Back to Journals » Clinical Ophthalmology » Volume 16
Reduction of Artificial Tears and Use of Adjunctive Dry Eye Therapies After Lifitegrast Treatment: Evidence from Clinical and Real-World Studies
Authors Nichols KK , Donnenfeld ED , Lau C, Syntosi A, Karpecki P , Hovanesian JA
Received 18 November 2021
Accepted for publication 17 February 2022
Published 25 March 2022 Volume 2022:16 Pages 909—916
DOI https://doi.org/10.2147/OPTH.S347496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Kelly K Nichols,1 Eric D Donnenfeld,2 Charis Lau,3 Annie Syntosi,4 Paul Karpecki,5 John A Hovanesian6,7
1School of Optometry, University of Alabama at Birmingham, Birmingham, AL, USA; 2New York University Medical Center, New York, NY, USA; 3Retina Global Medical Affairs, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 4Retina Global Patient Access, Novartis Pharma AG, Basel, Switzerland; 5Kentucky Eye Institute, Lexington, KY, USA; 6Harvard Eye Associates, Laguna Hills, CA, USA; 7UCLA Jules Stein Eye Institute, Los Angeles, CA, USA
Correspondence: John A Hovanesian, Harvard Eye Associates, 24401 Calle de la Louisa, Suites 300-312, Laguna Hills, CA, 92653, USA, Tel +1 949-742-3937, Fax +1 844-479-0584, Email [email protected]; [email protected]
Purpose: To assess the frequency of patients reducing the use of artificial tears (ATs) among patients with dry eye disease (DED) following lifitegrast treatment.
Patients and Methods: Two independent analyses were performed using the data from the 1-year, randomized, multicenter, Phase 3 SONATA trial and a noninterventional, real-world evidence (RWE) study conducted in patients with DED who were treated with lifitegrast in the United States and Canada. In SONATA, patients who had used ATs in the lifitegrast and placebo groups were included. The RWE study reviewed patients’ electronic medical records, prescribing patterns, and practices of physicians throughout the survey. These data were then used to compare the proportion of patients using ATs in the 6-month pre-index period versus the 12-month post-index period.
Results: Of 293 patients (lifitegrast, n=195; placebo, n=98) from SONATA, 107 (lifitegrast, n=64; placebo, n=43) used ATs during the on-therapy period while 186 (lifitegrast, n=131; placebo, n=55) did not. Of those not using ATs, the proportion of patients in the lifitegrast group at any time was higher (∼ 67% [n=131]) versus placebo (∼ 56% [n=55]); this was the case at all study time-points (Days 90, 180, 270, and 360). The RWE study included 600 patient charts (US, n=550; Canada, n=50); 75.5% (n=453) reported AT use. There was ∼ 40% decrease in the proportion of patients using ATs as adjunct DED therapy to lifitegrast in the post-index period (n=273) versus those in the pre-index period (n=453).
Conclusion: The findings show that the reliance on AT use can be gradually reduced with lifitegrast treatment, eventually leading to a reduction in disease burden.
Keywords: dry eye disease, lifitegrast, artificial tears, SONATA, real-world evidence, chart review
Introduction
Dry eye disease (DED) is a multifactorial, inflammatory disease of the ocular surface characterized by symptoms of dryness, irritation, or burning; these can damage the cornea and conjunctiva in the long term.1,2 Tear film instability, hyperosmolarity, and inflammation contribute to the physio-pathological process and trigger a loss of tear film homeostasis and a vicious cycle in DED.2 DED can substantially affect vision and quality of life (QoL), including activities such as reading, writing, social activities, or workplace productivity.3,4
Over the counter (OTC) artificial tears (ATs) are considered the first-line treatment in patients with DED.2,5,6 Due to the easy accessibility and immediate symptomatic relief provided by ATs, most patients initially self-manage their DED.6,7 As their symptoms become more pronounced, patients become more reliant on use of ATs. Even though OTC ATs can provide temporary symptomatic relief, they do not address the underlying disease mechanisms and provide negligible impact on disease intervention.8,9 A Cochrane Systematic Review of 43 randomized controlled trials found that most OTC ATs provide only symptomatic relief.5 In addition, many OTC ATs contain preservatives that may damage the ocular surface associated with allergic, toxic, or inflammatory reactions in the long term if used frequently.10,11
Disrupting the vicious cycle of inflammation on the ocular surface is hypothesized to be important in managing DED and preventing its progression. Besides ATs, current treatments include topical ophthalmic corticosteroids, which have been shown to rapidly improve both signs and symptoms in patients with DED, together with immunomodulators and non-steroidal anti-inflammatory drugs.11,12 However, frequent use of corticosteroids has safety concerns.11,12 Immunomodulators such as cyclosporine A (CsA) 0.05% eye drops/emulsions have been available in certain markets for many years and have resulted in a reduction of cytokines and inflammatory cells on the ocular surface; however, their slow onset of action and poor tolerability still remain challenging in some patients.11 Currently, there is no universally accepted standard treatment regimen for DED; it is generally personalized, based on the individual prescriber’s preference, belief, or experience,13 despite the International Tear Film & Ocular Surface Society (TFOS) Dry Eye Workshop II (DEWS II) guidelines.14
Lifitegrast 5.0% is a small molecule integrin competitive antagonist that inhibits T-cell–mediated inflammation by blocking lymphocyte function-associated antigen 1/intercellular adhesion molecule-1 binding, resulting in a decreased ocular inflammatory cycle.11,15 Lifitegrast was approved for use in DED in the United States (US) and Canada in 2016 and 2018, respectively.16,17 The efficacy and safety of lifitegrast 5.0% in improving the signs and symptoms of DED is well established in clinical trials.18–22 The long-term safety of lifitegrast in patients with DED was demonstrated in SONATA (Safety Of a 5.0% coNcentrATion of lifitegrAst ophthalmic solution).22 The recently published US/Canada real-world evidence (RWE) chart review study demonstrated that the majority of patients receiving lifitegrast treatment had a considerable reduction in overall treatment burden and improvement in DED signs and symptoms 6 months after lifitegrast initiation.21
The aim of this report is to evaluate whether treatment with lifitegrast can provide sustained symptomatic relief such that patients become less reliant on the overall use and frequency of ATs. Patient data from SONATA22 and the RWE chart review study21 have been analyzed separately.
Materials and Methods
Study Design
SONATA was an Independent Review Board (IRB)-approved 1-year, Phase 3, multicenter, randomized, prospective, double-masked, placebo-controlled, parallel-arm study conducted at 22 sites in the US (identifier, NCT01636206).22 The RWE chart review study was an IRB-approved non-interventional, retrospective, longitudinal cohort study using patient charts from both the US and Canada that were obtained through a healthcare provider panel.21 The study design and eligibility criteria for each study have been published previously.21,22 Details of the IRBs of the SONATA and RWE studies are provided in Appendix 1.
In the RWE study, the pre-index period was defined as the 6-month period prior to initiation of lifitegrast treatment (ie, no lifitegrast therapy), while the post-index period was defined as the 12-month period following lifitegrast treatment (along with other adjunct DED therapies).21
Efficacy Outcomes
The main outcome of this study was to measure changes in the proportion of patients using ATs post-initiation of lifitegrast therapy. Examining the concurrent use/reliance on ATs reflects patients’ needs to reach for a rescue therapy to quickly alleviate any uncontrolled symptoms associated with DED. Hence, a reduction in AT use could be an indicator that the associated symptoms are more controlled resulting in a gradual decline of AT use as a rescue therapy.
Statistical Analysis
Data from both the SONATA and RWE chart review studies were analyzed using descriptive statistics and expressed as proportions with two-sided 95% confidence intervals (CIs) for categorical variables and means, standard deviations (SDs), and medians for continuous variables. Ninety-five percent CIs for categorical variables were calculated using the Clopper–Pearson method.
Results
Demographics and Other Baseline Characteristics
SONATA
While the demographic characteristics of SONATA study are summarized in Table 1, data from a total of 293 patients (lifitegrast, n=195; placebo, n=98; safety population set) from the on-therapy period were used for this analysis. The mean age (SD) of patients who used or did not use ATs was 61.3 (11.5 years) and 58.8 years (12.8 years), respectively. The majority of analyzed patients who used (n=89; 83.2%) and did not use (n=132; 71.0%) ATs were female.
 |
Table 1 Demographic and Baseline Clinical Characteristics of SONATA and RWE Chart Review Studies |
RWE Chart Review
Overall, 600 patients (US, n=550; Canada, n=50) from the RWE chart review study were included in this analysis. In the RWE chart review, the mean age (SD) at baseline was 57.1 years (12.8 years); the majority were female (n=455; 75.8%). Among patients who were diagnosed with DED, 160 patients (26.7%) reported both evaporative and aqueous deficiencies, while 376 patients (62.7%) and 32 (5.3%) patients, respectively, were diagnosed with evaporative and aqueous deficiency alone. Before the index day, ≥75% of subjects (n=453) were using OTC ATs. At the time of lifitegrast initiation, ~28% of patients (n=167; 27.8%) in the RWE chart review study had a history of contact lens use (daily, weekly, and monthly disposable, disposable soft, extended wear, and gas permeable) (Table 1).
Efficacy Outcomes
During the on-therapy period of the SONATA study, 107 patients (36.5%) used ATs (lifitegrast, n=64 [59.8%]; placebo, n=43 [40.2%]) and 186 patients (63.5%) did not (lifitegrast, n=131 [70.4%]; placebo, n=55 [29.6%]). At any time during the on-therapy period, the proportion of patients who did not need ATs was always higher in the lifitegrast group compared with placebo (67.2% [n=131/195] vs 56.1% [n=55/98]) (Figure 1). A similar trend was observed during all study time-points (Figure 1).
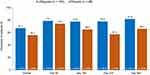 |
Figure 1 Proportion of patients who did not use artificial tears in the lifitegrast and placebo groups of the SONATA study (safety population). |
In the RWE chart review study, of the 281 patients with the 12-month follow-up, the majority of patients (n=238; 84.7%) remained on lifitegrast therapy at 12 months. During the post-index period, 226 patients (37.7%) were reported to be treated with lifitegrast alone, while among patients with lifitegrast plus adjunct combinations, the pre- versus post-index periods showed that there was an ~40.2% reduction in the use of OTC ATs (453 vs 271; 75.5% vs 45.2%), a 43.8% reduction in lid hygiene (313 vs 176; 52.2% vs 29.3%), and a 79.6% reduction in the use of topical corticosteroids (113 vs 23; 18.8% vs 3.8%) (Figure 2).
With lifitegrast alone or in combination with other adjunct therapies (autologous serum [n=20], OTC ATs [n=453], lid hygiene techniques [n=313], oral or topical CsA [n=123], and topical corticosteroids [n=113]), there was a reduction in patient-reported symptoms over the post-index versus pre-index period, including eye dryness (5%; 95% CI: 2.9–8.0 vs 87.5%; 95% CI: 84.6–90.0), blurred vision (3.4%; 95% CI: 1.7–6.1 vs 57.7%; 95% CI: 53.6–61.7), ocular stinging or burning (2.5%; 95% CI: 1.1–4.9 vs 56.0%; 95% CI: 51.9–60.0), foreign body sensation (2.5%; 95% CI: 1.1–4.9 vs 49.8%; 95% CI: 45.8–53.9), and ocular pain (0.6%; 95% CI: 0.1–2.2 vs 12.2%; 95% CI: 9.7–15.1) (Table 2).21
 |
Table 2 DED Symptoms Reported During the Preindex and Postindex Periods in the RWE Chart Review Study |
Discussion
The results from the SONATA and RWE chart review studies showed a consistent decreased use of OTC ATs as a rescue therapy following lifitegrast treatment in patients with DED. In the SONATA trial, a higher proportion of patients reported not using ATs in the lifitegrast group compared with placebo (67.2% vs 56.1%) during the 1-year period.22 In the RWE chart review study, the proportion of patients using ATs was reported to be 40.2% lower during the 12-month post-index period following lifitegrast treatment compared with the pre-index period.21
In the SONATA trial, the proportion of patients using ATs gradually decreased from Day 270–360 in those who were treated with lifitegrast; patients on placebo showed no decline. These data show that lifitegrast, apart from addressing the inflammation, also provides sustained relief of symptoms, which can help to reduce the reliance/dependence on ATs for temporary short-term symptomatic relief.
In the RWE chart review study, there was a reduction in the proportion of patients who were using lid hygiene and topical corticosteroids. Following lifitegrast treatment, the proportion of patients using lid hygiene was reduced by 43.8% compared with the pre-index period. This is important since lid hygiene has been considered the first-line therapy in patients with meibomian gland dysfunction and blepharitis.6,23 Lid hygiene is inconvenient and time-consuming,24 hence, a decline in its usage may also help reduce treatment burden in patients with DED. Moreover, there was an ~80% reduction in the use of topical corticosteroids in the post-index period compared with pre-index, reducing the risk of adverse events (AEs) from steroids.25,26
Treatment and management of DED pose a significant direct burden on healthcare and the indirect economic burden on patients and society is often ignored.27,28 A study conducted in the Singapore National Eye Center, which collected cost data from 54,052 patients, showed that lubricants alone accounted for 79.3% of the total treatment cost.27 A survey conducted in the US in 2171 respondents with DED showed that, from a societal perspective, the overall annual average burden of managing DED to US society was $55.4 billion – $11,302 per patient.28 Even though the results from the SONATA trial and RWE chart review study did not perform a cost estimation analysis, an overall reduction in the DED treatment economic burden of up to 80% can be estimated considering the proportion of patients in whom the use of ATs and other adjunct DED therapies were reduced following lifitegrast treatment. In the US, the usual price of bottled ATs (eg, Systane® Complete) is ~$15, whereas a preservative-free single-use AT (eg, Refresh Plus®) costs $23. Consider that patients with DED use bottled ATs 4 times daily, the cost per year would be $219. However, if such patients use preservative-free ATs every 2 hours, the cost increases to ~$959; use every 1 hour would further increase the cost to ~$1919 per year. Currently, the availability of socioeconomic data is limited, and the actual societal burden of DED is assumed to be much higher than estimated. Hence, consideration of costs while treating and managing patients with DED can prove to be beneficial to both the payers and society.
In this short report, the aim was to highlight whether lifitegrast treatment can provide sustained symptomatic relief in patients with DED and reduce dependency on ATs.11,12 Frequent use of ATs may indicate inadequate symptom control. For temporary relief from ocular discomfort while working in front of a screen, wearing contact lenses, or travelling, the use of ATs is convenient and more easily accessible compared with prescription therapy, and should continue to have a key role in management.5,6 However, ATs cannot be considered as the only resolutive treatment since they do not address the underlying inflammation.8,9
To date, this is the first such combined report from a randomized, Phase 3, clinical trial, SONATA and a RWE chart-review study involving ~900 patients, which evaluated the reduction of ATs in patients with DED following lifitegrast treatment. A limitation of this report is that the retrospectively collected data from patient charts can be biased based on patient reports, and the lack of statistical analysis due to initial study design and power. The RWE chart-review study from the US and Canada showed that >75% of recruited patients were using OTC ATs in the pre-index period; the numbers may be even higher considering that there might be under-reporting from either the patient or eye-care provider.
Conclusions
Findings from this short analysis suggest that following treatment with lifitegrast in patients with DED there is a gradual reduction in the use of ATs, indicating improved symptomatic relief and hence, a reduction in the dependency of ATs.
Abbreviations
AT, artificial tear; CI, confidence interval; CsA, cyclosporine A; DED, dry eye disease; IRB, independent review board; OTC, over-the-counter; RWE, real world evidence; SD, standard deviation.
Data Sharing Statement
The data can be made available upon request.
Ethics Approval and Informed Consent
Independent Review Board (IRB) has approved both SONATA and RWE chart review study. All participants of the SONATA study provided informed consent and the study was conducted according to the tenets of the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all the investigators of SONATA and RWE chart review study for their valuable contribution to this short analysis. The authors would also like to thank Brigitte Sloesen and Rebecca Piccolo for their valuable contribution in the interpretation of data. Medical writing and editorial assistance were provided by Sabyasachi Ghosh (Novartis Healthcare Pvt. Ltd., Hyderabad, India).
Author Contributions
All the listed authors have met the ICMJE guidelines. These include:
- Made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas.
- Have drafted or written, or substantially revised or critically reviewed the article. Have agreed on the journal to which the article will be submitted.
- Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
- Have agreed on the journal to which the article will be submitted.
- Agreed to take responsibility and be accountable for the contents of the article.
Funding
This analysis was funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure
Kelly K. Nichols is a consultant to Novartis for this and other work and reports personal fees from Allergan/AbbVie, Axim, Novartis, Sun, Visionology, Aerie, Bruder, Dompe, HanAll, Kala, Osmotica, Oyster Point, Sight Sciences, Alcon/Tear Film Innovations, Thea, Tarsus, and Topivert; personal fees; grants from Allergan, Kala, Tear Science, and NIH NEI, outside the submitted work. Eric D. Donnenfeld is a consultant to Allergan and Novartis. Charis Lau is an employee of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. Annie Syntosi is an employee of Novartis Pharma AG, Basel, Switzerland. Paul Karpecki reports consultant fees from Aerie, Akorn, Alcon, Allergan, Allysta, Avellino Labs, Azura Pharmaceuticals, Bausch & Lomb, Bio-Tissue, BlephEx, Bruder Healthcare, Inc., Cambium, EyeGate Pharma, Eyevance, Imprimis, Johnson & Johnson, Novartis, Neurolens, Nevakar, Novaliq, Oasis Medical, Oculus, OCuSOFT, Oyster Point Medical, Santen, Science Based Health, Sentiss; Sight Sciences, Silk Technologies, Sun Pharmaceutical Industries, Inc., Surface Pharmaceuticals, Tarsus Medical, Visant Medical, and Vital Tears; and personal fees from Sun Pharmaceutical Industries, Inc. outside the submitted work. John A. Hovanesian is a consultant to Allergan and Novartis for this and other work and has received grant support from Novartis. The authors report no other relevant conflicts of interest in this work.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
2. Şimşek C, Doğru M, Kojima T, Tsubota K. Current management and treatment of dry eye disease. Turk J Ophthalmol. 2018;48(6):309–313. doi:10.4274/tjo.69320
3. Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home Healthc Now. 2018;36(2):74–83. doi:10.1097/nhh.0000000000000652
4. Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. doi:10.1007/s40135-013-0009-1
5. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:Cd009729. doi:10.1002/14651858.CD009729.pub2
6. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
7. Foulks GN. Challenges and pitfalls in clinical trials of treatments for dry eye. Ocul Surf. 2003;1(1):20–30. doi:10.1016/s1542-0124(12)70004-6
8. Pflugfelder SC, Geerling G, Kinoshita S, et al. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163–178. doi:10.1016/s1542-0124(12)70085-x
9. Snapinn SM, Jiang Q. Responder analyses and the assessment of a clinically relevant treatment effect. Trials. 2007;8(1):31. doi:10.1186/1745-6215-8-31
10. Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 2015;59(1):2–5.
11. Abidi A, Shukla P, Ahmad A. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–198. doi:10.4103/0976-500X.195920
12. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):
13. Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol. 2016;10:1083–1094. doi:10.2147/opth.S110557
14. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
15. Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207–215. doi:10.1016/j.jtos.2016.01.001
16. Xiidra. (Lifitegrast ophthalmic solution) 5% - FDA. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208073Orig1s000Approv.pdf.
17. Xiidra. (Lifitegrast ophthalmic solution) 5% - Health Canada. Available from: https://www.multivu.com/players/English/8247751-shire-xiidra-canada/.
18. Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a Phase III, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124(1):53–60. doi:10.1016/j.ophtha.2016.09.025
19. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475–483. doi:10.1016/j.ophtha.2013.09.015
20. Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized Phase III OPUS-2 study. Ophthalmology. 2015;122(12):2423–2431. doi:10.1016/j.ophtha.2015.08.001
21. Hovanesian JA, Nichols KK, Jackson M, et al. Real-world experience with lifitegrast ophthalmic solution (Xiidra(®)) in the US and Canada: retrospective study of patient characteristics, treatment patterns, and clinical effectiveness in 600 patients with dry eye disease. Clin Ophthalmol. 2021;15:1041–1054. doi:10.2147/opth.S296510
22. Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of Lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35(6):741–748. doi:10.1097/ico.0000000000000803
23. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064. doi:10.1167/iovs.10-6997g
24. Kasetsuwan N, Suwajanakorn D, Tantipat C, Reinprayoon U. The efficacy between conventional lid hygiene and additional thermal pulsatile system in meibomian gland dysfunction patients treated with long-term anti-glaucoma medications in a randomized controlled trial. Clin Ophthalmol. 2020;14:2891–2902. doi:10.2147/opth.S259692
25. Cutolo CA, Barabino S, Bonzano C, Traverso CE. The use of topical corticosteroids for treatment of dry eye syndrome. Ocul Immunol Inflamm. 2019;27(2):266–275. doi:10.1080/09273948.2017.1341988
26. Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2(2):55–72. doi:10.1007/s40123-013-0020-5
27. Waduthantri S, Yong SS, Tan CH, et al. Cost of dry eye treatment in an Asian clinic setting. PLoS One. 2012;7(6):e37711. doi:10.1371/journal.pone.0037711
28. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–387. doi:10.1097/ICO.0b013e3181f7f363
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

