatment options.
It should be noted that linking bulky proteins to fusion targets will inevitably impacts the biological activity of the fusion target, which poses serious challenges for drug formulations and increases the occurrence of side effects.
In the construction of drug molecules, more attention should be paid to the manner in which the fusion protein binds to the target protein, the specific type and length of the linker, etc., avoiding damage the target protein activity.211,212 A fusion protein characterized by stable properties and favorable biological behavior holds substantial market potential, yet its development still presents formidable challenges.213
Diabetes mellitus is a global disease that can cause complications in multiple organs. It disrupts the metabolism of carbohydrates, proteins, and fats, negatively impacting both physical and mental health. Despite the availability of numerous drugs for the treatment of diabetes mellitus, there remains a dearth of fusion protein-based hypoglycemic drugs in development.
This review focuses on fusion protein-based drugs designed to prolong the half-life of functional proteins, including Fc fusion protein, albumin fusion protein, and transferrin fusion protein.
Nevertheless, some of the drugs mentioned have not been tested in clinical trials. Hence, their effects and adverse effects need to be further studied. In addition, protein-based drugs possess immunogenicity. And despite the high homology of fusion proteins, the possibility of immune reactions in allergic patients cannot be disregarded. It is anticipated that with the rapid development of bio-pharmaceutical technology, fusion protein-based drugs will have a bright future in the prevention and treatment of diabetes mellitus.
Acknowledgments
This research was supported by Provincial Natural Science Foundation of Hunan (#2019JJ50503 to J. Wang and #2021JJ70121 to T. Zou), and the National College Students Research Study and Innovative Experiment Project (#202110555100, to ZY.Zhao)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):117–124. doi:10.2174/1570161117666190502103733
2. Squadrito G, Cucinotta D. The late complications of diabetes mellitus. Ann Ital Med Int. 1991;6(1):126–136.
3. Schlienger JL. Type 2 diabetes complications. Presse Med. 2013;42(5):839–848. doi:10.1016/j.lpm.2013.02.313
4. Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10(7):501–513. doi:10.1038/nri2787
5. Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17(3):150–161. doi:10.1038/s41574-020-00443-4
6. Prevention CfDCa. National diabetes statistics report: what is Diabetes?; 2022. Available from: https://www.cdc.gov/diabetes/basics/diabetes.html.
7. Dash L, Bhasme P, Sumant O. Diabetes therapeutics market report; 2022. Available from: https://www.alliedmarketresearch.com/diabetes-therapeutics-market.
8. S O. Fusion protein and biosimilars market report; 2023. Available from: https://www.alliedmarketresearch.com/fusion-protein-and-biosimilars-market-A12017.
9. Geiser JS, Heathman MA, Cui X, et al. Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analyses of data from clinical trials. Clin Pharmacokinet. 2016;55(5):625–634. doi:10.1007/s40262-015-0338-3
10. Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK. Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther. 2010;12(6):790–797.
11. Yoo JH, Cho YK, Lee J, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diabetes Ther. 2019;10(4):1453–1463. doi:10.1007/s13300-019-0658-7
12. Frias JP, Wynne AG, Matyjaszek-Matuszek B, et al. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21(9):2048–2057. doi:10.1111/dom.13764
13. Rosenstock J, Sorli CH, Trautmann ME, et al. Once-weekly efpeglenatide dose-range effects on glycemic control and body weight in patients with type 2 diabetes on metformin or drug naive, referenced to liraglutide. Diabetes Care. 2019;42(9):1733–1741. doi:10.2337/dc18-2648
14. Moyers JS, Hansen RJ, Day JW, et al. Preclinical characterization of LY3209590, a novel weekly basal insulin fc-fusion protein. J Pharmacol Exp Ther. 2022;382(3):346–355. doi:10.1124/jpet.122.001105
15. Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244–1249. doi:10.2337/dc09-0054
16. Pan A, Gerriets V. Etanercept. In: StatPearls. Treasure Island (FL): StatPearls; 2023.
17. Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73(1):86–94. doi:10.1136/annrheumdis-2013-203843
18. Harigai M, Ishiguro N, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of Abatacept in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2016;26(4):491–498. doi:10.3109/14397595.2015.1123211
19. Schreiber S, Aden K, Bernardes JP, et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. 2021;160(7):2354–2366.e2311. doi:10.1053/j.gastro.2021.02.062
20. Vaishnaw AK, TenHoor CN. Pharmacokinetics, biologic activity, and tolerability of alefacept by intravenous and intramuscular administration. J Pharmacokinet Pharmacodyn. 2002;29(5–6):415–426. doi:10.1023/A:1022995602257
21. Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):284–294. doi:10.1016/S2213-8587(13)70111-6
22. White PC, Adhikari S, Grishman EK, Sumpter KM. A phase I study of anti-inflammatory therapy with rilonacept in adolescents and adults with type 1 diabetes mellitus. Pediatr Diabetes. 2018;19(4):788–793. doi:10.1111/pedi.12634
23. Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–297. doi:10.1016/S2213-8587(13)70214-6
24. Aroda VR, Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev. 2011;27(6):528–542. doi:10.1002/dmrr.1202
25. Ahren B, Burke B. Using albumin to improve the therapeutic properties of diabetes treatments. Diabetes Obes Metab. 2012;14(2):121–129. doi:10.1111/j.1463-1326.2011.01482.x
26. Duttaroy A, Kanakaraj P, Osborn BL, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54(1):251–258. doi:10.2337/diabetes.54.1.251
27. Wang Y, Shao J, Zaro JL, Shen WC. Proinsulin-transferrin fusion protein as a novel long-acting insulin analog for the inhibition of hepatic glucose production. Diabetes. 2014;63(5):1779–1788. doi:10.2337/db13-0973
28. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
29. Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19(1):31–44. doi:10.1038/nrm.2017.89
30. Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol. 1994;266(2):C319–334. doi:10.1152/ajpcell.1994.266.2.C319
31. Mackenzie RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:55–64. doi:10.2147/DMSO.S48260
32. Chen Y, Wang Y, Zhang J, et al. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198(4):545–560. doi:10.1083/jcb.201111091
33. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi:10.1038/nature08933
34. Russell WE, Bundy BN, Anderson MS, et al. Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: a randomized, double-masked, controlled trial. Diabetes Care. 2023;46(5):1005–1013. doi:10.2337/dc22-2200
35. Ehlers MR, Rigby MR. Targeting memory T cells in type 1 diabetes. Curr Diab Rep. 2015;15(11):84. doi:10.1007/s11892-015-0659-5
36. Bonifacio E, Achenbach P. Birth and coming of age of islet autoantibodies. Clin Exp Immunol. 2019;198(3):294–305. doi:10.1111/cei.13360
37. Hivert MF, Vassy JL, Meigs JB. Susceptibility to type 2 diabetes mellitus--from genes to prevention. Nat Rev Endocrinol. 2014;10(4):198–205. doi:10.1038/nrendo.2014.11
38. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi:10.1038/nrd4275
39. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabet Res Clin Pract. 2014;105(2):141–150. doi:10.1016/j.diabres.2014.04.006
40. van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71(4):174–187.
41. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi:10.1172/JCI29069
42. Kung YT, Du YC, Huang WT, Chen CC, Ke LT. Total synthesis of crystalline insulin. 科学通报:英文版 [Scientia Sinica]. 1966;15(4):544–561.
43. Institute, Biockemistry, Sinica. Resynthesis of insulin from its A And B chains. Semi Month J Sci. 1966;06:246–246.
44. Zhang Y. The first protein ever synthesized in vitro—a personal reminiscence of the total synthesis of crystalline insulin. Sci China. 2010;2010:1.
45. Institute, Sinica, Department, Peking. The synthesis of the: a chain of insulin and partial synthesis of crystalline insulin from the synthetic A Chain and the natural B Chain. Chin. Sci. Bull. 1966;06:256–256.
46. Mali S, Divekar M, Sumant O. Protein therapeutics market report; 2022. Available from: https://www.alliedmarketresearch.com/protein-therapeutics-market.
47. Walsh G, Walsh E. Biopharmaceutical benchmarks 2022. Nat Biotechnol. 2022;40(12):1722–1760. doi:10.1038/s41587-022-01582-x
48. Zaman R, Islam RA, Ibnat N, et al. Current strategies in extending half-lives of therapeutic proteins. J Control Release. 2019;301:176–189. doi:10.1016/j.jconrel.2019.02.016
49. Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180(6):2377–2381. doi:10.1084/jem.180.6.2377
50. Nakamura A, Sosa A, Komori H, Kita A, Miki K. Crystal structure of TTHA1657 (AT-rich DNA-binding protein; p25) from Thermus thermophilus HB8 at 2.16 A resolution. Proteins. 2007;66(3):755–759. doi:10.1002/prot.21222
51. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725. doi:10.1038/nri2155
52. Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The Neonatal Fc Receptor (FcRn): a Misnomer? Front Immunol. 2019;10:1540.
53. Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med. 2019;133:46–54. doi:10.1016/j.freeradbiomed.2018.06.037
54. Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22(3):225–250. doi:10.1002/med.10008
55. Amet N, Wang W, Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141(2):177–182. doi:10.1016/j.jconrel.2009.09.007
56. Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8(6):916–931.
57. Okuyama T, Eto Y, Sakai N, et al. A Phase 2/3 Trial of Pabinafusp Alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol Ther. 2021;29(2):671–679. doi:10.1016/j.ymthe.2020.09.039
58. Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10(21):1451–1458. doi:10.1016/S1359-6446(05)03575-0
59. Strohl WR. Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs. 2015;29(4):215–239. doi:10.1007/s40259-015-0133-6
60. Yang X, Bartlett MG. Glycan analysis for protein therapeutics. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1120:29–40. doi:10.1016/j.jchromb.2019.04.031
61. Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98(4):1223–1245. doi:10.1002/jps.21504
62. Borza B, Hajba L, Guttman A. N-glycan analysis in molecular medicine: innovator and biosimilar protein therapeutics. Curr Mol Med. 2020;20(10):828–839. doi:10.2174/1566524020999201203212352
63. Martins JP, Figueiredo P, Wang S, et al. Neonatal Fc receptor-targeted lignin-encapsulated porous silicon nanoparticles for enhanced cellular interactions and insulin permeation across the intestinal epithelium. Bioact Mater. 2021. doi:10.1016/j.bioactmat.2021.08.007
64. Miller MR, Raftis JB, Langrish JP, et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS nano. 2017;11(5):4542–4552. doi:10.1021/acsnano.6b08551
65. Mohammadpour R, Dobrovolskaia MA, Cheney DL, Greish KF, Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv Drug Delivery Rev. 2019;2019:144.
66. Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci. 2015;101(30):11076–11081. doi:10.1073/pnas.0402970101
67. Ward ES, Ober RJ. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol Sci. 2018;39(10):892–904. doi:10.1016/j.tips.2018.07.007
68. Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172(4):2021–2029. doi:10.4049/jimmunol.172.4.2021
69. Lohse S, Derer S, Beyer T, et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186(6):3770–3778. doi:10.4049/jimmunol.1003082
70. Karagiannis SN, Josephs DH, Karagiannis P, et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother. 2012;61(9):1547–1564. doi:10.1007/s00262-011-1162-8
71. Elders RC, Holder A, Smith KC, Baines SJ, Catchpole B. Recombinant canine IgE Fc and an IgE Fc-TRAIL fusion protein bind to neoplastic canine mast cells. Vet Immunol Immunopathol. 2014;159(1–2):29–40. doi:10.1016/j.vetimm.2014.02.018
72. Perez-Witzke D, Miranda-García MA, Suárez N, Becerra R, Montano RF. CTLA4Fcε, a novel soluble fusion protein that binds B7 molecules and the IgE receptors, and reduces human in vitro sCD23 production and lymphocyte proliferation. Immunology. 2016;148(1):40–55. doi:10.1111/imm.12586
73. Ammann JU, Jahnke M, Dyson MR, Kaufman J, Trowsdale J. Detection of weak receptor-ligand interactions using IgM and J-chain-based fusion proteins. Eur J Immunol. 2012;42(5):1354–1356. doi:10.1002/eji.201142151
74. Bettoni S, Maziarz K, Stone M, et al. Serum complement activation by C4BP-IgM fusion protein can restore susceptibility to antibiotics in Neisseria gonorrhoeae. Front Immunol. 2021;12(3511). doi:10.3389/fimmu.2021.726801
75. Kumar S, Singh VK, Vasam M, et al. An in vitro refolding method to produce oligomers of anti-CHIKV, E2-IgM Fc fusion subunit vaccine candidates expressed in E. coli. J Immunol Methods. 2020;487:112869. doi:10.1016/j.jim.2020.112869
76. Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22(6):868–876. doi:10.1016/j.copbio.2011.06.012
77. Damelang T, Rogerson SJ, Kent SJ, Chung AW. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019;40(3):197–211. doi:10.1016/j.it.2019.01.005
78. Bruggemann M, Williams GT, Bindon CI, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166(5):1351–1361. doi:10.1084/jem.166.5.1351
79. Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi:10.1182/blood-2008-09-179754
80. Honger G, Amico P, Arnold ML, Spriewald BM, Schaub S. Effects of weak/non-complement-binding HLA antibodies on C1q-binding. HLA. 2017;90(2):88–94. doi:10.1111/tan.13062
81. Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7(9):1401–1413. doi:10.1517/14712598.7.9.1401
82. Mayo KE, Miller LJ, Bataille D, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55(1):167–194. doi:10.1124/pr.55.1.6
83. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. doi:10.1016/S0140-6736(87)91194-9
84. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi:10.1016/S0140-6736(02)07952-7
85. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87(3):1239–1246. doi:10.1210/jcem.87.3.8355
86. Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38(4):665–673. doi:10.1007/BF01316798
87. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi:10.1152/physrev.00034.2006
88. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136(9):849–870. doi:10.1161/CIRCULATIONAHA.117.028136
89. Zimmerman RS, Hobbs TM, Wells BJ, et al. Association of glucagon-like peptide-1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system. Diabetes Obes Metab. 2017;19(11):1555–1561. doi:10.1111/dom.12969
90. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-The-art. Mol Metab. 2021;46:101102. doi:10.1016/j.molmet.2020.101102
91. Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovas Diabetol. 2022;21(1):1–16. doi:10.1186/s12933-022-01604-7
92. Chen K, Chen L, Shan Z, et al. Beinaglutide for weight management in Chinese individuals with overweight or obesity: a phase 3 randomized controlled clinical study. Diabetes Obes Metab. 2023;2023:1.
93. Liu L, Ruan Z, Ung COL, et al. Long-term cost-effectiveness of subcutaneous once-weekly semaglutide versus polyethylene glycol loxenatide for treatment of type 2 diabetes mellitus in China. Diabetes Ther. 2023;14(1):93–107. doi:10.1007/s13300-022-01336-7
94. Bettge K, Kahle M, El Aziz MS A, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336–347. doi:10.1111/dom.12824
95. Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: a real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol. 2022;13:1043789. doi:10.3389/fendo.2022.1043789
96. Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26(4):287–296. doi:10.1002/dmrr.1080
97. Umpierrez G, Blevins T, Rosenstock J, Cheng C, Anderson J. The effect of LY2189265 (GLP-1 analogue) once weekly on HbA(1c) and beta cell function in uncontrolled type 2 diabetes mellitus: the EGO study analysis. Paper presented at: 45th Annual Meeting of the 2009; 2009.
98. AdlP P, Loghin C, Cui X, et al. Once-weekly dulaglutide 1.5 mg restores insulin secretion in response to intravenous glucose infusion. Diabetes Obesity Metab. 2017;2017:1.
99. Wysham C, Blevins T, Arakaki R, et al. Erratum. efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37:2159–2167. doi:10.2337/dc13-2760
100. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168–2176. doi:10.2337/dc13-2759
101. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–858. doi:10.1111/dom.12479
102. MacIsaac RJ. Dulaglutide and Insulin: how Can the AWARD Studies Help Guide Clinical Practice? Diabetes Ther. 2020;11(8):1627–1638. doi:10.1007/s13300-020-00863-5
103. Leung K. (99m)Tc-Hydrazinonicotinamide-aminohexanoic acid-Lys(40)-exendin-4. In: Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD): MICAD; 2004.
104. Yoon KH, Kang J, Kwon SC, et al. Pharmacokinetic and dose-finding studies on efpeglenatide in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22(8):1292–1301. doi:10.1111/dom.14032
105. Pratley RE, Kang J, Trautmann ME, et al. Body weight management and safety with efpeglenatide in adults without diabetes: a Phase II randomized study. Diabetes Obes Metab. 2019;21(11):2429–2439. doi:10.1111/dom.13824
106. Del Prato S, Kang J, Trautmann ME, et al. Efficacy and safety of once-monthly efpeglenatide in patients with type 2 diabetes: results of a phase 2 placebo-controlled, 16-week randomized dose-finding study. Diabetes Obes Metab. 2020;22(7):1176–1186. doi:10.1111/dom.14020
107. Hompesch M, Kang J, Han O, et al. Effects of efpeglenatide versus liraglutide on gastric emptying, glucose metabolism and beta-cell function in people with type 2 diabetes: an exploratory, randomized phase Ib study. BMJ Open Diabetes Res Care. 2021;9(1):e002208. doi:10.1136/bmjdrc-2021-002208
108. Frias JP, Choi J, Rosenstock J, et al. Efficacy and safety of once-weekly efpeglenatide monotherapy versus placebo in type 2 diabetes: the AMPLITUDE-M randomized controlled trial. Diabetes Care. 2022;45(7):1592–1600. doi:10.2337/dc21-2656
109. Gerstein HC, Li Z, Ramasundarahettige C, et al. Exploring the relationship between efpeglenatide dose and cardiovascular outcomes in type 2 diabetes: insights from the AMPLITUDE-O trial. Circulation. 2023;147(13):1004–1013. doi:10.1161/CIRCULATIONAHA.122.063716
110. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi:10.1056/NEJMoa2108269
111. Pratley RE, Jacob S, Baek S, et al. Efficacy and safety of efpeglenatide in key patient subgroups from the BALANCE randomized trial, stratified by pre-diabetes status, BMI, and age at baseline. BMJ Open Diabetes Res Care. 2022;10(1):e002207. doi:10.1136/bmjdrc-2021-002207
112. Moyers JS, Hansen RJ, Day JW, et al. Preclinical characterization of once weekly basal insulin Fc (BIF). J Endocrine Soc. 2021;5:A442–A442. doi:10.1210/jendso/bvab048.903
113. Heise T, Chien J, Beals JM, et al. Pharmacokinetic and pharmacodynamic properties of the novel basal insulin Fc (insulin efsitora alfa), an insulin fusion protein in development for once-weekly dosing for the treatment of patients with diabetes. Diabetes Obes Metab. 2023;25(4):1080–1090. doi:10.1111/dom.14956
114. Frias J, Chien J, Zhang Q, et al. Safety and efficacy of once-weekly basal insulin Fc in people with type 2 diabetes previously treated with basal insulin: a multicentre, open-label, randomised, phase 2 study. Lancet Diabetes Endocrinol. 2023;11(3):158–168. doi:10.1016/S2213-8587(22)00388-6
115. Kazda CM, Bue-Valleskey JM, Chien J, et al. Novel once-weekly basal insulin fc achieved similar glycemic control with a safety profile comparable to insulin degludec in patients with type 1 diabetes. Diabetes Care. 2023;46(5):1052–1059. doi:10.2337/dc22-2395
116. Bue-Valleskey JM, Kazda CM, Ma C, et al. Once-weekly basal insulin fc demonstrated similar glycemic control to once-daily insulin degludec in insulin-naive patients with type 2 diabetes: a phase 2 randomized control trial. Diabetes Care. 2023;46(5):1060–1067. doi:10.2337/dc22-2396
117. Gilor C, Hulsebosch SE, Pires J, et al. An ultra-long-acting recombinant insulin for the treatment of diabetes mellitus in cats. J Vet Intern Med. 2021;35(5):2123–2130. doi:10.1111/jvim.16150
118. Hulsebosch SE, Pires J, Bannasch MJ, et al. Ultra-long-acting recombinant insulin for the treatment of diabetes mellitus in dogs. J Vet Intern Med. 2022;36(4):1211–1219. doi:10.1111/jvim.16449
119. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi:10.1146/annurev.iy.10.040192.002211
120. Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015;87(2):281–296. doi:10.1038/ki.2014.285
121. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi:10.1172/JCI117936
122. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–668. doi:10.1126/science.271.5249.665
123. Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46(12):1594–1603. doi:10.1007/s00125-003-1228-z
124. Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119(1):105–110. doi:10.1002/jcb.26174
125. Garrison L, McDonnell ND. Etanercept: therapeutic use in patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58(Suppl 1):I65–69. doi:10.1136/ard.58.2008.i65
126. Di KB, Bahcivan E, Eser Faki H, Uney K. Combined treatment with interleukin-1 and tumor necrosis factor-alpha antagonists improve type 2 diabetes in rats. Can J Physiol Pharmacol. 2018;96(8):751–756. doi:10.1139/cjpp-2017-0769
127. Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. 2008;35(2):355.
128. Corrao S, Pistone G, Scaglione R, Colomba D, Calvo L, Licata G. Fast recovery with etanercept in patients affected by polymyalgia rheumatica and decompensated diabetes: a case-series study. Clin Rheumatol. 2009;28(1):89–92. doi:10.1007/s10067-008-1026-6
129. Pfeifer EC, Saxon DR, Janson RW. Etanercept-induced hypoglycemia in a patient with psoriatic arthritis and diabetes. J Investig Med High Impact Case Rep. 2017;5(3):2324709617727760. doi:10.1177/2324709617727760
130. Doria A, Niewczas MA, Fiorina P. Can existing drugs approved for other indications retard renal function decline in patients with type 1 diabetes and nephropathy? Semin Nephrol. 2012;32(5):437–444. doi:10.1016/j.semnephrol.2012.07.006
131. Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi:10.1084/jem.192.2.295
132. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi:10.1084/jem.192.2.303
133. Ise W, Kohyama M, Nutsch KM, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11(2):129–135. doi:10.1038/ni.1835
134. Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8(2):153–160. doi:10.1038/nri2253
135. Haseda F, Imagawa A, Murase-Mishiba Y, et al. Low CTLA-4 expression in CD4+ helper T-cells in patients with fulminant type 1 diabetes. Immunol Lett. 2011;139(1–2):80–86. doi:10.1016/j.imlet.2011.05.003
136. Khalid Kheiralla KE. CTLA-4 (+49A/G) Polymorphism in Type 1 Diabetes Children of Sudanese Population. Glob Med Genet. 2021;8(1):11–18. doi:10.1055/s-0041-1723008
137. Wang CJ, Schmidt EM, Attridge K, et al. Immune regulation by CTLA-4--relevance to autoimmune diabetes in a transgenic mouse model. Diabetes Metab Res Rev. 2011;27(8):946–950. doi:10.1002/dmrr.1277
138. Teng GG, Turkiewicz AM, Moreland LW. Abatacept: a costimulatory inhibitor for treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2005;5(9):1245–1254. doi:10.1517/14712598.5.9.1245
139. Makino H, Kashihara N, Sugiyama H, et al. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes. 1996;45(4):488–495. doi:10.2337/diab.45.4.488
140. Herrera M, Soderberg M, Sabirsh A, et al. Inhibition of T-cell activation by the CTLA4-Fc Abatacept is sufficient to ameliorate proteinuric kidney disease. Am J Physiol Renal Physiol. 2017;312(4):F748–F759. doi:10.1152/ajprenal.00179.2016
141. Fujii M, Inoguchi T, Batchuluun B, et al. CTLA-4Ig immunotherapy of obesity-induced insulin resistance by manipulation of macrophage polarization in adipose tissues. Biochem Biophys Res Commun. 2013;438(1):103–109. doi:10.1016/j.bbrc.2013.07.034
142. Ursini F, Russo E, Letizia Hribal M, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine. 2015;94(21):e888. doi:10.1097/MD.0000000000000888
143. Ursini F, Russo E, Ruscitti P, Giacomelli R, De Sarro G. The effect of non-TNF-targeted biologics and small molecules on insulin resistance in inflammatory arthritis. Autoimmun Rev. 2018;17(4):399–404. doi:10.1016/j.autrev.2017.11.030
144. Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with Abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:9789):412–419. doi:10.1016/S0140-6736(11)60886-6
145. Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–3383. doi:10.1172/JCI57158
146. Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26(5):475–487. doi:10.1016/j.cytogfr.2015.07.004
147. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi:10.1016/j.bbamcr.2011.01.034
148. Wunderlich FT, Strohle P, Konner AC, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12(3):237–249. doi:10.1016/j.cmet.2010.06.011
149. Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi:10.2337/dc12-0702
150. Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685–698. doi:10.1007/s10787-018-0458-0
151. Jostock T, Mullberg J, Ozbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–167. doi:10.1046/j.1432-1327.2001.01867.x
152. Robinson R, Srinivasan M, Shanmugam A, et al. Interleukin-6 trans-signaling inhibition prevents oxidative stress in a mouse model of early diabetic retinopathy. Redox Biol. 2020;34:101574. doi:10.1016/j.redox.2020.101574
153. Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204. doi:10.1161/ATVBAHA.116.306256
154. Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int. 2017;2017:7839101. doi:10.1155/2017/7839101
155. Schulte DM, Waetzig GH, Schuett H, et al. Case report: arterial wall inflammation in atherosclerotic cardiovascular disease is reduced by olamkicept (sgp130Fc). Front Pharmacol. 2022;13:758233. doi:10.3389/fphar.2022.758233
156. Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(2):281–290. doi:10.1161/ATVBAHA.111.229435
157. Binder C, Cvetkovski F, Sellberg F, et al. CD2 Immunobiology. Front Immunol. 2020;11:1090. doi:10.3389/fimmu.2020.01090
158. Krueger GG. Selective targeting of T cell subsets: focus on alefacept - a remittive therapy for psoriasis. Expert Opin Biol Ther. 2002;2(4):431–441. doi:10.1517/14712598.2.4.431
159. Chamian F, Lin SL, Lee E, et al. Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J Transl Med. 2007;5:27. doi:10.1186/1479-5876-5-27
160. Pinckney A, Rigby MR, Keyes-Elstein L, Soppe CL, Nepom GT, Ehlers MR. Correlation among hypoglycemia, glycemic variability, and c-peptide preservation after alefacept therapy in patients with type 1 diabetes mellitus: analysis of data from the immune tolerance network T1DAL trial. Clin Ther. 2016;38(6):1327–1339. doi:10.1016/j.clinthera.2016.04.032
161. Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–290. doi:10.1016/j.cyto.2015.05.005
162. Peiro C, Lorenzo O, Carraro R, Sanchez-Ferrer CF. IL-1beta inhibition in cardiovascular complications associated to diabetes mellitus. Front Pharmacol. 2017;8:363. doi:10.3389/fphar.2017.00363
163. Fava AM, Reyaldeen R, Lo Presti S, et al. Rilonacept for the treatment of recurrent pericarditis. Expert Opin Biol Ther. 2022;22(1):7–16. doi:10.1080/14712598.2022.2005024
164. Klein AL, Imazio M, Brucato A, et al. RHAPSODY: rationale for and design of a pivotal Phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am Heart J. 2020;228:81–90. doi:10.1016/j.ahj.2020.07.004
165. Wang J, Zou T, Yang HX, et al. Insulin receptor binding motif tagged with IgG4 Fc (Yiminsu) works as an insulin sensitizer to activate Akt signaling in hepatocytes. Genet Mol Res. 2015;14(3):8819–8828. doi:10.4238/2015.August.3.5
166. Vico P, Dessy H. A case of lead poisoning in a rachitic child with pica. Critical review of the literature. Rev Med Brux. 1988;9(7–8):393–397.
167. Ying T, Feng Y, Wang Y, Chen W, Dimitrov DS. Monomeric IgG1 Fc molecules displaying unique Fc receptor interactions that are exploitable to treat inflammation-mediated diseases. MAbs. 2014;6(5):1201–1210. doi:10.4161/mabs.29835
168. Wang C, Wu Y, Wang L, et al. Engineered soluble monomeric IgG1 fc with significantly decreased non-specific binding. Front Immunol. 2017;8:1545. doi:10.3389/fimmu.2017.01545
169. Andersen JT, Dalhus B, Cameron J, et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun. 2012;3:610. doi:10.1038/ncomms1607
170. Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the Interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol. 2014;5:682.
171. Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45(15):4983–4990. doi:10.1021/bi052628y
172. Taguchi K, Chuang VT, Maruyama T, Otagiri M. Pharmaceutical aspects of the recombinant human serum albumin dimer: structural characteristics, biological properties, and medical applications. J Pharm Sci. 2012;101(9):3033–3046. doi:10.1002/jps.23181
173. Rogers B, Dong D, Li Z, Li Z. Recombinant human serum albumin fusion proteins and novel applications in drug delivery and therapy. Curr Pharm Des. 2015;21(14):1899–1907. doi:10.2174/1381612821666150302120047
174. Chuang VT, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharm Res. 2002;19(5):569–577. doi:10.1023/A:1015396825274
175. Maity N, Jaswal AS, Gautam A, Sahai V, Mishra S. High level production of stable human serum albumin in Pichia pastoris and characterization of the recombinant product. Bioprocess Biosyst Eng. 2022;45(2):409–424. doi:10.1007/s00449-021-02670-z
176. Chuang VT, Otagiri M. Recombinant human serum albumin. Drugs Today (Barc). 2007;43(8):547–561. doi:10.1358/dot.2007.43.8.1067343
177. Sheshukova KA, Wilken LR. Analysis of Recombinant Human Serum Albumin Extraction and Degradation in Transgenic Rice Extracts. Biotechnol Prog. 2018;34(3):681–691. doi:10.1002/btpr.2609
178. Chen Z, He Y, Shi B, Yang D. Human serum albumin from recombinant DNA technology: challenges and strategies. Biochim Biophys Acta. 2013;1830(12):5515–5525. doi:10.1016/j.bbagen.2013.04.037
179. Rosenstock J, Reusch J, Bush M, Yang F, Stewart M, Albiglutide Study G. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–1886. doi:10.2337/dc09-0366
180. Matthews JE, Stewart MW, De Boever EH, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(12):4810–4817. doi:10.1210/jc.2008-1518
181. Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with Type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012;29(10):1260–1267. doi:10.1111/j.1464-5491.2012.03745.x
182. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30(8):1448–1460. doi:10.1016/j.clinthera.2008.08.006
183. Fonseca VA, Alvarado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012;35(6):1225–1231. doi:10.2337/dc11-1935
184. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, Phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:9662):473–481. doi:10.1016/S0140-6736(08)61246-5
185. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi:10.1016/S0140-6736(18)32261-X
186. Ahren B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141–2148. doi:10.2337/dc14-0024
187. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475–2484. doi:10.1007/s00125-014-3360-3
188. Tan H, Su W, Zhang W, Zhang J, Sattler M, Zou P. Albumin-binding domain extends half-life of glucagon-like peptide-1. Eur J Pharmacol. 2021;890:173650. doi:10.1016/j.ejphar.2020.173650
189. Kemp SF, Creech RH, Horn TR. Glycosylated albumin and transferrin: short-term markers of blood glucose control. J Pediatr. 1984;105(3):394–398. doi:10.1016/S0022-3476(84)80011-6
190. Ma Y, Cai J, Wang Y, Liu J, Fu S. Non-Enzymatic Glycation of Transferrin and Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;14:2539–2548. doi:10.2147/DMSO.S304796
191. Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30(7):1926–1933. doi:10.2337/dc06-2625
192. Wlazlo N, van Greevenbroek MM, Ferreira I, et al. Iron metabolism is prospectively associated with insulin resistance and glucose intolerance over a 7-year follow-up period: the CODAM study. Acta Diabetol. 2015;52(2):337–348. doi:10.1007/s00592-014-0646-3
193. Kar M, Chakraborti AS. Release of iron from haemoglobin--a possible source of free radicals in diabetes mellitus. Indian J Exp Biol. 1999;37(2):190–192.
194. Cooksey RC, Jones D, Gabrielsen S, et al. Dietary iron restriction or iron chelation protects from diabetes and loss of β-cell function in the obese (ob/ob lep −/−) mouse. Am J Physiol Endocrinol Metab. 2010;298(6):E1236–E1243. doi:10.1152/ajpendo.00022.2010
195. Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T. Targeting the transferrin receptor for brain drug delivery. Prog Neurobiol. 2019;181:101665. doi:10.1016/j.pneurobio.2019.101665
196. Markowicz-Piasecka M, Markiewicz A, Darlak P, et al. Current Chemical, Biological, and Physiological Views in the Development of Successful Brain-Targeted Pharmaceutics. Neurotherapeutics. 2022;19(3):942–976. doi:10.1007/s13311-022-01228-5
197. Chen YS, Zaro JL, Zhang D, Huang N, Simon A, Shen WC. Characterization and oral delivery of proinsulin-transferrin fusion protein expressed using expresstec. Int J Mol Sci. 2018;19:2.
198. Banerjee D, Flanagan PR, Cluett J, Valberg LS. Transferrin receptors in the human gastrointestinal tract. Relationship to body iron stores. Gastroenterology. 1986;91(4):861–869. doi:10.1016/0016-5085(86)90687-6
199. Kim BJ, Zhou J, Martin B, et al. Transferrin fusion technology: a novel approach to prolonging biological half-life of insulinotropic peptides. J Pharmacol Exp Ther. 2010;334(3):682–692. doi:10.1124/jpet.110.166470
200. Brandsma ME, Diao H, Wang X, Kohalmi SE, Jevnikar AM, Ma S. Plant-derived recombinant human serum transferrin demonstrates multiple functions. Plant Biotechnol J. 2010;8(4):489–505. doi:10.1111/j.1467-7652.2010.00499.x
201. Wang Y, Chen YS, Zaro JL, Shen WC. Receptor-mediated activation of a proinsulin-transferrin fusion protein in hepatoma cells. J Control Release. 2011;155(3):386–392. doi:10.1016/j.jconrel.2011.06.029
202. Shao J, Zaro JL, Shen WC. Proinsulin-transferrin fusion protein exhibits a prolonged and selective effect on the control of hepatic glucose production in an experimental model of type 1 diabetes. Mol Pharm. 2016;13(8):2641–2646. doi:10.1021/acs.molpharmaceut.6b00168
203. Bergenstal RM, Cohen RM, Lever E, et al. The metabolic effects of biosynthetic human proinsulin in individuals with type I diabetes. J Clin Endocrinol Metab. 1984;58(6):973–979. doi:10.1210/jcem-58-6-973
204. Schatz H, Ammermann S, Laube H, Federlin K. Bioactivity and pharmacokinetics of human proinsulin in comparison to human insulin after intravenous and subcutaneous injection. Horm Metab Res. 1988;20(7):445–449. doi:10.1055/s-2007-1010856
205. Given BD, Cohen RM, Shoelson SE, Frank BH, Rubenstein AH, Tager HS. Biochemical and clinical implications of proinsulin conversion intermediates. J Clin Invest. 1985;76(4):1398–1405. doi:10.1172/JCI112116
206. Peavy DE, Abram JD, Frank BH, Duckworth WC. In vitro activity of biosynthetic human proinsulin. Receptor binding and biologic potency of proinsulin and insulin in isolated rat adipocytes. Diabetes. 1984;33(11):1062–1067. doi:10.2337/diab.33.11.1062
207. Liu Y, Wang HY, Zhou L, Su Y, Shen WC. Biodistribution, activation, and retention of proinsulin-transferrin fusion protein in the liver: mechanism of liver-targeting as an insulin prodrug. J Control Release. 2018;275:186–191. doi:10.1016/j.jconrel.2018.02.030
208. Liu Y, Wang HY, Shao J, Zaro JL, Shen WC. Enhanced insulin receptor interaction by a bifunctional insulin-transferrin fusion protein: an approach to overcome insulin resistance. Sci Rep. 2020;10(1):7724. doi:10.1038/s41598-020-64731-9
209. Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi:10.1038/nm1297-1362
210. Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi:10.1023/A:1026246500788
211. Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–1369. doi:10.1016/j.addr.2012.09.039
212. Chen X, Lee HF, Zaro JL, Shen WC. Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins. Mol Pharm. 2011;8(2):457–465. doi:10.1021/mp1003064
213. S O. Fusion protein and biosimilars market report; 2023.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.