Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Tildrakizumab for the Treatment of Moderate-to-Severe Psoriasis: Results from 52 Weeks Real-Life Retrospective Study
Authors Ruggiero A , Fabbrocicni G, Cacciapuoti S, Potestio L , Gallo L, Megna M
Received 5 January 2023
Accepted for publication 22 February 2023
Published 27 February 2023 Volume 2023:16 Pages 529—536
DOI https://doi.org/10.2147/CCID.S402183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 7
Editor who approved publication: Dr Jeffrey Weinberg
Angelo Ruggiero, Gabriella Fabbrocicni, Sara Cacciapuoti, Luca Potestio, Lucia Gallo, Matteo Megna
Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy
Correspondence: Angelo Ruggiero, Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Via Pansini 5, Naples, 80131, Italy, Tel +39-081-7462457, Fax +39-081-7462442, Email [email protected]
Background: Tildrakizumab, an anti-IL-23, showed promising efficacy and safety profiles in two randomized clinical-trials (reSURFACE-1 and reSURFACE-2), comparing tildrakizumab superiority to placebo and etanercept. Due to its recent availability in clinical-practice, real-life data are still limited.
Objective: To assess the efficacy and safety of tildrakizumab in a real-world-practice in patients suffering from moderate-to-severe psoriasis.
Methods: A 52-week observational retrospective study enrolled patients suffering from moderate-to-severe plaque-psoriasis, starting tildrakizumab treatment.
Results: A total of 42 patients were included in the study. Mean PASI showed a significant reduction at each follow-up (p< 0.001), reducing from 13.5± 5.9 at baseline, 2.8± 3.8 at week-28, resulting stable up to week-52. High rates of patients reached both PASI90 and PASI100 responses at both week 16 (PASI90: 52.4%, PASI100: 33.3%) and week 28 (PASI90: 76.1%, PASI100: 61.9%), maintaining these up to week 52 (PASI90: 73.8%, PASI100: 59.5%). The impact of treatment on patient’s quality of life has been evaluated with DLQI, which showed a significant reduction during follow-ups.
Conclusion: Our data confirm tildrakizumab as an effective and generally safe treatment for the management of moderate-to-severe psoriasis, with high rates of both PASI90 and PASI100 responses, and very few reported adverse events, up to 52 weeks of follow-up.
Keywords: guselkumab, risankizumab, tildrakizumab, real-world practice, psoriasis, anti-IL-23, biologics
Introduction
Psoriasis is a chronic inflammatory skin disease with a genetic background and autoimmune pathogenic traits, affecting 1–3% of the global population1,2 Even if the exact pathogenesis of psoriasis is still not fully understood, recent major research advantages lead to the development of biologic drugs targeting specific cytokines’ pathway which were found hyperactivated in psoriatic skin lesions.3 Biologic drugs deeply changed the approach to moderate-to-severe forms of psoriasis, showing promising results in terms of both safety and efficacy profiles.4 Among available biological therapies, anti-interleukin (IL)-23s (guselkumab, risankizumab and tildrakizumab) represent the latest class of biologics approved for the treatment of moderate-to-severe psoriasis. Several clinical trials evaluated the efficacy and safety of Ant-IL-23s, showing high response rates linked with an excellent safety profile.5–9 However, real-life data, showing their efficacy and safety profiles in daily dermatological practice, hence, in patients typically excluded by the rigid criteria of the clinical trials, such as patients suffering from several comorbidities, or other forms than plaque psoriasis (erythrodermic psoriasis), are mostly available for guselkumab and risankizumab, due to the later availability of tildrakizumab in clinical practice.10–20 Tildrakizumab is a high affinity humanized immunoglobulin (Ig) G1 κ antibody specifically targeting the p19 subunit of IL-23 without binding to IL-12 and the p40 subunit of IL-23.21 Its efficacy and safety have been evaluated in two randomized CT (reSURFACE 1 and reSURFACE 2), which showed tildrakizumab superiority to placebo and etanercept.22 However, tildrakizumab real-life data in literature are still limited1,23 Herein, we report the results of a 52-week observational retrospective study evaluating tildrakizumab efficacy and safety profiles in psoriatic patients in a real-life setting.
Methods
Study Design
A single-centre observational retrospective study was performed enrolling moderate-to-severe psoriatic patients treated with tildrakizumab, attending the Psoriasis Care Center of Dermatology at the University Federico II of Naples, between November-2019 to November 2022. Inclusion criteria were: (a) moderate-to-severe plaque psoriasis diagnosis since at least 1 year; (b) wash-out period of at least 2 weeks from topical and 4 weeks from systemic or UV treatment; (c) patients starting tildrakizumab, and for which data are available at least since week 52 of follow-up.
All enrolled patients were treated with the standard dose of tildrakizumab 100 mg. (1 subcutaneous injection at weeks 0 and 4, and then every 12 weeks). The aim of this study was to assess the efficacy and safety of tildrakizumab in a real-world setting.
At baseline, the following data were collected for each patient: i) personal and demographic data; ii) psoriasis duration and presence of psoriatic arthritis (PsA); iii) comorbidities; iv) previous treatments for psoriasis; v) psoriasis severity through Body Surface Area (BSA) and Psoriasis Activity Severity Index (PASI), and Dermatology Life Quality Index (DLQI) scores. PASI, BSA, DLQI, routine blood tests (blood count with formula, transaminases, creatinine, azotemia, glycaemia, erythrocyte sedimentation rate, C reactive protein, total cholesterol and triglycerides levels, protein electrophoresis), and adverse events (AEs), were assessed at each follow-up visit (weeks 16, 28, and 52). The efficacy of tildrakizumab treatment was evaluated at week 12, 28, and 52 in terms of mean percentage change from baseline and percentage of patients with a PASI reduction ≥75% (PASI 75), ≥90% (PASI 90) and 100% (PASI 100). Primary and secondary inefficacy were documented during follow-ups. Primary inefficacy was considered as the lack of PASI 75 response after 16 weeks of treatment, while secondary inefficacy was recorded when PASI 75 response was lost during follow-ups after week 16. Data about treatment efficacy were evaluated using a “last observation carried forward” method, where if a patient dropped out of the study the last available value was “carried forward” until the end of the treatment [last observation carried forward (LOCF) method].
At each follow-up visit, the safety was assessed recording treatment-emergent AEs, physical examinations, and routine laboratory monitoring (blood count with formula, transaminases, creatinine, azotaemia, glycaemia, erythrocyte sedimentation rate, C reactive protein, total cholesterol and triglycerides levels, protein electrophoresis). The occurrence of AEs was collected at each follow-up visit (weeks 16, 28 and 52).
The present study was conducted in the respect of the Declaration of Helsinki. The approval from the local institutional review board (University of Naples Federico II) was exempted because the protocol of the study did not deviate from the standard routine clinical practice. All patients included in the study signed a written informed consent form during routine clinical practice visits.
Statistical Analysis
Demographic and clinical variables were analysed through descriptive statistics. Data were presented as number and proportion of patients for categorical variables and as mean ± standard deviation in case of continuous ones. Student’s t-test and Chi-square test were used to assess the statistical significance of the quantitative and qualitative characteristics differences of the cohort of patients treated with tildrakizumab at different follow-up visits. A p-value of <0.05 was considered as statistically significant. The last observation carried forward (LOCF) method was used at each time point, where, if a person drops out of the study, then his or her last observed score on the dependent variable is used for all subsequent. All statistical analyses were performed using GraphPad-Prism 4.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
A total of 42 patients were enrolled in the study. The study population included 24 males (57.1%) and 18 females (42.9%), with a mean age of 49.5±9.2 years. All patients were affected by a moderate-to-severe form of plaque psoriasis, with a mean duration of 14.4±8.6 years. Psoriatic arthritis (PsA) was reported in 21.4% (n=9), with a mean duration of 3.9±4.2 years. The most frequently reported comorbidity were hypertension (45.2%, n=19) and dyslipidaemia (28.6%, n=12), followed by type 2 diabetes mellitus (16.6%, n=7), cardiopathy (14.2%, n=6), and depression (11.9%, n=5). All patients had previously been treated with at least one systemic conventional treatment (including methotrexate, cyclosporin, acitretin, and narrow band UVB phototherapy). As regard previous exposure to biologics, 45.2% (n=19) were bio-naïve, while 54.8% (n=23) have been previously treated with at least one biologic drug. Demographic, anamnestic, and clinical data were resumed in Table 1.
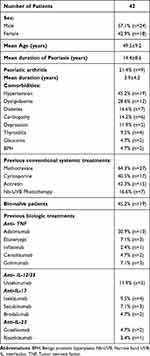 |
Table 1 Characteristics of the Study Population |
Regarding psoriasis severity, at baseline, mean PASI score was 13.5±5.9, which reduced to 5.4±4.2 at week 16 (p<0.001), 2.8.1±3.8 at week 28 (p<0.01), and up to 2.9±4.2 at week 52 (p<0.01). A similar trend was found for BSA, which reduced from 19.6±10.4 at baseline, to 10.4±6.3 at week 16 (p<0.001), 4.7 ± 4.9 at week 28 (p<0.01), and up to 4.9 ± 4.2 at week 52 (p<0.01). All these changes from baseline resulted statistically significant (p<0.001). (Table 2) A high rate of patients reached both PASI90 and PASI100 responses at both week 16 (PASI90: 52.4%, PASI100: 33.3%) and week 28 (PASI90: 76.1%, PASI100: 61.9%), maintaining these up to week 52 (PASI90: 73.8%, PASI100: 59.5%). (Table 2, Figure 1) Only 11.9% (n=5) discontinued the treatment: 2 of them due to treatment primary inefficacy; 3 of them for loss of response during follow-up (1 at week 28, and 2 at week 52). Hence, a total of 37 patients fully completed the 52 weeks of follow-up.
 |
Table 2 Clinical Outcomes During Tildrakizumab Treatment |
 |
Figure 1 Rates of PASI90 and PASI100 responses achieved during tildrakizumab treatment. |
The impact of treatment on patient’s quality of life has been evaluated with DLQI, which showed a significant reduction during follow-ups, reducing from 18.4 ± 8.5 at baseline, to 8.2 ± 4.2 at week 16, to 5.4 ± 3.6 at week 28, and up to 5.8 ± 4.2 at week 52.
Mean PASI, BSA, and DLQI, and PASI90 and PASI100 response rates were resumed in Table 2.
Concerning safety profile, tildrakizumab showed to be a generally safe treatment. Most frequently reported AEs were pharyngitis (11.9%, n=5) followed by flu-like illness (9.5%, n=4), headache (7.1%, n=3), diarrhoea (2.4%, n=1). Furthermore, 3 patients (7.1%) referred pain at the injection site. None of these AES required treatment discontinuation. No cases of serious AEs, candida, malignancy, cardiovascular events were reported during the study. Safety data were resumed in Table 3.
 |
Table 3 Reported Adverse Events During Tildrakizumab Treatment Up to 52 Weeks Follow-Up |
Discussion
The increased knowledge of psoriasis pathogenesis deeply changed the management of moderate-to-severe psoriasis. Indeed, the availability of biological drugs specifically targeting key cytokines implicated in psoriasis pathogenesis, deeply modified the treatment of moderate-to-severe disease, particularly in patients presenting contraindications and/or an inadequate response to conventional systemic treatments.24,25 In this scenario, the relatively recent findings of IL-23/IL-17 axis as the primary signalling pathway behind psoriatic skin changes, led to the development of new biologic drugs targeting these cytokines: anti IL-23.26 Tildrakizumab, a humanized antibody specifically targeting the p19 subunit of IL-23, represents the latest anti-IL-23 available in clinical practice on the Italian market.1 It received on March 20, 2018 the FDA approval for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.27 Tildrakizumab short and long term efficacy has been showed in two randomized placebo controlled studies: reSURFACE1 and reSURFACE2.23,28 In the reSURFACE studies, tildrakizumab was compared with placebo and etanercept, showing promising results in both comparisons.23,28 Particularly, a significantly higher proportion of patients in the tildrakizumab groups than in the placebo groups achieved PASI 75, PASI 90, and PASI 100 responses at week 12 in both studies (p<0·0001). In reSURFACE 2, a significantly higher proportion of patients in the tildrakizumab group than in the etanercept group achieved PASI 75 (p<0·0001) at week 12.28 Furthermore, also PASI90 and PASI100 responses in tildrakizumab treatment groups resulted significantly higher than etanercept, at both week 12 [tildrakizumab PASI90: 39% (n=119), PASI100: 12% (n=38) vs etanercept PASI90: 31% (n=85), PASI100 5% (n=15)] (p<0.0001), and week 28 [tildrakizumab PASI90: 56% (n=161), PASI100: 23% (n=66) vs etanercept PASI90: 21% (n=67), PASI100 11% (n=31)] (p<0.0001).23,28 A post hoc analysis of reSURFACE trials, reported data of 5 years of follow-up, focusing on psoriatic patients with metabolic syndrome treated with tildrakizumab. Interestingly, no significant differences were reported in terms of PASI response rates, and adverse events up to week 244 between patients with and without metabolic syndrome, confirming tildrakizumab efficacy and favorable safety profile even in long-term follow-up also in patients with metabolic syndrome.29 ReSURFACE studies also showed tildrakizumab as a safe treatment in moderate-to-severe psoriasis.23,28 Indeed, tildrakizumab, at both 100 and 200 mg dosages, did not raise any safety issues in both reSURFACE trials, resulting comparable to placebo in terms of reported AEs.31 Most reported AEs were nasopharyngitis (3.1%) and headache (1.3%) followed by injection site reaction (pain) in (1.4%), while reported malignancies consisted mostly of non-melanoma skin cancer, without any cases of melanoma skin cancer reported. Hence, reSURFACE trials showed tildrakizumab as an efficacy and generally safe option for the treatment of moderate-to-severe psoriasis.31 Data from real-life studies evaluating tildrakizumab treatment in daily dermatological practice, are still limited if compared to those available for other anti-IL-23s24 In a previous study, we analysed and compared guselkumab, risankizumab, and tildrakizumab safety and efficacy in real-world-practice in elderly patients.1 The study included 6 patients treated with tildrakizumab. Even in these fragile and complex class of patients, tildrakizumab was found to be a safe and effective treatment option, showing high rates of PASI90 and PASI100 responses at week 28 (PASI90: 50%; PASI100: 33.3%).1 Among other available real-life data, Burlando M. et al22 reported tildrakizumab efficacy and safety in 25 patients suffering from moderate-to-severe psoriasis.22 The authors confirmed the promising results of the clinical trials, showing even higher rates of PASI90 and PASI100 responses than resurface trials, at both week 12 (PASI90 and (71%) PASI100: 67%) and week 28 (PASI90: 91%, PASI100: 87%).22 Furthermore, in this study no AEs were reported, and no patients discontinued the treatment.22 Another recently published prospective study evaluated tildrakizumab efficacy in real-life settings in 150 patients30 Interestingly, these patients strongly differed from patients included in reSURFACE studies, for both clinical and demographic data. Indeed, the baseline PASI reported by authors was of 8.6 ± 4.2 in comparison to the PASI of the reSURFACE studies that was significantly 20.5 ± 7.63. This was due to the absence of a washout periods in the study.30 The authors reported a rapid decrease of mean PASI, with a huge improvement of DLQI, showing results in line with reSURFACE studies.30 Another recent study by Narcisi A. et al31 retrospectively evaluated and compared the efficacy anti-IL23 drugs and IL17 or IL17RA inhibitors in a real-life population with scalp psoriasis.31 Interestingly, although responders’ rate was slightly higher at Week 24 and 48 in patients treated with anti-IL17, no statistically significant differences were reported if compared to anti-IL-23s.31 Concerning tildrakizumab, even if no specific data were reported in this study, in the sub-analysis among the three anti-IL-23, patients treated with tildrakizumab seemed to respond later than risankizumab and guselkumab.31 Recently published real-life studies also confirmed the promising results of tildrakizumab showing high rates of PASI 90 and PASI 100 responses, a sustainable response over time, linked with an excellent safety profile, reporting a significative clinical improvement also in difficult to treat area, such as nails and scalps.32–39 Previously, we published a preliminary real-life data evaluating tildrakizumab in the management of plaque psoriasis up to 28 weeks of treatment, showing huge improvements obtained also at earlier follow-up visits (PASI90 and PASI100 were reached by 52.9% and 35.3% patients at week 12, and by 76.5% and 61.8% at week 28).40 A recently published retrospective, multicenter real-life study, enrolled a total of 237 patients treated with tildrakizumab up to 52 weeks.41 Authors’ results were in line with our study, showing comparable rates of both PASI 90 and PASI 100 at week 52, which were reached by 73.55% and 58.68% of patients, respectively.41 An interesting data regarded the difference between bio-naïve patients, in which the efficacy of tildrakizumab resulted to be significantly higher than bio-experienced patients.41 Indeed, although our study found a slightly higher improvement in bio-naïve patients, this resulted a not statistically significant difference; this may come from the higher number of patients enrolled in this study (n=237 vs n=42).41 In line with our experience, tildrakizumab resulted a generally safe treatment, with no serious AE reported, no discontinuations due to AE, and with nasopharyngitis and upper respiratory infections as the most reported AEs.41 The results of our study confirmed tildrakizumab as an effective and generally safe treatment option for the management of moderate-to-severe psoriasis. Indeed, we reported a significant reduction in mean PASI during follow-ups, with even higher rates of PASI90 and PASI100 responses than reSURFACE studies, at both week 16 (PASI90: 52.4%, PASI100: 33.3%) and week 28 (PASI90: 76.1%, PASI100: 61.9%), maintaining these responses up to week 52 (PASI90: 73.8%, PASI100: 59.5%). Interestingly, no significant differences were found among bio-naïve and bio-experienced patients, as well as among patients with different comorbidities. Notably, our results were also maintained over 1-year of follow-up, showing a stable disease until last follow-up visit, linked with an important positive impact on patients’ quality of life, showed by a significative reduction of DLQI during treatment (which reduced from 18.4 ± 8.5 at baseline, to 5.4 ± 3.6 at week 28, and 5.8 ± 4.2 at week 52). (Table 2, Figure 1) Our data also confirmed the safety of tildrakizumab, reporting only few AEs [pharyngitis (11.9%, n=5) followed by flu-like illness (9.5%, n=4), headache (7.1%, n=3), diarrhoea (2.4%, n=1)], and without any cases of serious AEs, candidiasis, malignancy, and cardiovascular events reported during the study. (Table 3).
Limitations
The major limitations of the study include the small sample size of the study population, the retrospective nature of the study, and the high percentage of bio naïve patients that may limit the generalizability of the results.
Conclusions
Our results confirm tildrakizumab as an effective and generally safe treatment for the management of moderate-to-severe psoriasis, showing higher PASI90 and PASI100 responses, and a comparable safety profile, if compared to reSURFACE trials, linked to a sustained clinical response up to 52 weeks of treatments. Therefore, even if our data confirmed the promising results of tildrakizumab trials, more real-life studies with a larger study population and a longer follow-up period may be needed to confirm its efficacy and safety in long term, and to better clarify its role in the management of moderate-to-severe psoriasis.
Ethical Approval
This clinical study is adhered to the tenets of the Declaration of Helsinki and its later amendments. The approval from the local institutional review board (University of Naples Federico II) was exempted because the protocol of the study did not deviate from the standard routine clinical practice. All patients included in the study signed a written informed consent form during routine clinical practice visits.
Funding
There is no funding to report.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Ruggiero A, Fabbrocini G, Cinelli E, Ocampo Garza SS, Camela E, Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2021;47:561–567. doi:10.1111/ced.14979
2. Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl2):ii18–ii25. doi:10.1136/ard.2004.033217
3. Megna M, Tommasino N, Potestio L, et al. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an Italian 28-week retrospective study. J Dermatolog Treat. 2022;33(6):2813–2820. doi:10.1080/09546634.2022.2081655
4. Kamata M, Tada Y. Efficacy and Safety of Biologics for Psoriasis and Psoriatic Arthritis and Their Impact on Comorbidities: a Literature Review. Int J Mol Sci. 2020;21(5):1690. doi:10.3390/ijms21051690
5. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled Phase 3 trials. Lancet. 2018;392(10148):650–661. doi:10.1016/S0140-6736(18)31713-6
6. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with Adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the Phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi:10.1016/j.jaad.2016.11.041
7. Gerdes S, Bräu B, Hoffmann M, et al. Real-world effectiveness of guselkumab in patients with psoriasis: health-related quality of life and efficacy data from the noninterventional, prospective, German multicenter PERSIST trial. J Dermatol. 2021;48(12):1854–1862. doi:10.1111/1346-8138.16128
8. Megna M, Ruggiero A, Camela E, Fabbrocini G, Marasca C. A case of erythrodermic psoriasis successfully treated with guselkumab. Dermatol Ther. 2020;33(2):e13238. doi:10.1111/dth.13238
9. Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Psoriasis: a Review of the Real-World Evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi:10.2147/CCID.S364640
10. Snast I, Sherman S, Holzman R, Hodak E, Pavlovsky L. Real-life experience of guselkumab in patients with psoriasis. Dermatol Ther. 2020;33(6):e13964. doi:10.1111/dth.13964
11. Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Guselkumab is efficacious and safe in psoriasis patients who failed anti-IL17: a 52-week real-life study. J Dermatolog Treat. 2022;33(5):2560–2564. doi:10.1080/09546634.2022.2036674
12. Ruggiero A, Fabbrocini G, Cinelli E, Megna M. Efficacy and safety of guselkumab in psoriasis patients who failed ustekinumab and/or anti-interleukin-17 treatment: a real-life 52-week retrospective study. Dermatol Ther. 2021;34(1):e14673. doi:10.1111/dth.14673
13. Megna M, Cinelli E, Gallo L, Camela E, Ruggiero A, Fabbrocini G. Risankizumab in real life: preliminary results of efficacy and safety in psoriasis during a 16-week period. Arch Dermatol Res. 2021;314:619–623. doi:10.1007/s00403-021-02200-7
14. Ruiz-Villaverde R, Rodriguez-Fernandez-Freire L, Pérez-Gil A, Font-Ugalde P, Galán-Gutiérrez M. Risankizumab: efficacy, Safety, and Survival in the Mid-Term (52 Weeks) in Real Clinical Practice in Andalusia, Spain, According to the Therapeutic Goals of the Spanish Psoriatic Guidelines. Life. 2022;12(11):1883. doi:10.3390/life12111883
15. Ruggiero A, Camela E, Potestio L, Fabbrocini G, Megna M. Drug safety evaluation of tildrakizumab for psoriasis: a review of the current knowledge. Expert Opin Drug Saf. 2022. doi:10.1080/14740338.2022.2160447
16. Gargiulo L, Ibba L, Pavia G, et al. Real-Life Effectiveness and Safety of Risankizumab in 131 Patients Affected by Moderate-to-Severe Plaque Psoriasis: a 52-Week Retrospective Study. Dermatol Ther (Heidelb). 2022;12(10):2309–2324. doi:10.1007/s13555-022-00795-x
17. Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Risankizumab treatment in psoriasis patients who failed anti-IL17: a 52-week real-life study. Dermatol Ther. 2022;35(7):e15524. doi:10.1111/dth.15524
18. Ruggiero A, Potestio L, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate to severe psoriasis: results from a single center preliminary real-life study. Dermatol Ther. 2022;35(12):e15941. doi:10.1111/dth.15941
19. Ruggiero A, Fabbrocini G, Cinelli E, Megna M. Guselkumab and risankizumab for psoriasis: a 44-week indirect real-life comparison. J Am Acad Dermatol. 2021;85(4):1028–1030. doi:10.1016/j.jaad.2021.01.025
20. Ruggiero A, Fabbrocini G, Cinelli E, Megna M. Real world practice indirect comparison between guselkumab and risankizumab: results from an Italian retrospective study. Dermatol Ther. 2022;35(1):e15214. doi:10.1111/dth.15214
21. Sinclair R, Thirthar Palanivelu V. Tildrakizumab for the treatment of psoriasis. Expert Rev Clin Immunol. 2019;15(1):5–12. doi:10.1080/1744666X.2019.1544493
22. Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–288. doi:10.1016/S0140-6736(17)31279-5
23. Burlando M, Castelli R, Cozzani E, Parodi A. Treatment of moderate-to-severe plaque psoriasis with tildrakizumab in the real-life setting. Drugs Context. 2021;10:548.
24. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:2297. doi:10.3390/ijms18112297
25. Tonini A, Gualtieri B, Panduri S, et al. A new class of biologic agents facing the therapeutic paradigm in psoriasis: anti-IL-23 agents. Expert Opin Biol Ther. 2018;18(2):135–148. doi:10.1080/14712598.2018.1398729
26. Markham A. Tildrakizumab: first global approval. Drugs. 2018;78(8):845–849. doi:10.1007/s40265-018-0917-3
27. Griffiths CEM, Thaci D, Iversen L, et al. Tildrakizumab results in significant and sustained improvements in health-related quality of life in patients with moderate to severe psoriasis in a phase 3 trial (reSURFACE 1) [e-poster 8047]. AAD Annual Meeting. 2019;1:3265.
28. European Medicines Agency. Assessment Report. Ilumetri. International Non-Proprietary Name: Tildrakizumab. 2018. Procedure no. EMEA/H/C/004514/0000.
29. Thaçi D, Gerdes S, Du Jardin KG, Perrot JL, Puig L. Efficacy of Tildrakizumab Across Different Body Weights in Moderate-to-Severe Psoriasis Over 5 Years: pooled Analyses from the reSURFACE Pivotal Studies. Dermatol Ther (Heidelb). 2022;12(10):2325–2341. doi:10.1007/s13555-022-00793-z
30. Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: results of a Real-World Prospective Cohort Study in Nonselected Patients. Dermatology. 2021;1–5.
31. Narcisi A, Valenti M, Cortese A, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: a single-center retrospective comparative study. Dermatol Ther. 2022;35(2):e15228. doi:10.1111/dth.15228
32. Mastorino L, Cariti C, Susca S, et al. Tildrakizumab in real-life shows good efficacy in moderate-to-severe psoriasis regardless of previous use of biologic drugs and joint involvement. Dermatol Ther. 2022;35(11):e15818. doi:10.1111/dth.15818
33. Potestio L, Genco L, Villani A, et al. Reply to ‘Cutaneous adverse effects of the available COVID-19 vaccines in India: a questionnaire-based study’ by Bawane J et al. J Eur Acad Dermatol Venereol. 2022;36(11):e863–e864. doi:10.1111/jdv.18341
34. Becher G, Conner S, Ingram JA, et al. A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis. Dermatol Ther. 2022;12(10):2343–2354. doi:10.1007/s13555-022-00800-3
35. Zagaria O, Villani A, Ruggiero A, Potestio L, Fabbrocini G, Gallo L. New-onset lichen planus arising after COVID-19 vaccination. Dermatol Ther. 2022;35(5):e15374. doi:10.1111/dth.15374
36. Tsianakas A, Schwichtenberg U, Pierchalla P, Hinz T, Diemert S, Korge B. Real-world effectiveness and safety of tildrakizumab in long-term treatment of plaque psoriasis: results from the non-interventional, prospective, multicentre study TILOT. J Eur Acad Dermatol Venereol. 2023;37(1):85–92. doi:10.1111/jdv.18572
37. Marasca C, Ruggiero A, Napolitano M, Fabbrocini G, Megna M. May COVID-19 outbreaks lead to a worsening of skin chronic inflammatory conditions? Med Hypotheses. 2020;143:109853. doi:10.1016/j.mehy.2020.109853
38. Brunasso A. Nail Psoriasis Improvement During Tildrakizumab Therapy: a Real-Life Experience. J Drugs Dermatol. 2022;21(8):914–916. doi:10.36849/JDD.6828
39. Galluzzo M, Talamonti M, Cioni A, et al. Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: scalp, Nail, Palmoplantar and Genital Psoriasis. J Clin Med. 2022;11(9):2631. doi:10.3390/jcm11092631
40. Ruggiero A, Potestio L, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate to severe psoriasis: results from a single center preliminary real-life study. Dermatol Ther. 2022;35(12):e15941.
41. Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J Eur Acad Dermatol Venereol. 2023;37(1):93–103. doi:10.1111/jdv.18594
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
