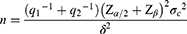Back to Journals » Journal of Pain Research » Volume 16
Remifentanil at a Relatively Elevated Dose in Active Phase is Safe and More Suitable Than Fixed Lower Dose for Intravenous Labor Analgesia
Authors Cai M, Liu J, Lei XF, Li YL, Yu J
Received 16 May 2023
Accepted for publication 17 July 2023
Published 24 July 2023 Volume 2023:16 Pages 2543—2552
DOI https://doi.org/10.2147/JPR.S419076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Meng Cai,1,* Jie Liu,2,* Xiao-Feng Lei,1 Yun-Long Li,3 Jin Yu1
1Department of Anesthesiology, Women and Children’s Hospital of Chongqing Medical University, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China; 2Department of Respiratory, The Affiliated Banan Hospital of Chongqing Medical University, People’s Hospital of Chongqing Banan District, Chongqing, People’s Republic of China; 3Department of Obstetrics and Gynecology, Women and Children’s Hospital of Chongqing Medical University, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin Yu, Department of Anesthesiology, Women and Children’s Hospital of Chongqing Medical University, Chongqing Health Center for Women and Children, No. 120, Longshan Road, Yubei District, Chongqing, 401147, People’s Republic of China, Tel +86-18623117820, Fax +86-23-63702844, Email [email protected]
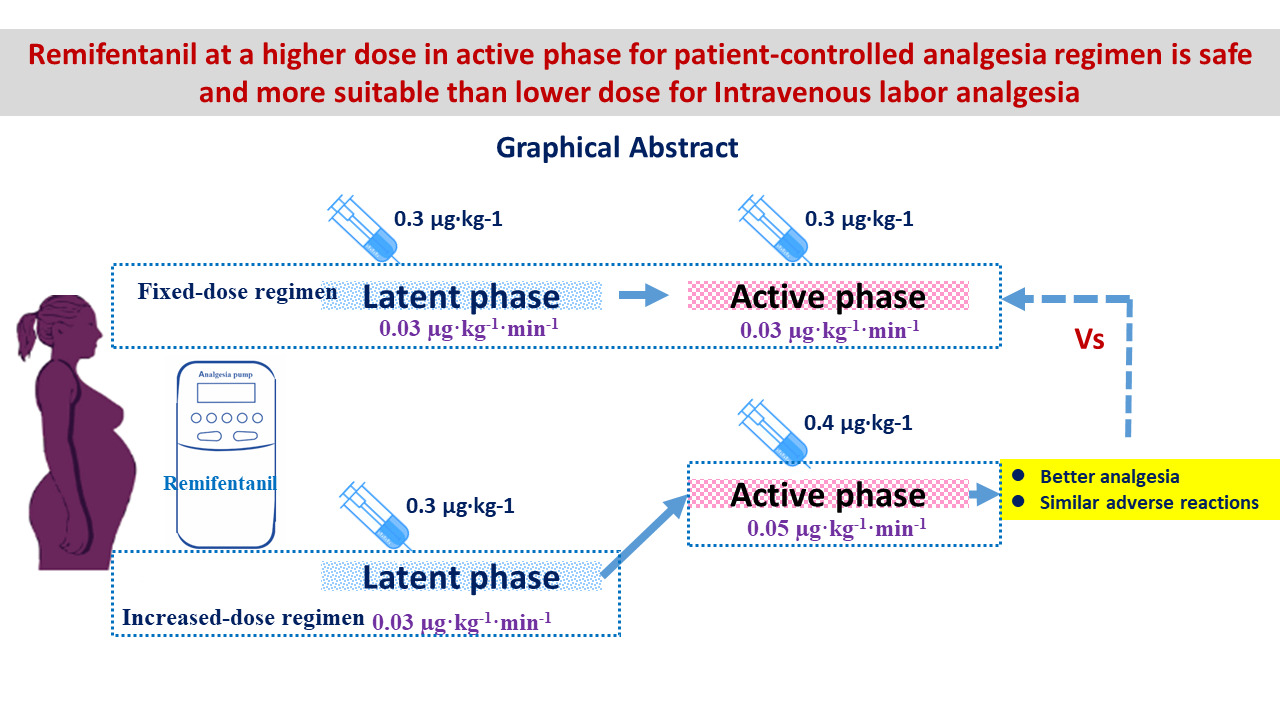
Background: Intravenous labor analgesia is recommended as an alternative for parturients who have contraindications to epidural analgesia. There are several opioid analgesics and different administering regimens used in the clinic. This study aimed to compare the effectiveness and safety of two intravenous remifentanil dosage regimens in the first labor stage.
Patients and Methods: One hundred and fifteen parturients with a contraindication to epidural analgesia but were willing to receive systemic labor analgesia were randomized into group A received a fixed dose of remifentanil throughout the first stage of labor, and group B received an elevated dose of remifentanil during the active phase of the first stage both by patient-controlled analgesia (PCA). Maternal numerical rating scale (NRS) pain score and oxygen desaturation, sedation efficacy, satisfaction, as well as maternal and fetal adverse reactions were recorded and compared.
Results: The mean NRS pain scores before analgesia and in the latent phase showed no statistically significant difference between the two groups (P > 0.05). However, during the active phase, group B demonstrated significantly lower mean NRS pain scores and lowest pain score compared to group A (P < 0.05). Furthermore, group B exhibited higher overall sedation scores and satisfaction scores in comparison to group A (P < 0.05). The incidence of adverse reactions between the two groups was similar (P > 0.05).
Conclusion: Relatively elevated intravenous dosage of remifentanil with PCA during the active phase in the first stage of labor is safe and more effective than a fixed-dosage regimen for labor analgesia.
Trial Registration: This study was registered with ChiCTR on 24/08/2021 with trial identification number: ChiCTR2100050247. First participant was recruited on 31/08/2021. The last patient was recruited on 12/08/2022.
Keywords: patient-controlled analgesia, PCA, remifentanil, labor analgesia, intravenous administration, opioids
Graphical Abstract:
Introduction
Epidural analgesia is commonly regarded as definitely effective and safe for pain management during labor. However, certain absolute or relative contraindications exist for this analgesia strategy, including spinal abnormalities or surgeries, anticoagulant usage, bleeding disorders, infection, progressive neurological disorders or even maternal preference.1 According to clinical management guidelines (American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Obstetrics), intravenous labor analgesia was suggested as an alternative to intraspinal labor analgesia for parturients with related contraindications.2 Pethidine and fentanyl are used intravenous labor analgesics previously but could potentially lead to side effects such as nausea, excessive sedation, respiratory depression, and other adverse reactions.3
Remifentanil possesses a rapid onset and offset pharmacokinetic properties and has been recently introduced for labor analgesia to overcome the side effects of meperidine and fentanyl. Moreover, remifentanil exhibits swift redistribution and elimination characteristics in the fetus through placental and fetal esterase mechanisms. Thus, remifentanil is increasingly considered a suitable alternative for labor analgesia by intravenous patient-controlled analgesia (PCA).2 Previous studies have demonstrated that PCA with remifentanil for labor is more effective than meperidine intramuscular application, obtaining declines in pain scores and fewer epidural conversions.4–8 However, the use of intravenous remifentanil PCA is limited to specific situations where epidural analgesia is contraindicated. This restriction is primarily due to the lack of high-quality evidence supporting its benefits and concerns of potential maternal respiratory depression.9–11 Fixed doses of remifentanil were employed through pump injection in the past. Based on the fact that the intensity of pain increased as labor progressing especially in active stage, the present study was designed to compare the analgesia effectiveness and safety of remifentanil at a relatively elevated dose in active phase or fixed lower dose in the first stage of labor.
Materials and Methods
This trial was approved by the Medical Ethics Committee of Chongqing Health Center for Women and Children (reference number: 2021–028 on 03/08/2021) and registered with Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn, registration number: ChiCTR2100050247). The trial was performed in accordance with International Conference on Harmonization – Guidelines for Good Clinical Practice (ICH-GCP) and Declaration of Helsinki. Informed consents were obtained from all participants.
Participants
One hundred and twenty parturients with a contraindication to performing epidural analgesia but willing to receive systemic analgesia during labor were enrolled. The inclusion criteria were (1) patients aged 18–40 years; (2) patients with single-term pregnancy at 37–42 weeks in cephalic presentation; (3) patients with ongoing uterine contractions; (4) patients whose labor was initiated with cervical dilatation of >2 cm; (5) patients with normal cardiotocographic function; and (6) patients categorized as ASA II. Exclusion criteria were (1) fetal distress; (2) meconium-stained amniotic fluid; (3) placental abnormalities; (4) intrauterine growth retardation or suspected fetal weight of <2000 g; (5) non-cephalic fetal presentation; (6) known allergy or hypersensitivity to opioid analgesics or suspicion of opioid abuse ⁄addiction; (7) other analgesics usage before or during the study. Parturients were free to withdraw from the study at any time.
Enrolled parturients were randomized into group A (fixed-dose of intravenous remifentanil by PCA through the first stage of labor) and group B (increased dose of intravenous remifentanil by PCA in the active phase) via a simple randomization strategy. The group allocation was blinded via sealed envelopes until PCA initiation. The participated parturients, obstetricians, and data collecting nurses were all blinded to the grouping arrangement. Only the anesthesiologist who was responsible for the PCA pump speed adjusting knew the specific grouping information. Valid participation in the study procedures was verified, and valid data were available for 115 participants who were included in the study sample (see Figure 1). The CONSORT checklist is available as a Supplementary File.
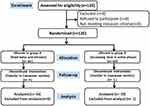 |
Figure 1 Flow chart of the study. |
Study Outcomes
The primary outcome was maternal pain control efficacy (NRS score). The secondary outcomes included maternal sedation, satisfaction, and maternal and fetal adverse reactions.
Study Protocol
Venous access was established in the delivery room from left upper limb. The parturient routinely received oxygen inhalation through a nasal catheter at a rate of 3 L/min. The hemodynamic parameters, including heart rate (HR), noninvasive blood pressure (BP), and respiratory rate (RR) every 30 min and continuous blood oxygen saturation (SpO2) by pulse oximetry were monitored. Uterine activity and fetal heart rate (FHR) were recorded continuously using an external tocodynamometer monitor (Avalon FM30, Germany, Philips). All parturients received biunique nursing. Airway equipment with pressurized oxygen supply air bag, laryngoscope, intubation tube and oxygen mask were available in case of respiratory depression. Hypoxemia (pulse oxygen saturation of <94%) or excessive maternal sedation (sedation score of ≥4) was the threshold for mandatory discontinuous infusion of remifentanil.7 Abnormal indicators were recorded and included in the statistical analysis.
Three milligram remifentanil was diluted in 300-mL normal saline (10 µg·mL−1 solution) and administered via the proximal port of the intravenous extension set using a patient-controlled analgesia pump (ZZB-III, Jiangsu Apon Medical Technology, China). Group A received a fixed-dose regimen of remifentanil with a PCA bolus of 0.3 µg·kg−1 and a baseline infusion of 0.03 µg·kg−1·min−1 through the first labor stage. Group B received the same regimen as group A in the latent phase of the first stage, but received an increased remifentanil dosage with a PCA bolus of 0.4 µg·kg−1 and an infusion of 0.05 µg·kg−1·min−1 in the active phase. The lockout interval of PCA was set at 2 minutes, the 4-hour limits were 3 mg, and the PCA bolus speed was set at 6 mL/min in both groups. At the start of uterine contractions, parturients were advised to press the PCA button. The analgesia pump was removed by the end of the first labor stage and no analgesic was given when the second labor stage started. The latent phase is commonly defined as cervical dilation of 0–6 cm, and the active phase commences from 6 cm to full dilation.12 Cervix was checked every 4 hours for the latent stage and every 2 hours for the active stage by a midwife. If the parturients had a sense of bowel movement, the cervix dilation was immediately checked.
Measurements
Pain intensity was measured by a NRS ranging from 0 to 10 (0 = no pain, 10 = worst pain). The level of sedation was assessed by a 5-point sedation scale13 (1 = fully awake, 2 = mild sedation, 3 = lethargic, 4 = sleeping but can be awakened, and 5 = deep sleep, cannot be awakened). NRS, sedation scores, BP, HR, RR, SpO2, FHR and uterine activity values were recorded before the start of intravenous remifentanil and then every 30 min until the end of the first labor stage. Two hours after delivery, the parturients were asked to feedback their overall satisfaction on a 10-point scale (0 = no satisfaction and 10 = complete satisfaction), and a score of >7 was considered good management.
The duration of the first labor stage, resuscitation, non-reassuring FHR (NRFHR), umbilical artery pH, PaCO2 and PaO2 immediately after delivery, naloxone requirement and Apgar scores at 1, 5, and 10 min for newborns were also recorded. Asphyxia was defined as an Apgar score <8.14 NRFHR was defined as tachycardia, baseline FHR variability, bradycardia not accompanied by absent baseline variability, absence of induced accelerations after fetal stimulation and periodic or episodic deceleration.15 After administering intravenous remifentanil for analgesia, Naloxone will be employed as an antagonist if respiratory depression occurs in newborns and proves difficult to rectify. Maternal hypotension was defined as a 20% decrease in the noninvasive mean BP compared to the baseline value. Hypoxemia was defined as SpO2 of <94% at oxygen inhalation or RR of <10 breaths/minute. Bradycardia was defined as HR of <60 beats/minute and urinary retention was defined as an inability to urinate with a full bladder. Additionally, other associated adverse effects of remifentanil such as itching, nausea and vomiting were also documented.
Statistical Analysis
Statistical analysis was conducted with SPSS for Windows version 22.0 (SPSS Inc, Chicago, IL, USA). The primary outcome was a decrease in NRS score. The sample size was prospectively determined by giving 80% power with a type I error of 0.05 to detect the predicted difference in NRS score in the active stage between the two groups. According to the formula
Where σc = 0.5 and δ= 0.92 based on literature data,16 the lowest sample size obtained by the sample size calculation formula was 55 cases in each group.
Normally distributed data were analyzed by the Kolmogorov–Smirnov test. Data were expressed as mean ±standard deviation (SD). Measurement data of the two groups were compared by the Student’s t-test. Due to the different lengths of labor between individual cases in the group, repeated-measures ANOVA could not be implemented for pain score and sedation score. The Student’s t-test was used to evaluate the difference between the two groups in pain score and sedation score at each time point. The maternal adverse events and FHR tracings were analyzed by the chi-squared test and calibration chi-square test. The Mann–Whitney U-test was used to compare Apgar scores and umbilical artery pH. A P value of <0.05 was considered statistically significant.
Results
Four parturients in group A and one parturient in group B were removed because of the prolonged first labor stage and switched to cesarean section. For the finally analyzed 115 parturients, there was no statistical difference between the two groups regarding demographic characteristics such as weight, height, body mass index (BMI), gestational weeks, multiparous parity, multiple pregnancies and labor induction history (P > 0.05) (see Table 1).
 |
Table 1 Demographic Characteristics of Parturients |
The trend of NRS pain score and sedation scores of the two groups in the latent phase and active phase are shown in Figure 2. In the latent phase and before analgesia, there was no significant difference between the two groups in the NRS pain score and sedation scores at each time point (P > 0.05). Compared to group A, the NRS pain score and the lowest pain score of group B in the active phase were significantly lower (P < 0.05). The sedation score in the active phase and overall satisfaction score of group B was higher compared with group A (P < 0.05). There was no significant difference between the two groups in the duration of the first labor stage, the number of boluses and total remifentanil consumption (P > 0.05) (see Table 2).
 |
Table 2 Comparison of Pain Score, Sedation Score and Satisfaction Score |
The incidence of other adverse reactions (hypoxemia, nausea, vomiting, drowsiness, dizziness, confusion, hypotension, bradycardia and itching) between the two groups was comparable (P > 0.05) (see Table 3). Bradycardia occurred in three parturients (5.4%) in group A and seven parturients (11.9%) in group B. But all cases last less than 30 seconds having HR at least 50 bpm. Bradycardia was managed by lowering the medication dose and giving external stimulation.
 |
Table 3 Maternal Adverse Reactions of Two Groups |
There was no statistical difference in fetal and neonatal adverse reactions between the two groups (P > 0.05). No neonate developed respiratory depression or required naloxone in both groups. The umbilical artery blood gas analysis was within a normal range in every neonate. The Apgar scores at 1, 5, and 10 min of two groups were all full marks (see Table 4).
 |
Table 4 Comparison of the Fetal and Neonatal Adverse Reactions |
Discussion
This clinical trial revealed that better analgesia effects, more effective sedation and higher satisfaction scores were obtained by an elevated remifentanil dose in active phase of the first labor stage comparing with a fixed lower dose both by PCA. The positive outcomes were achieved without adverse effects on the fetus or neonates.
According to the guidelines of the American Society of Anesthesiologists (ASA) and the College of Obstetricians and Gynecologists (ACOG), epidural labor analgesia is the most dependable and widely used labor analgesia. It is well established that intraspinal anesthesia is superior effective for labor analgesia compared to intravenous opioid drugs.11,17 So, intravenous labor analgesia is only an alternative when there is contraindication for intraspinal puncture. Furthermore, remifentanil by PCA was associated with a lower risk for the requirement of additional analgesics when compared to other opioids (IV/IM).11,18 But when remifentanil is used for second-stage labor analgesia, a case series observed three out of eight newborns needed initial respiratory support.19 Another study also found that desaturation episodes of parturients per hour were twice as common during the second stage of labor as compared with that in the first stage.20 With the concerns of safety of parturients and neonates, in this study, no analgesic was given in the second labor stage.
Labor pain intensifies in both frequency and intensity as labor progressing. The ideal labor analgesia should provide satisfied analgesic effects with minimal side effects to parturients and neonates. A fixed-dose regimen through the first stage might underestimate or overestimate patient requirements.21 Based on the fast onset of remifentanil within 30–60 seconds,22 a continuous infusion was needed and administered as a baseline dose to provide constant analgesia, combing rescue bolus by PCA to match the pain pattern.
Intravenous remifentanil 0.2–1 µg·kg−1·min−1 with a variable lockout interval by PCA bolus has been studied and reported. But a high rate of epidural analgesia switch, incomplete analgesia and side effects such as maternal desaturation, pruritus and excessive sedation have been observed.23–26 Compared to remifentanil 0.05–0.2 µg·kg−1·min−1 continuous infusion in the latent phase of labor, increasing stepwise boluses by PCA from 0.1 to 0.4 µg·kg−1 provided better pain relief and similar placental transfer for parturients.27 However, there was no comparison between different bolus dosages combining infusion dosage in previous studies. In this study, the efficacy and safety of two dosage regimens of remifentanil by PCA for labor analgesia in the first stage was designed and conducted.
During the latent phase of labor, the average pain scores in both of the studied groups decreased to approximately 3. Since pain severity increases in line with uterine contractions intensity in the active phase, the dose of an infusion of 0.05 µg·kg−1·min−1 and a PCA bolus of 0.4 µg·kg−1 was proved to provide more analgesia than an infusion of 0.03 µg·kg−1·min−1 and a PCA bolus of 0.3 µg·kg−1. The number of boluses and total remifentanil consumption in both groups were similar. Thus, the better analgesic effect is not due to the higher frequency of bolus giving in group B. Compared with a previous study,6 the mean pain scores during the latent phase of the first labor stage were lower, and the remifentanil administering regimen was simpler and more feasible.
By this regimen, the NRS value was kept at around 5.7 in the active phase in group B, which is more severe than that in the latent phase. However, the mean pain score of 5.7 did not affect the parturient satisfaction, suggesting that safety and a significant reduction of pain were achieved. Considering that the safe dosage of remifentanil has been reported to be 0.25–0.5 µg·kg−1 for bolus administration and 0.025–0.05 µg·kg−1·min−1 for infusion for labor analgesia,22 an infusion of 0.05 µg·kg−1·min−1 and a PCA bolus of 0.4 µg·kg−1 administering in active phase was an applicable regimen.
The primary concern related to remifentanil PCA is the potential adverse reactions experienced by the mother.25,28,29 The dosage of remifentanil in group B approached the upper limits of the safe dose. The parturients in this study had no respiratory depression or hypoxemia, which might attribute to the bolus dose being given at a constant speed of 6 mL·min−1 rather than pulse infusion and prophylactic oxygen administration.
Because of the sudden increase in blood concentration in response to remifentanil bolus dose, maternal bradycardia incidence was higher in group B. There was no statistical difference compared with group A, and all bradycardias lasted less than 30 seconds and had at least 50 bpm. Clinicians need to be vigilant when administering high dose of remifentanil in practice although the rapid onset and offset pharmacokinetic properties of remifentanil resulting in a self-recoverable effect. Dizziness, drowsiness and restricted parturients’ movements occurred in a few cases during labor analgesia but were similar between the two administering regimens. Therefore, close observation and biunique nursing including continuous monitoring of SpO2 and oxygen supplementation are necessary and recommended during analgesia with intravenous remifentanil. The maternal and fetal adverse reactions were comparable between the two regimens which was consistent with previous findings.30,31 Although umbilical artery lactate values in both groups were increased, it is reasonable and understandable that the lactate level was higher in neonates born by vaginal delivery compared to those born by cesarean section because of continuous uterine contractions.
The limitations of this study were: (1) our study did not specifically focus on analgesia in the second stage of labor. (2) No comparison was made with blank controls or parturients who received conventional epidural analgesia.
Conclusions
Increasing intravenous remifentanil bolus PCA and constant infusion dosage to a relatively elevated level in the active phase of the first labor stage is superior to fixed-lower dose administration in terms of analgesia effectiveness and is safe for parturients and fetuses.
Abbreviations
PCA, patient-controlled analgesia; NRS, numerical rating scale; ChiCTR, Chinese Clinical Trial Registry; HR, heart rate; BP, blood pressure; RR, respiratory rate; SpO2, blood oxygen saturation; FHR, fetal heart rate; NRFHR, non-reassuring FHR; SD, standard deviation; BMI, body mass index.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author (Jin Yu; [email protected]). The data are not publicly available because this was a clinical trial containing information that could compromise the privacy of research participants.
Ethics Approval and Consent to Participate
This trial was approved by the Medical Ethics Committee of Chongqing Health Center for Women and Children (reference number: 2021-028 on 03/08/2021) and registered with Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn, registration number: ChiCTR2100050247). The trial was performed in accordance with International Conference on Harmonization – Guidelines for Good Clinical Practice (ICH-GCP) and Declaration of Helsinki. Informed consents were obtained from all participants.
Acknowledgments
We are grateful to Prof. Yongchun Su at Chongqing Youyoubaobei Women and Children’s Hospital for his manuscript preparation assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Chongqing Maternal and Child Health Scientific Research and Cultivation Project (No. 2021FY106), Natural Science Foundation of Chongqing of China (No. cstc2021jcyj-msxmX0763) and National Key Clinical Speciality Construction Project (Obstetrics and Gynecology).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Zhang P, Yu Z, Zhai M, et al. Effect and safety of remifentanil patient-controlled analgesia compared with epidural analgesia in labor: an updated meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2021;86(3):231–238. doi:10.1159/000515531
2. Zhang P, Yu Z, Zhai M, et al. ACOG practice bulletin No. 209: obstetric analgesia and anesthesia. Obstet Gynecol. 2019;2019:e208–e225.
3. Ching Wong SS, Cheung CW. Analgesic efficacy and adverse effects of meperidine in managing postoperative or labor pain: a narrative review of randomized controlled trials. Pain Phys. 2020;23(2):175–201. doi:10.36076/ppj.2020/23/175
4. Van de Velde M, Carvalho B. Remifentanil for labor analgesia: an evidence-based narrative review. Int J Obstet Anesth. 2016;25:66–74. doi:10.1016/j.ijoa.2015.12.004
5. Lu G, Yao W, Chen X, et al. Remifentanil patient-controlled versus epidural analgesia on intrapartum maternal fever: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):151. doi:10.1186/s12884-020-2800-y
6. Balki M, Kasodekar S, Dhumne S, et al. Remifentanil patient-controlled analgesia for labour: optimizing drug delivery regimens. Can J Anaesth. 2007;54(8):626–633. doi:10.1007/BF03022956
7. Wilson MJA, MacArthur C, Hewitt CA, et al. Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labour (RESPITE): an open-label, multicentre, randomised controlled trial. Lancet. 2018;392(10148):662–672. doi:10.1016/S0140-6736(18)31613-1
8. Leong WL, Sng BL, Sia AT. A comparison between remifentanil and meperidine for labor analgesia: a systematic review. Anesth Analg. 2011;113(4):818–825. doi:10.1213/ANE.0b013e3182289fe9
9. Schnabel A, Hahn N, Broscheit J, et al. Remifentanil for labour analgesia: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2012;29(4):177–185. doi:10.1097/EJA.0b013e32834fc260
10. Kranke P, Girard T, Lavand’homme P, et al. Must we press on until a young mother dies? Remifentanil patient controlled analgesia in labour may not be suited as a “poor man’s epidural”. BMC Pregnancy Childbirth. 2013;13(139). doi:10.1186/1471-2393-13-139
11. Weibel S, Jelting Y, Afshari A, et al. Patient-controlled analgesia with remifentanil versus alternative parenteral methods for pain management in labour. Cochrane Database Syst Rev. 2017;4:CD011989. doi:10.1002/14651858.CD011989.pub2
12. Hutchison J, Mahdy H, Hutchison J. Stages of Labor. Treasure Island (FL): StatPearls; 2022.
13. Lim TW, Choi YH, Kim JY, et al. Efficacy of the bispectral index and Observer’s assessment of alertness/sedation scale in monitoring sedation during spinal anesthesia: a randomized clinical trial. J Int Med Res. 2020;48(4):300060519893165. doi:10.1177/0300060519893165
14. Cnattingius S, Norman M, Granath F, et al. Apgar score components at 5 minutes: risks and prediction of neonatal mortality. Paediatr Perinat Epidemiol. 2017;31(4):328–337. doi:10.1111/ppe.12360
15. Macones GA, Hankins GD, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. J Obstet Gynecol Neonatal Nurs. 2008;37(5):510–515. doi:10.1111/j.1552-6909.2008.00284.x
16. Bin D, Chun F, Shan J, et al. Comparison of different administration methods of remifentanil in the intravenous labor analgesia. Shanghai Med J. 2016;39(8):470–475.
17. Jia Z, Li Y, Jia H, et al. Curative effect of remifentanil on labor analgesia in newborns. J Matern Fetal Neonatal Med. 2020;33(11):1913–1918. doi:10.1080/14767058.2018.1533946
18. Lavand’homme P, Roelants F. Patient-controlled intravenous analgesia as an alternative to epidural analgesia during labor: questioning the use of the short-acting opioid remifentanil. Survey in the French part of Belgium (Wallonia and Brussels). Acta Anaesthesiol Belg. 2009;60(2):75–82.
19. Schwarz GL, Volmanen P, Albrechtsen S, et al. Remifentanil target-controlled infusion during second stage labour in high-risk parturients: a case series. Acta Anaesthesiol Scand. 2013;57(6):802–808. doi:10.1111/aas.12096
20. Messmer AA, Potts JM, Orlikowski CE. A prospective observational study of maternal oxygenation during remifentanil patient-controlled analgesia use in labour. Anaesthesia. 2016;71(2):171–176. doi:10.1111/anae.13329
21. Devabhakthuni S. Efficacy and safety of remifentanil as an alternative labor analgesic. Clin Med Insights Womens Health. 2013;6:37–49. doi:10.4137/CMWH.S8015
22. Ohashi Y, Baghirzada L, Sumikura H, et al. Remifentanil for labor analgesia: a comprehensive review. J Anesth. 2016;30(6):1020–1030. doi:10.1007/s00540-016-2233-y
23. Blair JM, Hill DA, Fee JP. Patient-controlled analgesia for labour using remifentanil: a feasibility study. Br J Anaesth. 2001;87(3):415–420. doi:10.1093/bja/87.3.415
24. Moran VH, Thomson G, Cook J, et al. Qualitative exploration of women’s experiences of intramuscular pethidine or remifentanil patient-controlled analgesia for labour pain. BMJ Open. 2019;9(12):e032203. doi:10.1136/bmjopen-2019-032203
25. Volmanen P, Akural EI, Raudaskoski T, et al. Remifentanil in obstetric analgesia: a dose-finding study. Anesth Analg. 2002;94(4):913–917. doi:10.1097/00000539-200204000-00026
26. Tveit TO, Halvorsen A, Seiler S, et al. Efficacy and side effects of intravenous remifentanil patient-controlled analgesia used in a stepwise approach for labour: an observational study. Int J Obstet Anesth. 2013;22(1):19–25. doi:10.1016/j.ijoa.2012.09.003
27. Shen MK, Wu ZF, Zhu AB, et al. Remifentanil for labour analgesia: a double-blinded, randomised controlled trial of maternal and neonatal effects of patient-controlled analgesia versus continuous infusion. Anaesthesia. 2013;68(3):236–244. doi:10.1111/anae.12098
28. Dhileepan S, Stacey RG. A preliminary investigation of remifentanil as a labor analgesic. Anesth Analg. 2001;92(5):1358–1359. doi:10.1097/00000539-200105000-00062
29. Owen MD, Poss MJ, Dean LS, et al. Prolonged intravenous remifentanil infusion for labor analgesia. Anesth Analg. 2002;94(4):918–919. doi:10.1097/00000539-200204000-00027
30. Anderson B. The use of remifentanil as the primary agent for analgesia in parturients. Crit Care Nurs Clin North Am. 2017;29(4):495–517. doi:10.1016/j.cnc.2017.08.009
31. Yin W, Jung F, Adams D, et al. Case report of remifentanil labor analgesia for a pregnant patient with congenital methemoglobinemia type 1. AA Pract. 2021;15(1):e01373. doi:10.1213/XAA.0000000000001373
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.