Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Reflections on Drivers for the Emergence and Spread of Antimicrobial Resistant Bacteria Detected from Chickens reared on Commercial Layer Farms in Mukono District, Uganda
Authors Kakooza S , Tayebwa DS, Njalira KR, Kayaga EB, Asiimwe I , Komugisha M, Wanyana M, Kisekka R, Kyabarongo A , Kiryabwire DH, Nabatta E, Eneku W
Received 24 April 2023
Accepted for publication 27 November 2023
Published 6 December 2023 Volume 2023:14 Pages 209—219
DOI https://doi.org/10.2147/VMRR.S418624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Steven Kakooza,1 Dickson Stuart Tayebwa,1 Kassim Rashid Njalira,1 Edrine Beatrice Kayaga,1 Ismail Asiimwe,2 Mariam Komugisha,3 Mariam Wanyana,1 Raymond Kisekka,1 Alex Kyabarongo,1 David H Kiryabwire,4 Esther Nabatta,3 Wilfred Eneku1
1Central Diagnostic Laboratory, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda; 2Bukomansimbi District Veterinary Office, Bukomansimbi, Uganda; 3Ministry of Agriculture, Animal Industry and Fisheries, Entebbe, Uganda; 4Mukono District Veterinary Office, Mukono, Uganda
Correspondence: Dickson Stuart Tayebwa, Central Diagnostic Laboratory, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, P.O Box 7062, Kampala, Uganda, Tel +256 776005428, Email [email protected]
Purpose: We investigated the fecal carriage of antimicrobial resistant Escherichia coli and potential practices influencing antimicrobial resistance (AMR) dynamics among poultry farm settings in Mukono District, Uganda.
Methods: Twenty-nine commercial layer farms were visited and samples collected from 10 birds. The samples were then subjected to culture and sensitivity testing. The investigative framework for antimicrobial stewardship practices (IFAP) was used as a participatory tool to generate data through interviews and observations on antimicrobial use, drivers for use, players, and actions following non-responsive treatment outcomes.
Results: The cultures done on 290 cloacal swabs yielded a total of 273 Escherichia coli isolates (94.1% recovery rate) which were tested in vitro for their sensitivity to different antibiotics. The prevalence of multi-drug resistant E. coli was 59.3% (162/273). A high prevalence of resistance to tetracycline (91.6%, n = 250) and trimethoprim sulphamethoxazole (70.3%, n = 192) was noted. In this collection of isolates, the prevalence of molecular determinants associated with the predominant phenotypes was; tetA (79.3%; 138/174), tetB (17.2%; 30/174), tetC (7.5%; 13/174), sul1 (11.5%; 20/174), and sul2 (60.3%; 105/174). Responses derived using the IFAP revealed several vices related to misuse and overuse of antibiotics, a threat to the poultry industry. The farmers also reported habits of selling off sick birds for slaughter when treatment outcomes were non-responsive. Such a practice could drive dissemination of antimicrobial resistant organisms and antibiotic residues to the consumers of those poultry products.
Conclusion: The IFAP tool was useful and can be modified, and adopted for use in engaging agricultural communities in participatory AMR surveillance. A high carriage of multi-drug resistant E. coli was detected in the birds. On these farms, the worrying antimicrobial stewardship practices discovered could be sponsoring the emergence and spread of antimicrobial resistant bacteria in the Ugandan context.
Keywords: antimicrobial resistance, Escherichia coli of chicken, antimicrobial stewardship, Uganda’s poultry sector
Introduction
The emergence of antimicrobial resistant bacteria (ARB) is a threat to the current exponentially growing poultry sector in Uganda.1,2 Although not well documented, reports exposing antimicrobial resistance (AMR) effects on production and farmers’ livelihoods exist, particularly the increasing prevalence of ARB-mediated treatment failures and deaths.1 This problem could be attributed to the irrational use of antibiotics for treatment, prophylaxis, and growth promotion by Ugandan farmers. Antimicrobials are also promptly gained by numerous individuals in need over the counter since guidelines to restrict admittance to veterinary medications are liberal. As farmers strive to have profitable agriculture, they will do anything to hamper production barriers and in the long run also try out risky interventions that have been whistle blown or suspected to have spectacular gains on production. With such levels of anxiety in the community, it is not shocking that now stressing reports document the utilization of profoundly critical human medications like antiretrovirals in poultry and other commercial agricultural systems.3,4
Poultry is a possible source of multi-drug resistant (MDR) Escherichia coli with the potential to spread ARB in the environment (through feces) and human populations (via the food chain).5 Existing data on antibiograms of E. coli isolated from both apparently healthy and sick birds portray high resistance rates to majorly tetracyclines and sulphonamides.1,6,7 It is remarkable that findings from a recent Ugandan study presented a case of an MDR E. coli isolate resistant to over thirteen drugs of both medical and veterinary relevance.1 There exists a zoonotic possibility of such dangerous bugs also causing a couple of infections in humans, especially those in close contact with sentinel animal hosts of ARB such as poultry. This explains the need for continuous AMR surveillance in both humans and animals as stressed by the global and Uganda AMR action plans.
Studies so far done give minimal insights into the forces at work influencing agriculture-mediated AMR in Uganda’s communities. The current national trends and regional patterns of antimicrobial consumption in agriculture have also been under studied. In the long run, there is lack of evidence-based data to inform planning and policy decisions, thus incapacitating government exertions aimed at controlling the development and spillover of MDR bacteria in livestock into the environment and human population. We investigated the fecal carriage of antimicrobial reistant bacteria and the role of agricultural practices in mediating AMR in the Ugandan context, with special emphasis on poultry production. This report supports the implementation of the Uganda National AMR action plan (2018–2023).
Materials and Methods
Study Design, Sampling, and Data Collection
This was a cross-sectional study conducted on 29 commercial layer chicken farms in Mukono district, central Uganda. Sample size calculations were based on recommendations from a previous study by Wang and Cheng.8 Calculations gave a total of 222 samples taking into account a reported prevalence of 17.5% cloacal carriage of a known multidrug resistant E. coli phenotype,9 at a confidence level of 95% and precision of 0.05. Only farms with flock sizes of 500 and above chickens were included in the study as such intensive settings have been associated with heavy use of antibiotics.10 The farms were selected by simple random sampling from a list of farms provided by the district veterinary authorities. From each farm with apparently healthy flocks, 10 birds were randomly selected and then sampled, thus yielding a total of 290 cloacal swab specimens. The swabs (in transport media) (DELTALAB, Spain) were transported on ice in a cool box and referred to the Central Diagnostic Laboratory (CDL), College of Veterinary medicine, Animal resources and Biosecurity (COVAB), Makerere University for bacterial culture and antimicrobial sensitivity testing. The samples were cultured within 24 hours after collection. A line list was used to capture bird demographics (age, sub-county) tagged to each sample. The investigative framework for antimicrobial stewardship practices (IFAP) (Figure 1) was used as a participatory tool to generate data on antimicrobial use, drivers for use, players, and actions following non-responsive treatment outcomes. The participant submissions from conversations were recorded.
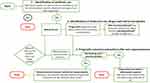 |
Figure 1 The graphical representation of the investigative framework for antimicrobial stewardship (IFAP) tool. |
Ethical Issues
Before commencement, farm owners or managers were given consent forms prior to inclusion in the study. The forms explained the study, stipulated the roles of contributing farms and benefits from participation in the research. The study received approval from the Makerere University School of Veterinary medicine and Animal Resources (SVAR), Institutional Review Board (Ref Number: SVAR_IACUC/41/2020).
Isolation and Identification of Escherichia coli
Briefly, swab samples were pre-enriched in 10ml of buffered peptone water (Condalab, Spain) and incubated aerobically at 37°C for 18 hours. The overnight cultures were then streaked on to Mac Conkey agar (Condalab, Spain) and incubated at 37°C for 18 hours. Bacterial colonies with an intense pink color and surrounded by a pink zone (indicating precipitation of bile salts) were suspected to be E. coli. The suspected colonies were further identified biochemically using indole, methyl red, urease, Voges Proskauer, lactose fermentation, and citrate utilization tests.11
Antimicrobial Susceptibility Testing
In vitro phenotypic analyses were conducted using the Kirby Bauer disk diffusion test12 according to the Clinical Laboratory Standards Institute (CLSI) guidelines.13 A bacterial suspension adjusted to match the turbidity standard of 0.5 Mc Farland was prepared by resuspending some fresh colonies in 2 mL of 0.85% physiological saline. A sterile swab was then soaked in the mixture and used to spread the suspension evenly on to the Mueller Hinton agar (BBL, Becton Dickinson and Company, France). Seven antibiotic discs (of clinical relevance in both humans and animals) at a given disk content were chosen and placed on to the surface of the agar at approximately 2mm apart. The antibiotics (Oxoid, United States of America) were: tetracycline (30µg), ciprofloxacin (5µg), neomycin (30µg), gentamicin (10µg), trimethoprim-sulfamethoxazole (25µg), ceftriaxone (30µg), and ampicillin (10µg). The plates were then incubated at 37°C for 24 hours. After incubation, the growth inhibition zones were measured in mm using a ruler. The results were then interpreted and recorded as resistant, susceptible, and intermediate basing on the CLSI cut-offs. Quality controls for susceptibility testing were performed according to the CLSI using E. coli 25922 ATCC reference strain.
Bacterial Multidrug Resistance (MDR) and Multiple Antibiotic Resistance (MAR) Index
An isolate was considered to be multi-drug resistant if the strain was resistant to at least three different families of antimicrobials.14 The MAR index for each isolate was calculated using the formula; a/b where a is the number of antibiotics to which the test isolate was resistant to and b is the total number of antibiotics to which the test isolates were subjected.15
Genotypic Detection of AMR Genes
To determine antimicrobial resistance genes carried by the E. coli strains isolated from the chicken, a conventional colony PCR was done as described before16,17 but with modifications. Both naïve and E. coli resistant to either tetracycline or sulfonamides were tested. The steps involved recovery of bacteria in nutrient broth, bacterial DNA extraction, conduction of PCR, agarose gel electrophoresis, and the illumination of PCR products. Primers sets targeting the tet and sul genes were used, and the PCR conditions were adopted from previous studies.18,19 Known positive DNA with the targeted genes was used as a positive control when running PCR and visualization of the products in 2% agarose gels.
Statistical Analyses
Data was entered and cleaned in Microsoft Excel (version 2019) before importation into STATA version 17 (StataCorp, College Station, TX) and R statistical software for analysis. Descriptive statistics were used to report the data where statistics were presented as frequencies and percentages. The prevalence of specific antibiotic or multi-drug resistant E. coli was calculated as the proportion of positive cases out of the number of isolates tested from the birds. The corresponding confidence intervals of prevalence were computed as exact binomial 95% confidence intervals. The Pearson chi-square (X2) test was also used to evaluate significant differences (p < 0.05) between the prevalence of resistance phenotypes in MDR and non-MDR strains. Bivariate logistic regression modelling was used to demonstrate associations between individual phenotypes and the risk of an isolate being MDR. Analysis of variance (ANOVA) test at 5% level of significance was done to compare the means of the MAR Indexes of the variables.
Results
Descriptive Statistics of the Poultry Farms
The 29 visited farms were all rearing birds under an intensive production system with 22 farms having flock sizes of 500 to less than 1000 chickens, while 7 farms had 1000 chickens and above. The majority of these farms were managed by women (21/29, 72.4%) and to a lesser extent by men (8/29, 27.6%). The residences of 93.1% (n = 27) of the managers were on the farm, while 6.9% (n = 2) resided off the farm. Of the 29 farm managers interviewed, 16 (55.2%) had attained secondary education, 9 (31.0%) had attended tertiary, and 4 (13.8%) stopped at primary level of education. The distribution of the farms by the sub-counties of Mukono district was: Goma (7), Kyampisi (5), Mukono town council (6), Nabaale (1), Nagojje (5), Nakisunga (3) and Nama (2). Ten farms had been operating for 3 years and below, 10 for 4 to 6 years and 9 farms for 7 years and above. 86.2% (n = 25) of the farms sourced their birds from one supplier, 6.9% (n = 2) from two suppliers, 3.4% (n = 1) from three suppliers and one was not sure of the source of their birds. All farms housed their birds under a deep litter system. Fourteen (14) (48.3%) of the farms had well-kept treatment records and 15 (51.7%) did not. Some of the classes of antibiotics in use on the farms were tetracyclines, macrolides, quinolones, aminoglycosides, and potentiated sulphonamides. Antibiotic co-formulations being administered included; doxycycline-sulphacetamide (Drug brand A), sulfadiazine-trimethoprim (Drug brand B), colistin-tetracycline-streptomycin-erythromycin, and gentamicin-tylosin (Drug brand C). Noteworthy, on these farms, we found use of drug brands having both multi-vitamins and antibiotics in them. Due to diseases and production losses, we established that 14 farms had used one antibiotic class in the past 3 months, 5 farms had used two classes, 1 had used three, 6 had used four, 1 had used five and 2 were not sure if they had used antibiotics.
Antimicrobial Resistance of E. coli
The cultures done on 290 cloacal swabs yielded a total of 273 isolates (94.1% recovery rate) which were tested in vitro for their sensitivity to different antibiotics. The prevalence of resistance from highest to lowest was tetracycline (TE) (91.6%, n = 250), trimethoprim sulphamethoxazole (SXT) (70.3%, n = 192), ampicillin (AP) (52.4%, n = 143), ciprofloxacin (CIP) (27.5%, n = 75), neomycin (N) (19.4%, n = 53), gentamicin (GM) (6.2%, n = 17), and ceftriaxone (CTR) (2.2%, n = 6).
The prevalence of MDR E. coli was 59.3% (162/273). Of the total 162 MDR strains, 112 (69.1%) were resistant to 3 antibiotic families (MDR3), 37 (22.8%) were resistant to 4 antibiotic families (MDR4), 11 (6.8%) were resistant to 5 antibiotic families (MDR5) and 2 (1.2%) were resistant to 6 antibiotic families (MDR6). There was no pan resistant isolate in the tested collection. Upon profiling of MDR, the two most frequent profiles were TE-SXT-AP (MDR3), followed by TE-SXT-AP-CIP (MDR4). In Figure 2, the clustered bar chart shows the numbers of the identified phenotypic patterns of MDR E. coli isolates (N = 162) based on phenotypic resistance to 7 antibiotics. We identified 23 groups belonging to the four MDR classes (MDR3,4,5,6), with the most prevalent profile being TE-SXT-AP and the 2 broadest spectra (MDR6) exhibited by two isolates.
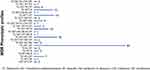 |
Figure 2 The phenotypic patterns of MDR E. coli isolates (n = 162) based on phenotypic resistance to 7 antibiotics. |
Comparison of AMR in MDR and Non-MDR E. coli Populations
The analyses depicted significantly higher prevalence of six resistance phenotypes in the population of MDR E. coli (p < 0.001) except for ceftriaxone resistance. The average ranking showed that ceftriaxone and gentamicin resistant E. coli strains were minimal in MDR and non-MDR strain populations (Table 1).
 |
Table 1 Anti-Microbial Resistance in Multi-Drug and Non-Multi-Drug E. coli |
Modelling Individual Resistance Outcomes Influencing an Isolate’s MDR Status
Using the X2 test, an association was noticed between all the antibiotic resistance phenotypes for all drugs and MDR (p<0.001) except for ceftriaxone resistance. The bivariate logistic regression modelling found out that a plausible risk of an isolate being multidrug resistant was increased depending on the resistance phenotype portrayed. The significant odds (in descending order) were found with tetracycline resistance (OR = 18.7, CI = 4.3–81.4), trimethoprim sulphamethoxazole resistance (OR = 14.3, CI = 7.5–27.6), ampicillin resistance (OR = 8.9, CI = 5.1–15.7), neomycin resistance (OR = 4.8, CI = 2.2–10.7), and ciprofloxacin resistance (OR = 3.4, CI = 1.8–16.3) (Table 2).
 |
Table 2 Univariate Screening and Bivariate Logistic Regression Analysis of Resistance Phenotypes Related to Isolate MDR |
Bacterial Genetics of Antimicrobial Resistance
Molecular determinants were studied for the two prevalent resistance phenotypes (tetracycline and sulphonamide). Only 174 randomly sampled isolates from the collection were tested. The prevalence of AMR genes was tetA (79.3%; 138/174), tetB (17.2%; 30/174), tetC (7.5%; 13/174), tetD (0.0%; 0/174), tetE (0.0%; 0/174), tetG (0.0%; 0/174)sul1 (11.5%; 20/174) and sul2 (60.3%; 105/174).
Multiple Antibiotic Resistance Indices
This study observed that 87.9% (n = 240) of the isolates had multiple antibiotic resistance (MAR) index greater than 0.2, while 12.1% (n = 33) isolates had MAR index less than 0.2. The mean, range, and median of the bacteria MAR indexes were 0.38 (n = 273), 0.00–0.86, and 0.43, respectively. There was no significant difference in the mean MAR indexes of isolates from farms across the seven sub-counties (p = 0.858); Mukono town council (n= 60; mean = 0.38; 95% CI = 0.34–0.42), Nama (n= 20; mean = 0.38; 95% CI = 0.32–0.44), Kyampisi (n= 45; mean = 0.40; 95% CI = 0.35–0.46), Goma (n= 69; mean = 0.38; 95% CI = 0.34–0.40), Nabaale (n= 10; mean = 0.39; 95% CI = 0.34–0.42), Nakisunga (n= 30; mean = 0.41; 95% CI = 0.35–0.48) and Nagojje (n= 39; mean = 0.37; 95% CI = 0.32–0.43).
Investigative Framework for Antimicrobial Stewardship Practices (IFAP)
Briefly, this study prototyped the IFAP graphical tool (Figure 1) that was used to: 1) detect antibiotic use on farms, 2) identify drivers for antibiotic use, human players, and the drugs used, and 3) probe prognostic outcomes and actions after alleged non-responsive outcomes of treatment given in problematic flocks.
Identification of antibiotic use: We identified use by presence of drug sachets on farms coupled with interviews (Figure 3). Out of the 29 farms, 23 farms were using antibiotics in the period that spanned from 3 months back to the day we visited the farm.
 |
Figure 3 Evidence of antibiotic use on farms. |
Reasons for antibiotic use: the majority (17 respondents) had flocks presenting with clinical signs such as diarrhea, cough, shivering, flu, respiratory conditions, general weakness, deaths, swollen abdomen. Three respondents reported having sick flocks and also production losses (soft egg shells, egg drop), six farmers had confirmed diseases (Newcastle disease, Newcastle disease-colibacillosis co-infections, calcium deficiency, coccidiosis), 2 had only production issues and only one gave antibiotics for prophylaxis.
Drugs used: As explained in section 3.1 (descriptive statistics), the use of inappropriate combination antibiotics was rampant. Some drug brands sold as vitamins to farmers also contained antibiotics, a practice that qualifies as antibiotic misuse and overuse in these settings. The drugs were mostly administered by casual farm personnel/workers.
Who recommended use? In one case of prophylactic use, advice came from a farm worker as a precaution based on previous happenings. On 17/29 farms (58.6%), upon having disease signs or production losses, diagnosis, and decision to use antibiotics was made by a farm personnel based on previous experiences. Three farms submitted samples for necropsy and laboratory testing, whereas 7 had veterinarians who visited the farms to diagnose the disease by physical examination and syndromes.
Did the drugs work: On 13 farms (44.8%), there was improvement after treatment in the scenarios of drug use in the past 3 months, whereas 16 farms (55.2%) reported non-responsive health outcomes in their flocks.
Action after none improvement outcomes: When asked about their response to none improvement outcomes, some sought for advice from a veterinarian and an agro vet outlet (n=2), others submitted samples for testing (n=4), whereas majority changed to a new antibiotic (n=7). They also gave their views on what is done on some other farms in such cases, which included: buying of a new antibiotic (n=10), reporting to a veterinarian (n=2), selling the sick birds for slaughter (5 responses) and referral of cases to the laboratory (4 responses).
What could cause poor prognostic outcomes after treatment? The responses included: too much infection load, counterfeit and expired drugs, poor monitoring and management of sick birds, source of birds, breed, treating a viral disease, and germs that are stubborn to drugs.
Proposed Interventions to Reverse AMR Outcomes
The data from the IFAP interviews gave us an understanding of current forces at work so as to support and advise on the enactment of interventions to boost good antimicrobial stewardship at a poultry farm. We focused on improving farmers’ knowledge through awareness on good infection prevention and control (sensitizations) (Figure 4) and access to veterinary services (including laboratories) and professionals.
 |
Figure 4 Poster designed to sensitize farmers on AMR control. |
Discussion
Multiple antibiotic resistance indexing has been shown to be a cost-effective and valid method of bacteria source tracking.20 On almost all farms, we found E. coli with MAR indices above 0.2, an indication that the studied bacteria originated from potentially dangerous sources where antibiotics are regularly used. High AMR prevalence rates were noted with tetracycline and trimethoprim sulphamethoxazole. This was in agreement with previous studies.1,2,21 A recent study by Mwansa et al22 reported patterns of E. coli from workers, which were similar to the poultry patterns reported in our study. The researchers (Mwansa et al)22 also documented almost similar poultry production systems and antimicrobial use dynamics as in our study results. However, in our study, low E. coli resistance to ceftriaxone was noted, which could be due to its low application in the treatment of poultry bacterial infections. This also gives optimism for treating zoonotic E. coli that could cross to humans and cause infections. The modelling of phenotypes linked with MDR also ranked ceftriaxone as a non-significant contributor to MDR patterns. The study detected prevalence of tetA (79.3%), and sul2 (60.3%) as the predominant molecular determinants of tetracycline and sulphonamide resistance. The tetA and sul2 gene high abundance was also found in soil samples from animal farms (chicken farms inclusive) in Uganda23 thus the high influx could also be mitigated by genetic exchanges among bacteria at the human-animal-environment interface.
We piloted the IFAP framework as a cheaper alternative surveillance method, to understand antimicrobial use dynamics in the community. The information collected using this tool was useful in developing sensitization material for the farmers involved in this study. Data on AMR (usually antibiograms) is acquired from health institutions through active and passive surveillance. Locally, active surveillance has been associated with research fatigue because collecting samples is tedious and the resources are limited. Therefore, this prototype tool can be modified and validated as a tool for surveillance in low-middle-income countries where structural, cultural, and socioeconomic factors affecting AMR emergence may be unique.24,25
The IFAP framework showed that farmers in Mukono used antibiotics injudiciously which culminated into the high prevalence of multi-drug resistance. Non-responsive outcomes in a flock under treatment were a major driver for misuse and overuse of antibiotics. As we see retrospectively, farmers that treated disease and got good outcomes consulted both the veterinarians and took samples to the laboratory for diagnosis. Consulting veterinarians prevents misuse of antibiotics.26 As documented, one of the farmers used antibiotics to treat calcium deficiency. From the latter scenario, farmers need to be encouraged to seek diagnostics services to guide judicious antibiotic use.27 Furthermore, regulations need to be taken on selling antibiotics containing vitamins to reduce the misguided use.28
Some farmers sold sick birds for slaughter when treatment outcomes were non-responsive. This practice could drive dissemination of resistant organisms and expose antibiotic residues to the consumers. In this study, we were limited in quantitative approaches to understanding AMR dynamics such as thorough scoring of infection prevention and control on farms so as to craft linkages with the burden of AMR. Future studies could also delve more into the epidemiology of non-responsive treatment outcomes in animals so as to generate more data for quantifying AMR impacts in agriculture. The hypothesis to be tested as we noted was – “Is the problem with the drugs on market or the pathogens or the management of sick birds?” as suggested by farmers.
Conclusion
A high carriage of multi-drug resistant E. coli was detected in the birds. On these farms, there existed worrying antimicrobial stewardship practices being done that could sponsor the development and spread of antimicrobial resistant bacteria. We encourage more integrated interventions using a one-health approach to address the current misuse and overuse of antimicrobials in poultry farming so as to reverse the occurrence of forecasted public health setbacks.
Acknowledgments
We are grateful for the support rendered to us by the Mukono district veterinary offices during sampling and data collection. All bacterial testing was done by the staff in the unit of bacteriology, CDL, COVAB, found at Makerere University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was implemented under the project, Enhancing the Role of Farmers in the fight Against Antimicrobial resistance (EROFAM). The EROFAM received financial support from the Makerere University Research and Innovation Fund (MAK-RIF), an initiative of the Government of Uganda.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kakooza S, Muwonge A, Nabatta E, et al. A retrospective analysis of antimicrobial resistance in pathogenic Escherichia coli and Salmonella spp. isolates from poultry in Uganda. Int J Vet Sci Med. 2021;9(1):11–21. doi:10.1080/23144599.2021.1926056
2. Samuel M, Wabwire TF, Tumwine G, Waiswa P. Antimicrobial Usage by Small-Scale Commercial Poultry Farmers in Mid-Western District of Masindi Uganda: patterns. Public Health Implications Antimicrobial Resistance E Coli. 2023;2023:67.
3. Ndoboli D, Nganga F, Lukuyu B, et al. The misuse of antiretrovirals to boost pig and poultry productivity in Uganda and potential implications for public health. Int J One Heal. 2021;7(1):88–95. doi:10.14202/IJOH.2021.88-95
4. Nakato R, Tumwine JK, Nanzigu S, et al. Antiretroviral drugs found in pork on Ugandan market: implications for HIV/AIDS treatment. One Heal. 2020;9.
5. Mitchell NM, Johnson JR, Johnston B, Curtiss IIIR, Mellata M. Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs. Appl Environ Microbiol. 2015;81(3):1177–1187. doi:10.1128/AEM.03524-14
6. Kabiswa W, Nanteza A, Tumwine G, Majalija S. Phylogenetic Groups and Antimicrobial Susceptibility Patterns of Escherichia coli from Healthy Chicken in Eastern and Central Uganda. J Vet Med. 2018;2018:1–6. doi:10.1155/2018/9126467
7. Okubo T, Yossapol M, Maruyama F, et al. Phenotypic and genotypic analyses of antimicrobial resistant bacteria in livestock in Uganda. Transbound Emerg Dis. 2019;66(1):317–326. doi:10.1111/tbed.13024
8. Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020;158(1):S65–71. doi:10.1016/j.chest.2020.03.012
9. Kakooza S, Munyiirwa D, Ssajjakambwe P, et al. Epidemiological Dynamics of Extended-Spectrum β-Lactamase-or AmpC β-Lactamase-Producing Escherichia coli Screened in Apparently Healthy Chickens in Uganda. Scientifica. 2021;2021. doi:10.1155/2021/3258059
10. Hosuru Subramanya S, Bairy I, Nayak N, et al. Detection and characterization of ESBL-producing Enterobacteriaceae from the gut of healthy chickens, Gallus gallus domesticus in rural Nepal: dominance of CTX-M-15-non-ST131 Escherichia coli clones. PLoS One. 2020;15(5):e0227725. doi:10.1371/journal.pone.0227725
11. Kakooza S, Ssajjakambwe P, Nabatta E, et al. Investigating Probable Causes of Bacterial Loss in a Biobank at a Ugandan Research Institute. Biopreserv Biobank. 2021;19(6):465–466. doi:10.1089/bio.2021.0075
12. Elkenany RM, Eladl AH, El-Shafei RA. Genetic characterisation of class 1 integrons among multidrug-resistant Salmonella serotypes in broiler chicken farms. J Glob Antimicrob Resist. 2018;14:202–208. doi:10.1016/j.jgar.2018.04.009
13. Wayne PA; Clinical and laboratory standards institute. Performance Standards for Antimicrobial Susceptibility Testing.
14. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
15. Aworh MK, Kwaga J, Okolocha E, Mba N, Thakur S. Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PLoS One. 2019;14(11):e0225379. doi:10.1371/journal.pone.0225379
16. Mudenda S, Malama S, Munyeme M, et al. Antimicrobial resistance profiles of Escherichia coli isolated from laying hens in Zambia: implications and significance on one health. JAC-Antimicrobial Resist. 2023;5(3):dlad060.
17. Abu M, Mostofa H, Priya S, et al. Ultra-High Efficient Colony PCR for High Throughput Screening of Bacterial Genes. Indian J Microbiol. 2017;57(3):365–369. doi:10.1007/s12088-017-0665-1
18. Thi P, Hoa P, Nonaka L, Hung P, Suzuki S. Detection of the sul1, sul2, and sul3 genes in sulfonamide-resistant bacteria from wastewater and shrimp ponds of North Vietnam. Int J Med. 2008;5:1–8.
19. Jin L, Bum J, Huh M, Chung J. Detection of tetracycline-resistance determinants by multiplex polymerase chain reaction in Edwardsiella tarda isolated from fish farms in Korea. Annals of Surgery. 2004;240(1):89–100. doi:10.1097/01.sla.0000129492.95311.f2
20. Mir R, Salari S, Najimi M, Rashki A. Determination of frequency, multiple antibiotic resistance index and resistotype of Salmonella spp. in chicken meat collected from southeast of Iran. Vet Med Sci. 2022;8(1):229–236. doi:10.1002/vms3.647
21. Islam M, Hossain M, Sobur M, Punom SA, Rahman AMM, Rahman M. A Systematic Review on the Occurrence of Antimicrobial-Resistant Escherichia coli in Poultry and Poultry Environments in Bangladesh between 2010 and 2021. Biomed Res Int. 2023;2023:1–18. doi:10.1155/2023/2425564
22. Mwansa M, Mukuma M, Mulilo E, et al. Determination of antimicrobial resistance patterns of Escherichia coli isolates from farm workers in broiler poultry production and assessment of antibiotic resistance awareness levels among poultry farmers in Lusaka, Zambia. Front Public Heal. 2022;10.
23. Kakooza S, Cruz AF, Wampande E, Okubo T, Tsuchida S, Ushida K. Occurrence of Selected Bacterial Antibiotic Resistance Genes in Soils of Animal Farms in Uganda. Afr Study Monogr. 2021;41(2):51–59.
24. Sulis G, Sayood S, Gandra S. Antimicrobial resistance in low-and middle-income countries: current status and future directions. Expert Rev Anti Infect Ther. 2022;20(2):147–160. doi:10.1080/14787210.2021.1951705
25. Kiggundu R, Lusaya E, Seni J, et al. Identifying and addressing challenges to antimicrobial use surveillance in the human health sector in low-and middle-income countries: experiences and lessons learned from Tanzania and Uganda. Antimicrob Resist Infect Control. 2023;12(1):9. doi:10.1186/s13756-023-01213-3
26. Ogwuche A, Ekiri AB, Endacott I, et al. Antibiotic use practices of veterinarians and para-veterinarians and the implications for antibiotic stewardship in Nigeria. J S Afr Vet Assoc. 2021;92(1):1–14. doi:10.4102/jsava.v92i0.2120
27. McAdams D, Wollein Waldetoft K, Tedijanto C, Lipsitch M, Brown SP. Resistance diagnostics as a public health tool to combat antibiotic resistance: a model-based evaluation. PLoS Biol. 2019;17(5):e3000250. doi:10.1371/journal.pbio.3000250
28. Ha DR, Haste NM, Gluckstein DP. The role of antibiotic stewardship in promoting appropriate antibiotic use. Am J Lifestyle Med. 2019;13(4):376–383. doi:10.1177/1559827617700824
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
