Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Rapid Response of Palmoplantar Psoriasis to Risankizumab: A Case Report
Authors Al Muqrin AM , Alghamdi AA, AlShaalan ZM
Received 4 August 2022
Accepted for publication 23 September 2022
Published 4 October 2022 Volume 2022:15 Pages 2129—2132
DOI https://doi.org/10.2147/CCID.S384990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Abdullah Muqrin Al Muqrin,1 Abdulaziz A Alghamdi,1 Ziad M AlShaalan2
1Dermatology, Prince Mohammed Medical City, Skaka, AlJouf, Saudi Arabia; 2Department of Internal Medicine, College of Medicine, Jouf University, Skakak, AlJouf, Saudi Arabia
Correspondence: Abdullah Muqrin Al Muqrin, Dermatology, Prince Mohammed Medical City, Skaka City, 72345, AlJouf, Saudi Arabia, Tel +96654777446, Email [email protected]
Abstract: Palmoplantar psoriasis, a clinical variant of plaque psoriasis, has a significant impact by causing deterioration in the social and functional aspects of patients’ lives. Numerous therapeutic interventions are available for palmoplantar psoriasis. Although emerging biological agents have had an enormous positive impact on chronic plaque psoriasis, studies assessing their effectiveness in the palmoplantar phenotype are limited in the literature. We therefore present a case report of a patient with a 10-year history of palmoplantar psoriasis, which has significantly impacted her occupational life. She was treated with Risankizumab, showing a significant and rapid improvement in her symptoms. We believe that Risankizumab could be one of the most effective therapeutic interventions in the clinical context where rapid clearance of palmoplantar psoriasis is required.
Keywords: psoriasis, Risankizumab, palmoplantar, rapid onset, biologic, biological agent
Introduction
Palmoplantar psoriasis is a regional phenotype of plaque psoriasis that could either occur as an isolated condition or as a consequence of chronic plaque psoriasis, with an estimated prevalence of 10.1–76% in all patients with psoriasis.1 A significant aspect of this regional phenotype is its adverse effects on patients’ quality of life (QoL). According to one study, the QoL of approximately 82% of patients with palmoplantar psoriasis was moderately to severely affected.2 This is because patients with palmoplantar psoriasis demonstrate impaired activities of daily living and physical disability.3,4 Moreover, it has been shown that palmoplantar psoriasis is significantly associated with mood disorders, as compared to generalized plaque psoriasis.1 Fortunately, there are numerous therapeutic interventions, such as topical treatments, classical systemic therapy, and biological agents. However, the palmoplantar psoriasis phenotype has been classified as a “difficult to treat psoriasis” due to its frequently reported poor response to therapy.5 Furthermore, evidence-based guidelines addressing the management of this variant are lacking. To the best of our knowledge, there have only been three published randomized controlled trials (Infliximab, Secukinumab, and Ixekizumab) regarding this topic.6–8 Risankizumab, a humanized immunoglobulin G (IgG1) designed to bind the p19 subunit of the IL23 cytokine, is another biological agent that has been approved by the Food and Drug Association (FDA) for chronic plaque psoriasis in 2019.11 In spite of its proven efficacy in chronic plaque psoriasis, there is no dedicated clinical trial assessing its efficacy in restricted palmoplantar psoriasis. Herein, we report the case of a patient with palmoplantar psoriasis to highlight the efficacy of Risankizumab in terms of clinical clearance and rapid onset of action, thereby assessing its therapeutic potential for this phenotype of psoriasis.
Case Report
The patient was a healthy, 25-year-old, Saudi woman, who was diagnosed with palmoplantar psoriasis in 2012. During the disease course, she received multiple, intermittent, super-potent, topical corticosteroids, but did not show improvement. Currently, she is a practicing medical doctor, and her condition prevented her from applying to the general surgery residency program due to functional disability. On physical examination, thick erythematous scaly plaques, with few overlying fissures, on her palms and soles were noted (Figure 1). The patient was also noted to be extremely frustrated by her condition, which has negatively impacted her social and occupational life. Accordingly, we discussed with her the benefits and risks of the available therapeutic interventions, including systemic and biological agents, and suggested the use of an interleukin-23 inhibitor agent (Risankizumab) that was available. She agreed to start Risankizumab and received a total of two doses (baseline and Week 4). By Week 6, the psoriasis on her palms had almost cleared, with only a few remnant scales (Figure 2). The patient was satisfied with the results and was functionally motivated to pursue her occupational objectives.
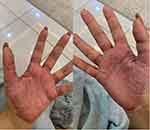 |
Figure 1 Well-defined thick erythematous scaling plaques involving both palms of the patient. |
 |
Figure 2 Faint erythematous patch with minimal scaling involving both palms of the patient. |
Discussion
The severity of palmoplantar psoriasis is well reported in the literature. Despite occurring as an isolated condition, palmoplantar psoriasis can still significantly impact patient QoL.2–4 Thus, its severity should be assessed cautiously, and the surface area of the affected region should not be taken as the sole measure of severity in this regional variant.9 Few randomized clinical trials have been published regarding the efficacy of biological agents in managing palmoplantar psoriasis. An Infliximab pilot study demonstrated failure in reaching the primary endpoint of m-PPPASI75, as compared to that of placebo in a randomized controlled trial, despite its effective superiority to placebo at Week 14 in terms of surface area reduction and m-PPPASI50.7 Similarly, the GESTURE trial revealed that 33.3% and 22.1% of patients on 300-mg and 150-mg Secukinumab, respectively, significantly improved at Week 16, as compared to those with placebo.8 Furthermore, the UNCOVER trials have shown that 80%, 70%, and 50% of patients taking Ixekizumab achieved PPASI50, 75, and 100, respectively, at Week 12.6
In the current era of biologics and molecular treatment, we believe that individualized approaches are needed to address different psoriasis variants. Fortunately, there are many systemic options for psoriasis treatment. Despite these rapid advancements, other issues have risen, such as factors that can affect treatment selection, including insurance systems and health care leaders in the hospital.12 Moreover, with the rapid advancements in biological agents, investigators have begun paying more attention into exploring other outcomes. For example, a previous study reported that TNF-alpha inhibitors may exhibit stronger suppressive properties on inflammatory markers, whereas newer interleukin inhibitors agents may exhibit favorable safety profiles.13 Given this, we hope that this article will contribute to the accumulating evidence that further explores the outcomes for each individual biological agent. We believe that this will help in establishing the unique profile of each agent, such as its efficacy in different phenotypes of psoriasis, with consideration to cost-effectiveness. Such publications may help in providing bases to synthesize evidence-based guidelines that positively affect patient outcomes.
Risankizumab has been reported to be effective in clearing palmoplantar psoriasis after the fourth dose.10 In our case, we documented an almost complete clearance of palmoplantar psoriasis after the second dose at Week 6. This suggests that Risankizumab has a potentially rapid onset of action, making it a highly effective biological agent that could be used in appropriate clinical scenarios where rapid clearance may be needed. However, it should be noted that the main limitation of this article is its nature as a case report. Prospective clinical trials that consider the onset of action and efficacy of Risankizumab are therefore warranted in the future.
Conclusion
Palmoplantar psoriasis should be assessed carefully due to its detrimental effects on patient QoL. Furthermore, a guided protocol is required for managing this particular phenotype. In this era of biological treatment, rapid escalation of management plans may be indicated in the appropriate context. Particularly, Risankizumab can be a potential medication with rapid onset of action against palmoplantar psoriasis, which can lead to significant QoL improvements in the functional aspect of this regional variant.
Data Sharing Statement
Data sharing is not applicable to this article, since no datasets were generated or analyzed.
Ethics Approval and Informed Consent
A written informed consent was obtained from the patient to publish the case details and the images. Institutional approval is not required to publish the case details.
Consent for Publication
Consent for publication was taken.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Timotijević ZS, Trajković G, Jankovic J, et al. How frequently does palmoplantar psoriasis affect the palms and/or soles? A systematic review and meta-analysis. Postepy Dermatol Alergol. 2019;36(5):595–603. doi:10.5114/ada.2019.89508
2. Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–1031. doi:10.1016/j.jaad.2008.11.910
3. Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623–632. doi:10.1016/j.jaad.2014.04.063
4. Pettey AA, Balkrishnan R, Rapp SR, Fleischer AB, Feldman SR. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49(2):271–275. doi:10.1067/s0190-9622(03)01479-8
5. Sánchez-Regaña M, Aldunce Soto MJ, Belinchón Romero I, et al. Evidence-based guidelines of the Spanish psoriasis group on the use of biologic therapy in patients with psoriasis in difficult-to-treat sites (nails, scalp, palms, and soles). Actas Dermo Sifiliogr. 2014;105(10):923–934. doi:10.1016/j.ad.2014.02.015
6. Menter A, Warren RB, Langley RG, et al. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three Phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol. 2017;31(10):1686–1692. doi:10.1111/jdv.14237
7. Bissonnette R, Poulin Y, Guenther L, Lynde CW, Bolduc C, Nigen S. Treatment of palmoplantar psoriasis with infliximab: a randomized, double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2011;25(12):1402–1408. doi:10.1111/j.1468-3083.2011.03984.x
8. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80. doi:10.1016/j.jaad.2016.07.058
9. Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589. doi:10.1111/dth.12589
10. Alajlan AM, Qadoumi TA. Palmoplantar psoriasis successfully treated with risankizumab. Cureus. 2021;13(8):e17434. doi:10.7759/cureus.17434
11. Li W, Ghamrawi R, Haidari W, Feldman SR. Risankizumab for the treatment of moderate to severe plaque psoriasis. Ann Pharmacother. 2020;54(4):380–387. doi:10.1177/1060028019885836
12. Carrascosa JM, Puig L, Belinchón Romero I, et al. Practical update of the recommendations published by the psoriasis group of the Spanish academy of dermatology and venereology (GPS) on the treatment of psoriasis with biologic therapy. part 1. concepts and general management of psoriasis with biologic T. Actas Dermosifiliogr. 2022;113(3):261–277. doi:10.1016/j.ad.2021.10.003
13. Ataseven A, Temiz SA, Eren G, Özer İ, Dursun R. Comparison of anti-TNF and IL-inhibitors treatments in patients with psoriasis in terms of response to routine laboratory parameter dynamics. J Dermatolog Treat. 2022;33(2):1091–1096. doi:10.1080/09546634.2020.1801975
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
