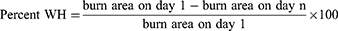Back to Journals » Journal of Inflammation Research » Volume 16
Radix Salvia miltiorrhiza Ameliorates Burn Injuries by Reducing Inflammation and Promoting Wound Healing
Authors Tian S, Guo L, Song Y, Yang H, Wang J, Qiao J , Wu X , Bai M, Miao M
Received 21 June 2023
Accepted for publication 16 September 2023
Published 25 September 2023 Volume 2023:16 Pages 4251—4263
DOI https://doi.org/10.2147/JIR.S427024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Shuo Tian,1,2 Lin Guo,3 Yagang Song,1,2 Huiyan Yang,1 Jia Wang,3 Jingyi Qiao,1,2 Xiangxiang Wu,1,2 Ming Bai,1,2 Mingsan Miao1,2
1Academy of Traditional Chinese Medicine, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China; 2Henan Collaborative Innovation Center for Research and Development on the Whole Industry Chain of Yu-Yao, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China; 3Department of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China
Correspondence: Mingsan Miao, Henan Collaborative Innovation Center for Research and Development on the Whole Industry Chain of Yu-Yao, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China, Tel/Fax +86 371 65962546, Email [email protected]
Purpose: Radix Salvia miltiorrhiza (RSM), a commonly used medicinal plant, has been reported to have anti-inflammatory effects, but relevant studies on burn injuries are lacking. We investigated the anti-inflammation and wound healing (WH) effects of an aqueous extract of RSM on a burn model in rats.
Methods: The effects of RSM were studied by heat-induced burns in rats, treatment with vehicle, Jinwanhong ointment, and RSM (1.5 or 0.75 g/mL). Indicators of burn tissue (BT) were photographed by digital machines and analyzed. The microcirculation in BT was detected by scattered full-frame real-time imaging. Levels of inflammatory mediators and growth factors were measured by enzyme-linked immunosorbent assay (ELISA) and immunohistochemical staining. Local pathologic changes in BT were observed by hematoxylin-and-eosin (HE) staining. Ultrahigh pressure liquid chromatography–linear ion trap–Orbitrap mass spectrometry (UHPLC–LTQ–Orbitrap-MS) was used to explore the absorption of RSM in local skin, subcutaneous tissue, muscle tissue, serum, liver tissue, and kidney tissue.
Results: RSM treatment could reduce the wound area, increase percent WH, increase blood perfusion in BT, reduce serum levels of interleukin (IL)-6, IL-1, tumor necrosis factor-α (TNF-α), increase levels of epidermal growth factor (EGF), transforming growth factor (TGF)-β, and hydroxyproline (Hyp) in serum, and increase protein expression of basic fibroblast growth factor (bFGF), TGF-β 1, EGF, and insulin-like growth factor-1 (IGF)-1 in skin tissues. RSM treatment led to micro-absorption in the skin, subcutaneous tissues, and muscle, but not in the blood, liver, or kidney.
Conclusion: RSM may promote WH by exerting anti-inflammatory effects, improving local-wound microcirculation, and accelerating the metabolism at the wound surface.
Keywords: Radix Salvia miltiorrhiza, burns, anti-inflammatory, wound healing
Introduction
Burns can lead to trauma with enormous damage to the structure and function of skin.1 According to the World Health Organization, ~180,000 people die from a burn injury each year. Wound healing (WH) involves three overlapping stages: inflammation, proliferation, and maturation.2 Treatments for burns include pharmaceutical agents and antibiotics. The expense of synthetic drugs and increased antibiotic resistance poses challenges for developed and developing countries. Medicinal plants can be biocompatible, safe, and affordable treatment compared with synthetic drugs. Some medicinal plants have shown good effects in the treatment of burns, such as Sanguisorba officinalis L,3 Calendula officinalis,4 and plant extracts (eg, isorhamnetin,5 oleanolic acid, ursolic acid, irisol).
Medicinal plants with WH activity have attracted widespread attention, but topical application of medicinal plants is, in general, not well studied. Their mechanism of action is not clear. In Latin America, only 35% of the pharmacological effects of plants used in traditional medicine for WH have been tested and verified.6 Many medicinal plants with great potential for WH in India and Africa that have not been validated either.7,8 Numerous studies have shown that topical application of medicinal plants acts leads to regulation of the local skin microenvironment through various pathways (eg, anti-inflammatory, decontamination) and improved blood circulation to the wounded surface.9
Radix Salvia miltiorrhiza (RSM) is a plant used in traditional Chinese medicine formulations. The parts of RSM with medicinal properties are the dried root or rhizome.10 Salvianolic acids and tanshinones are the main active ingredients of RSM. The Chinese Pharmacopoeia uses the content of tanshinone IIA and salvianolic acid B as landmark components to measure the efficacy of RSM. Ancient Chinese texts have shown RSM to be used externally to treat burns, such as “Qian Jin Yao Fang”, “Taiping Huimin and Pharmaceutical Bureau Formula”. Topical patterns of RSM based on analysis of association rules and factor analysis suggest that burns are the top-three ranked diseases.11 Clinical studies has shown that topical application of compound-Salvia injection can accelerate local blood perfusion and improve tissue hypoxia, which has a therapeutic effect on neonatal sclerosis.12 Studies have shown that topical application of Salvia miltiorrhiza essential oil can promote WH by modulating inflammatory factors, growth factors, and antioxidant properties.13 We found that topical application of RSM has good efficacy against burns14 and skin wounds,15 but the specific mechanism of action is not clear.
We investigated the effects of topical application of RSM on burns with regard to WH promotion. We measured the amount of blood perfusion, level of proinflammatory factors in serum, expression of growth factors, as well as examining local skin histopathology.
Materials and Methods
Materials
RSM was added to water (10× volume and 8× volume) and decocted twice. The filtrate was combined in a container and concentrated to contain 1.5 g/mL and 0.75 g/mL of raw herbs at 70°C, which were used high- and low-dose groups, respectively.
The content of salvianolic acid B in drugs was determined by ultrahigh pressure liquid chromatography (UHPLC). We used the UltiMate™ 3000 system (Dionex, Thermo, CA, USA). We employed a C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase was acetonitrile–0.1% phosphoric acid solution (22:78 ratio). The column temperature was 20°C. The flow rate was 1.2 mL/min. The detection wavelength was 286 nm.
The standard curve of salvianolic acid B was made by using external standards. The content of salvianolic acid B in samples was calculated, which showed that the RSM content at the high dose was 15.84 mg/mL, and the RSM content at the low dose was 6.85 mg/mL.
Animal Model
The protocol for animal experiments was approved (DWLL2018030008) by the Animal Use and Care Committee of Henan University of Chinese Medicine (Henan, China).
Male and female Sprague–Dawley rats (200~220 g) were obtained from Jinan Pengyue Experimental Animal Breeding (certificate of conformity of laboratory animals: 3700200016069; Shandong, China). Rats were fed for 7 days before experimentation. The temperature and relative humidity of the breeding environment was 22±2°C and 50%–60%, respectively.
Twelve rats were selected randomly as a blank group (BG). The remaining rats were prepared as burn models according to methods described previously.16 An area of fur ~25 cm2 was removed from the back of each rat. The next day, 2% sodium pentobarbital (45 mg/kg, i.p.) was used for the induction of anesthesia. Bare skin was scalded at 65°C for 10s with a burn instrument (YLS-5Q, Yiyan Technology, Jinan, China; scalding head diameter = 3.5 cm; pressure = 0.5 kg), the burn area was 9.6 cm2 (equivalent to a shallow second-degree burn).
Grouping and Administration
After preparation of the burn model, rats were divided into a model group (MG), positive control group (treated with Jinwanhong ointment and called the Jinwanhong group (JWHG)), RSM high-dose group (RSMHG), and RSM low-dose group (RSMLG). Twelve rats were in each group. Treatment started on the first day of modeling (6 h after preparation of the burn model).
Rats in the BG were not processed further. Rats in the MG were treated with an equal volume of physiologic (0.9%) saline; saline was adsorbed with an antiseptic sponge and applied to the wound surface. The JWHG was treated with Jinwanhong ointment (0.1 mg/cm2). In the RSMHG and RSMLG, the volume applied was 0.5 mL/cm2, and the treatment method was identical to that of the MG: keep the drug in contact with burn tissue (BT) for 6 h, remove the dressing after 6 h, and wash the medicated area with saline. Treatment was undertaken once a day for 12 days.
Observation of the Burn Area
During the treatment period, wounds were observed for color, swelling, inflammatory exudation, infection, crusting, and debridement. On day 12 of treatment, wounds were graded according to the area of crust shedding: I, >90%; II, 60%–90%; III, 30%–60%; IV, <30%.
Measurement of the Burn Area
The burns incurred by rats were photographed with a digital camera (focal length = 28 mm; object distance = 5 cm) pretreatment as well as on days 6 and 12 of treatment. The area of the burn wound was calculated using an image-analysis system (ImageJ, US National Institutes of Health, Bethesda, MD, USA). Percent WH for each group of rats after 12 days of treatment was calculated using the following formula:
Microcirculation Testing
Blood perfusion in local skin tissue was detected by a scattered full-frame, real-time scanning imaging system pretreatment as well as on days 6 and 12 of treatment. The detection height was 15 cm.
Biochemical Indices
Rats in each group were anesthetized by intraperitoneal injection. Two hours after the final treatment of agent, blood was collected from the abdominal aorta. After centrifugation (1328× g, 10 min, 4°C), plasma and serum were separated, and the latter stored at −80°C. Levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, transforming growth factor (TGF)-β, epidermal growth factor (EGF), and hydroxyproline (Hyp) in serum were determined by enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer instructions.
Immunohistochemical Analyses
After the final treatment, the skin lesion was cut, fixed in 10% formaldehyde solution, and embedded in paraffin. It was cut into sections of thickness 5 μm. Then, immunohistochemical staining was undertaken. Expression of basic fibroblast growth factor (bFGF), TGF-β1, EGF, and insulin-like growth factor (IGF)-1 in each group was measured.
Histomorphology
After the final treatment, the skin lesion was cut, fixed in 10% formaldehyde solution, and embedded in paraffin. It was cut into sections of thickness 5 μm. Then, staining with hematoxylin and eosin was carried out. The changes in histomorphology in rats of each group were observed under a light microscope (BX63, OLYMPUS, TOKYO, JAPAN).
Measurement of Levels of Tanshinone IIA and Salvianolic Acid B in Tissue Samples
First, biological samples were prepared. The skin of BT, subcutaneous tissue, as well as muscle, kidney, and liver tissues were homogenized by pre-cooled 0.9% saline (ratio of tissue:0.9% saline = 1:3). Tissue homogenates were prepared by pulverization with a tissue grinder, and centrifugation (1328× g, 10 min, 4°C) enabled separation of homogenates, which were stored at −80°C. The content of tanshinone IIA and salvianolic acid B in tissue homogenates (skin, subcutaneous, muscle, kidney, liver) as well as blood was determined by ultrahigh pressure liquid chromatography–linear ion trap–Orbitrap mass spectrometry (UHPLC–LTQ–Orbitrap-MS).
Next, biological samples were processed. Briefly, 100 μL of the sample was added to methanol (1:3 ratio). After vortex-mixing and centrifugation (10,845 × g, 10 min, 4°C), the supernatant was removed and freeze-dried at low temperature. Next, methanol (100 μL) was added, followed by re-solubilization and centrifugation (10,845 × g, 10 min, 4°C). The supernatant was removed for testing.
Next, standard solutions were prepared. Briefly, a standard of tanshinone IIA was placed in a brown measuring bottle. Methanol was added to create a standard solution of 20 μg/mL. We weighed the standard of salvianolic acid B precisely. Then, we added a mixture of methanol and water (8:2) to make a standard solution of salvianolic acid B of 0.1 mg/mL.
UHPLC–LTQ–Orbitrap-MS was undertaken using a Thermo Fisher Scientific (Waltham, MA, USA) system. UHPLC–LTQ–Orbitrap-MS was used to determine the content of tanshinone IIA and salvianolic acid B in tissue samples. An Acclaim™ RSLC 120 C18 column was employed. Mobile phase A was acetonitrile. Mobile phase B was 0.1% aqueous formic acid. The column temperature was 30°C. The flow velocity was 0.3 mL/min. The injection volume was 1 µL.
For MS, the ion source was ESI. Positive-ion mode was used for detection. The scan range was m/z 100–1000. The ion temperature was 80°C. The capillary voltage was 2.8 kV. The cone hole voltage was 40 V. The gas flow rate was 500 L/h. The conditions for gradient elution are shown in Table 1.
 |
Table 1 Gradient Elution Condition |
Statistical Analyses
Data are the mean ± SD. Statistical analyses were carried out using one-way analysis of variance by SPSS 21.0 (IBM, Armonk, NY, USA). The Ridit test was used for between-group analyses. P < 0.05 was considered significant. ImageJ was used to analyze the burn area and immunohistochemical results. Data-processing software (Xcalibur 3.0 and Mass Frontier 7.0) was employed for the analysis and processing of UHPLC–LTQ–Orbitrap-MS results.
Results
Crusting
After model rats incurred burns on their back, the color of the trauma surface was white and edema was clearly visible. The trauma surface began to turn red and edema was most obvious 6 h after the burn had been incurred. At days 3–4 of treatment, the burn area began to scab gradually, and inflammatory exudation and infection improved.
At 5 days after treatment, three rats in the JWHG and one rat in the RSMHG started to show upward curling and curling at the edge of the crust, but this phenomenon was not seen in the other groups.
At 7–8 days after treatment, one rat in the MG showed inflammatory exudation. Rats in the JWHG and RSMHG started to show shedding of the crust, and RSMLG rats started to show upward curling and curling of crust edges. At 12 days after treatment, the crusts fell off to different degrees in all groups (Table 2).
 |
Table 2 Effects on Apparent Scores of Rats in Burn Injury Model |
Burn Area
The burn area of MG rats increased significantly compared with that in the BG (P < 0.01) (Figure 1), indicating that the burn model had been replicated. On the first day of treatment, there was no significant difference in the burn area of each group compared with that of the MG (Figure 1a and 1b), indicating that the burn rats were grouped rational. Compared with rats in the MG, rats in the JWHG, RSMHG, and RSMLG had a significantly reduced burn area on days 6 and 12 of treatment (P < 0.01) (Figure 1a, c and d), indicating a potent therapeutic effect on rats who had suffered burns. Compared with the MG, we found significantly increased percent WH of burns in the JWHG, RSMHG, and RSMLG after 12 days of treatment (Figure 1a and e).
Blood Perfusion in BT
The average blood-perfusion volume of the burned surface in MG rats was significantly lower than that of rats in the BG (P < 0.01) (Figure 2), which indicated that the burn model affected the blood circulation in local skin tissue. On the first day of treatment, there was no significant difference in BT perfusion in each group compared with that in the MG (Figure 2a and 2b). The JWHG had significantly increased blood perfusion in BT on days 6 and 12 of treatment compared with that in the MG (P < 0.01) (Figure 2a, c and d). On day 6 of treatment, the RSMHG had significantly increased blood perfusion in BT (P < 0.05), and the RSMLG tended to have increased blood perfusion. On day 12 of treatment, the RSMHG had significantly increased blood perfusion (P < 0.01), as did the RSMLG (P < 0.05). These data indicated that RSM treatment could improve the blood circulation of burned skin tissue.
Biochemical Indicators in Serum
Serum levels of TNF-α, IL-1, and IL-6 were increased significantly in MG rats compared with those in BG rats (P < 0.01) (Figure 3a–c), which suggested that burns caused significant inflammatory symptoms. Compared with the MG, the JWHG, RSMHG, and RSMLG showed significantly reduced serum levels of TNF-α, IL-1, and IL-6 after 12 days of treatment (P < 0.01), indicating that RSM therapy could reduce the inflammatory response of rats who had suffered burns.
Compared with BG rats, the serum levels of hydroxyproline, EGF, and TGF-β were significantly lower in MG rats (P < 0.01) (Figure 3d–f). Compared with the MG, the JWHG, RSMHG, and RSMLG had significantly increased levels of Hyp, EGF, and TGF-β in serum 12 days after treatment (P < 0.01). This result indicated that RSM could promote the release of growth factors and collagen production to different degrees, promote the growth and proliferation of tissue cells, and aid WH.
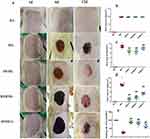
Changes in Histopathology
The skin of rats in the BG showed a neatly arranged squamous epithelium with 2–3 layers of cells, skin appendages were intact, and collagen fibers (CFs) and blood vessels were distributed evenly (Figure 4a). In MG rats, a large amount of inflamed necrotic tissue was seen in BT, the number of CF bundles and fibroblasts was low, and new granulation tissue was seen in some rats. In JWHG rats, a large amount of new granulation tissue was visible, the epithelium was covered (or four-fifths covered), the number of CF bundles and fibroblasts was increased, and the number of inflammatory cells reduced. In RSMHG rats, epidermal defects were repaired, formation of granulation tissue increased, infiltration of new epithelial cells was seen at the edge of the injury, epithelial cover was about three-fifths, and the number of CF bundles and fibroblasts increased. In RSMLG rats, there was formation of new granulation tissue, infiltration of new epithelial cells was seen at the edge of the injury, epithelial cover was about two-fifths, and the number of CF bundles and fibroblasts was increased slightly.
According to the Ridit test, compared with the BG, MG rats had significant histopathologic changes in skin lesions (P < 0.01) (Figure 4b and c). Compared with the MG, skin-tissue necrosis and the number of inflammatory cells were reduced significantly in the JWHG, RSMHG, and RSMLG. Also, the extent of epithelial coverage of BT was increased significantly, CF formation increased, and skin-tissue damage was reduced significantly (P < 0.01), which promoted WH.
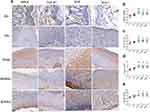
Protein Expression of Growth Factors
Compared with the BG, protein expression of bFGF, TGF-β1, EGF, and IGF-1 in the local skin tissues of MG rats was increased to different extents (Figure 5), which indicated an increase in local release of growth factors in rats who had suffered burns. Compared with the MG, the JWHG, RSMHG, and RSMLG had increased protein expression of bFGF, TGF-β1, EGF, and IGF-1 in local skin tissue 12 days after treatment (P < 0.01, P < 0.05). These data suggested that RSM treatment could promote the release of growth factors in skin tissues at the burn site to different degrees, promote the growth and proliferation of tissue cells, and aid WH.
Absorption of Tanshinone IIA and Salvianolic Acid B in Different Tissues
UHPLC–LTQ–Orbitrap-MS showed that the retention time of the standard of salvianolic acid B was 7.29, m/z was 736.1872, and molecular formula was C36H34O16[M + NH4] (Figure 6a, c and d). The corresponding ion peaks were extracted for the skin, subcutaneous tissue, corresponding muscle of subcutaneous tissue, serum, liver tissue, and kidney tissue of the BG, RSMHG, and RSMLG. None of the corresponding ion peaks appeared at the positions of the standards in the BG. The homogenates of skin, subcutaneous tissue, and muscle in the RSMHG and RSMLG showed the corresponding ion peaks, whereas those for the serum, liver, and kidney did not. The prediction of relative content based on peak area suggested that, in the RSMHG, the average peak area of skin tissue, subcutaneous tissue, and muscle tissue was 2,942,265, 157,522, and 87,218 whereas, in the RSMLG, it was 1,364,709, 16,996, and 16,412, respectively.
UHPLC-LTQ-Orbitrap-MS showed that the retention time of the standard of tanshinone IIA was 19.75, m/z was 295.1324, and the molecular formula was C19H18O3[M + H] (Figure 6b, e and f). The corresponding ion peaks were extracted for the skin, subcutaneous tissue, corresponding muscle of subcutaneous tissue, serum, liver tissue, and kidney tissue of the BG, RSMHG, and RSMLG. None of the corresponding ion peaks appeared at the positions of the standards in the BG. The skin homogenates in the RSMHG and RSMLG showed the corresponding ion peak, whereas those for subcutaneous tissue, muscle tissue, serum, liver tissue, and kidney tissue did not. In the RSMHG, the average peak area of homogenates of skin tissue was 868,683, and was 448,034 in the RSMLG.
Discussion
After a burn injury, the normal structure and function of the skin are destroyed, the defense function of the skin is damaged, nerve endings are freed from the body surface, many inflammatory factors are released from the burn surface, capillary dilation and permeability in traumatized tissue increase, and traumatized tissue is edematous, ischemic, and hypoxic. Healing of a burn wound involves complete and orderly repair by inflammatory cells, the extracellular matrix, and various tissues under regulation by the body.
Studies have shown that topical application of botanical agents can promote the healing of burn wounds thanks to regulation of the local microenvironment of the skin (micro-action, micro-circulation, micro-absorption). This involves anti-inflammatory and antibacterial actions, regulation of the immune function of the wound, improving local blood circulation, promoting the proliferation of fibroblasts, and influencing the release of growth factors.17,18
The skin is the largest organ of the body. It is the most vulnerable body-surface tissue to be damaged by burning. The healing of skin wounds is the key to the treatment of burns. Our study showed that RSM therapy could promote WH by calculating percent WH before as well as 6 and 12 days after treatment.
The microcirculation is composed of very small vessels that supply oxygen and energy to tissue cells and the transfer of metabolic waste.19 Microcirculatory disorders are important pathophysiologic changes caused by burns. After a burn, many inflammatory mediators are released. They cause damage to vascular endothelial cells, increase vascular permeability, and decrease the effective blood volume. These actions lead to decreased tissue perfusion and burn-site ischemia and hypoxia. Such phenomena result in progressive necrosis of BT, which hampers treatment and affect the prognosis,20 and may cause loss of macrocirculation–microcirculation coupling and progressive deterioration, leading to organ failure.21 Improving microcirculatory disorders is a key factor in promoting WH,22 maintaining hemodynamics,23 and improving the prognosis after burn treatment. It is also important for the clinical treatment of shock and septic shock after incurring a burn.24 We observed the effect of RSM therapy on improving the microcirculation in BT by detecting blood perfusion after a burn injury 6 days and 12 days after treatment. The average blood perfusion in rats 6 days and 12 days after treatment of a burn was increased significantly compared with that before treatment, and RSM improved the microcirculation significantly.
After a skin burn, the healing process first enters an inflammatory phase, where uncontrolled and excessive inflammation leads to tissue damage and delays healing.25 TNF-α, IL-1, and IL-6 play a leading part in the systemic inflammatory response. On the one hand, they interact with inflammatory cells to aggravate the local inflammatory response. On the other hand, they activate the cytokine cascade, which further induces the release of inflammatory mediators, leading to a severe systemic inflammatory response. The latter can lead to the development of irreversible multi-organ dysfunction syndrome.26 In the present study, serum levels of TNF-α, IL-1, and IL-6 were measured by ELISA on day 12 of treatment. The serum levels of these inflammatory factors were reduced significantly in the RSM group, which suggested that RSM therapy could promote the healing of burn wounds by reducing the levels of inflammatory factors.
WH is regulated by various growth factors. EGF is a potent and widely present growth factor in the body that stimulates the division and proliferation of different types of cells.27 It can reduce the inflammatory response of traumatized tissues, prevent infection, has an analgesic effect, and can accelerate WH. bFGF is a mitogenic factor of vascular endothelial cells and fibroblasts. It can promote the division and proliferation of cells, induce the proliferation of smooth muscle cells, increase the distribution of vascular endothelial cells, participate in neovascularization, accelerate formation of granulation tissue, enable repair of damaged endothelial cells, and promote WH.28 TGF-β is produced and released by lymphocytes, macrophages, and platelets. TGF-β1 has a very important regulatory role in WH. It can promote fibroblast production and synthesize a large amount of elastin, type-I collagen, and type-III collagen.29 IGF-1 can participate in regulation of the growth and metabolism of fibroblasts during WH, induce fibroblast proliferation, stimulate regeneration of connective tissue, and has a role in the final stage of WH.30 In the present study, serum levels of EGF and TGF-β were measured by ELISA. Protein expression of bFGF, TGF-β1, EGF, and IGF-1 in skin tissues was measured by immunohistochemical staining after 12 days of treatment. Compared with the model group, RSM treatment could increase levels of bFGF, TGF-β1, EGF, and IGF-1 in rats who had incurred burns. These data suggest that RSM could accelerate WH by promoting the release of growth factors.
Hydroxyproline is the main constituent of CFs in connective tissue. Hence, the amount of hydroxyproline is also crucial for WH.31,32 We measured the serum level of hydroxyproline by ELISA after 12 days of treatment. Compared with the model group, the RSM groups had a significantly increased hydroxyproline level, which suggests that RSM could promote WH by increasing collagen synthesis.
Studies have shown that, after topical application of botanical agents, they are absorbed in small amounts by local skin tissue. Such administration can relieve tissue adhesions, increase the local supply of blood and oxygen, accelerate the metabolism at the wound surface, and promote WH. We examined the absorption of tanshinone IIA and salvianolic acid B (representative components of RSM) in different types of wounds by UHPLC–LTQ–Orbitrap-MS. Salvianolic acid B was absorbed in small amounts in local skin, subcutaneous tissue, and muscle. Tanshinone IIA was absorbed in small amounts only in local skin. These data are inconsistent with the traditional perception that “drugs should be absorbed into blood”. This phenomenon could be because most botanical medicines are large-molecule compounds, which hampers skin penetration and entry into the blood circulation. Also, botanical medicines applied topically could exert an effect through direct action to regulate the skin microenvironment, which is consistent with our previous report.33
Conclusions
RSM treatment improved blood circulation in a burn wound on the skin. It had an anti-inflammation action, promoted the release of growth factors, accelerated the metabolism in the burn wound, improved the local skin microenvironment, and promoted WH.
Acknowledgments
We thank all the study participants, research staff, and students who contributed to this study. We thank The Charlesworth Group (https://www.cwauthors.com/) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Natural Science Project of China (82074038), the Postdoctoral Research project of Henan Province (202001053), the Henan Major Science and Technology Special Projects (201300310100), the Henan Province Key R&D Special Project (International Cooperation Category) (231111521200), the Henan Province Chinese Medicine “double first-class” to create a special project of scientific research (HSRP-DFCTCM-2023-7-01).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Elfawy LA, Ng CY, Amirrah IN, et al. Sustainable approach of functional biomaterials-tissue engineering for skin burn treatment: a comprehensive review. Pharmaceuticals. 2023;16(5):701. doi:10.3390/ph16050701
2. Kamolz LP, Hecker A. Molecular mechanisms related to burns, burn wound healing and scarring. Int J Mol Sci. 2023;24(10):8785. doi:10.3390/ijms24108785
3. Siddique R, Mehmood MH, Hussain L, et al. Role of medicinal herbs and phytochemicals in post burn management. Inflammopharmacology. 2023;31(4):1695–1714. doi:10.1007/s10787-023-01246-5
4. Rezai S, Rahzani K, Hekmatpou D, Rostami A. Effect of oral Calendula officinalis on second-degree burn wound healing. Scars Burn Heal. 2023;9:20595131221134053. doi:10.1177/20595131221134053
5. Pu X, Cao X, Liu H, Huang W, Zhang L, Jiang T. Isorhamnetin attenuates the proliferation, invasion, migration and fibrosis of keloid fibroblasts by targeting S1PR1. Exp Ther Med. 2023;26(1):310. doi:10.3892/etm.2023.12009
6. Salazar-Gomez A, Alonso-Castro AJ. Medicinal plants from latin America with wound healing activity: ethnomedicine, phytochemistry, preclinical and clinical studies-A review. Pharmaceuticals. 2022;15(9):1095. doi:10.3390/ph15091095
7. Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing--exploring medicinal plants of India. J Ethnopharmacol. 2007;114(2):103–113. doi:10.1016/j.jep.2007.08.010
8. Agyare C, Boakye YD, Bekoe EO, Hensel A, Dapaah SO, Appiah T. Review: African medicinal plants with wound healing properties. J Ethnopharmacol. 2016;177:85–100. doi:10.1016/j.jep.2015.11.008
9. Tian S, Miao M. Mechanism of external use of traditional Chinese medicine—“three micro-regulations for balance” based on neural-endocrine-immune network. Chin J Exper Trad Med Form. 2019;25(04):6–12.
10. XD ME, Cao YF, Che YY, et al. Danshen: a phytochemical and pharmacological overview. Chin J Nat Med. 2019;17(1):59–80. doi:10.1016/S1875-5364(19)30010-X
11. Bai M, Liu H, Mingsan M. Analysis of Salvia miltiorrhiza external application based on association rules and factor analysis. World Chin Med. 2021;16(21):3237–3240+3245.
12. Deng P, Wei H. Overview of clinical applications of Salvia preparations for neonatal sclerosis. J Mod Med Health. 2014;30(05):710–712.
13. Farahpour MR, Pirkhezr E, Ashrafian A, Sonboli A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed Pharmacother. 2020;128:110120. doi:10.1016/j.biopha.2020.110120
14. Liang X, Liang X, Miao M. Effect of water solution of Radix Salviae Militiorrhizae in external application on rat and mouse scald model. J Tradit Chin Med Pharm. 2013;28(01):56–59.
15. Liang X, Bai M, Yan X, Miao M. External curative effect of Salviae militiorrhizae Radix decoction on skin wounds. Chin J Mod Appl Pharm. 2013;30(05):486–490.
16. Miao M, Wang T, Tian S. Specifications for preparation of burn and scald injury models (draft). J Exper Trad Med Form. 2017;23(24):11–16. Chinese.
17. Zhao Q, Zhao X, Wu J. Research progress in the wound healing mechanisms of external use traditional Chinese medicine for burns. Med Recapit. 2014;20(16):3003–3005.
18. Cao L, Bai M, Miao M, Fang X, Miao Y. Explorated the therapeutic mechanism of Chinese material medica external application based on ‘double micro modulation balance’. J Tradit Chin Med Pharm. 2018;33(3):819–823.
19. Guven G, Hilty MP, Ince C. Microcirculation: physiology, pathophysiology, and clinical application. Blood Purif. 2020;49(1–2):143–150. doi:10.1159/000503775
20. Ling S, Yang L, Tao J, Wang X, Su X, Wang J. Improvement effect of allicin on microcirculation of deep II degree burn wounds in rats. Acta Chin Med. 2022;37(2):371–375.
21. Lucius J, Jensen JO, Tasar RR, et al. Acute microcirculatory effects of remote ischemic conditioning in superficial partial thickness burn wounds. J Burn Care Res. 2022;2022:1.
22. Zhang P, Zhang K. Effect of nourishing yin healing formula as an adjunct to the treatment of patients with severe burns and its effect on microcirculatory status. J Serv Theory Pract. 2022;35(20):3492–3494.
23. Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. doi:10.1186/cc14726
24. Huan J, Zhang L. Inspiration from the research advances in microcirculatory dysfunction to the treatment of burn shock and burn septic shock. Chin J Burns Trauma Repair. 2022;38(05):401–407.
25. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol. 2017;199(1):17–24. doi:10.4049/jimmunol.1700223
26. Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17(5):266–274. doi:10.1006/cyto.2001.1003
27. Xu L, Tong T. Regulation of cell growth and proliferation by epidermal growth factor. Prog Physiol Sci. 1988;19(1):36–38.
28. Mao W, Wang W, Chen Q, Liu L, Yan Z. Effects of Sheng Ji Yu Yang cream on the levels of basic fibroblast growth factor bFGF and epidermal growth factor EGF in chronic ulcer wounds of lower limbs. Lishizhen Med Mater Med Res. 2019;30(02):404–406.
29. Sun L, Zhu L, Yang S. Effect of negative pressure wound therapy on the expression of transforming growth factor-beta1 in granulation tissue with mild and moderate ischemia wound of diabetic foot. Chin J Injury Repair Wound Heal. 2019;14(01):20–25.
30. Yao C, Chen F. Expression of inflammation and fibrosis markers in the development of acne hypertrophic scar. Genomics Appl Biol. 2020;39(6):2890–2896.
31. Akcakaya A, Aydogdu I, Citgez B. Investigation into the optimal prosthetic material for wound healing of abdominal wall defects. Exp Ther Med. 2018;15(2):1622–1625. doi:10.3892/etm.2017.5551
32. Krishnappa P, Venkatarangaiah K, Shimoga Rajanna SK, Kayattukandy Balan R, Kayattukandy Balan R. Wound healing activity of Delonix elata stem bark extract and its isolated constituent quercetin-3-rhamnopyranosyl-(1-6) glucopyranoside in rats. J Pharm Anal. 2016;6(6):389–395. doi:10.1016/j.jpha.2016.05.001
33. Tian S, Xu E, Wu Y, Li M, Bai M, Miao M. Mechanism of the body surface: new thinking on the mechanism of traditional Chinese medicine external treatment. J Tradit Chin Med Pharm. 2021;36(08):4433–4438.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.