Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Qualitative Validation of COPD Evidenced Care Pathways in Japan, Canada, England, and Germany: Common Barriers to Optimal COPD Care
Authors Meiwald A , Gara-Adams R, Rowlandson A, Ma Y , Watz H, Ichinose M, Scullion J , Wilkinson T, Bhutani M, Weston G, Adams EJ
Received 3 February 2022
Accepted for publication 9 June 2022
Published 1 July 2022 Volume 2022:17 Pages 1507—1521
DOI https://doi.org/10.2147/COPD.S360983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Anne Meiwald,1 Rupert Gara-Adams,1 Aleix Rowlandson,1 Yixuan Ma,1 Henrik Watz,2 Masakazu Ichinose,3 Jane Scullion,4 Tom Wilkinson,5,6 Mohit Bhutani,7 Georgie Weston,1 Elisabeth J Adams1
1Aquarius Population Health, London, UK; 2Pulmonary Research Institute, LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Grosshansdorf, Schleswig-Holstein, Germany; 3Academic Center, Osaki Citizen Hospital, Miyagi, Japan; 4Bush & Co, Northamptonshire, UK; 5Faculty of Medicine, Southampton University, Southampton, Hampshire, UK; 6Respiratory and Allergy, NIHR Southampton Biomedical Research Centre, Southampton, Hampshire, UK; 7Department of Medicine, University of Alberta, Edmonton, Alberta, Canada
Correspondence: Elisabeth J Adams, Aquarius Population Health, Unit 29 Tileyard Studios, London, N7 9AH, UK, Tel +44 (0)207 993 2930, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. A comprehensive and detailed understanding of COPD care pathways from pre-diagnosis to acute care is required to understand the common barriers to optimal COPD care across diverse health systems.
Methods: Country-specific COPD care pathways were created for four high-income countries using international recommendations and country-specific guidelines, then populated with published epidemiological, clinical, and economic data. To refine and validate the pathways, semi-structured interviews using pre-prepared discussion guides and country-specific pathway maps were held with twenty-four primary and secondary care respiratory healthcare professionals. Thematic analysis was then performed on the interview transcripts.
Results: The COPD care pathway showed broad consistency across the countries. Three key themes relating to barriers in optimal COPD management were identified across the countries: journey to diagnosis, treatment, and the impact of COVID-19. Common barriers included presentation to healthcare with advanced COPD, low COPD consideration, and sub-optimal acute and chronic disease management. COVID-19 has negatively impacted disease management across the pathway but presents opportunities to retain virtual consultations. Structural factors such as insurance and short duration of appointments also impacted the diagnosis and management of COPD.
Conclusion: COPD is an important public health issue that needs urgent prioritization. The use of Evidenced Care Pathways with decision-makers can facilitate evidence-based decision making on interventions and policies to improve care and outcomes for patients and reduce unnecessary resource use and associated costs for the healthcare provider/payer.
Keywords: health policy, COPD management, COPD diagnosis, exacerbations, qualitative, pathway mapping
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable, progressive respiratory disease, characterized by irreversible airflow limitation and symptoms such as dyspnea, persistent cough, and sputum production. COPD was the third leading cause of mortality worldwide in 2019,1 responsible for 3.23 million deaths.2 Exacerbations, defined as ‘periods of acute worsening of respiratory symptoms’,3 are responsible for much of the morbidity and mortality, by accelerating decline in lung function and causing a reduction in health-related quality of life (HRQoL).4
COPD affects approximately 384 million people globally5 and is associated with significant resource burden. In the United States alone, the combined estimated direct and indirect costs of COPD are $52 billion6 and in Japan $6.8 billion.7 Despite this, chronic respiratory diseases, including COPD, often receive less funding and attention than other major causes of global morbidity and mortality.8,9
Although there are international recommendations for treatment (Global Initiative for Chronic Obstructive Lung Disease; GOLD),3 it is unclear how care is currently delivered within individual countries. No previous studies offer comprehensive mapping of the entire care pathway informed by both qualitative and quantitative data. Previous studies have focused on specific components of the COPD pathway (particularly inpatient or prescription pathways) or offered descriptive pathway overviews without quantitative data.10–12
To gain a comprehensive understanding of the full care pathway and barriers to optimal COPD care (including the impact of COVID-19) across different countries, national COPD care pathways in four countries (Japan, Canada, England and Germany) were developed. These four high-income countries are geographically diverse, with different, although well-structured, healthcare structures, and mapping their care pathways could be an important step in understanding barriers to optimal management. Improved understanding of the common barriers could inform international approaches to improving care standards and patient outcomes.
Materials and Methods
Quantitative data from published sources were collected in a targeted literature review and informed the focus of the questions used in the semi-structured interviews. Qualitative data were analyzed thematically and used to validate and supplement the findings from the literature. Consolidated criteria for reporting qualitative studies (COREQ) were followed.13
Research Team and Reflexivity
Information on the research team and reflexivity are found in Table 1.
 |
Table 1 Report on the Accordance with the COREQ Checklist for Domain 1: Research Team and Reflexivity |
Participant Selection
Respiratory healthcare professionals (HCPs) directly involved in the care of adults with COPD in the four countries were selected using purposive sampling. One clinician from Germany was recruited via snowball sampling. To ensure all aspects of COPD care were considered, HCPs were recruited from a range of professions within primary (general practice and community services) and secondary care (hospitals and specialists). This included specialist nurses, general practitioners (GPs), respiratory therapists, and respiratory specialists. Twenty-four HCPs (six from each country) were interviewed. Potential interviewees were contacted via email.
Data Collection
The research was conducted in two phases (phase one: initial qualitative interviews with HCPs and collection of published data; phase two: validation exercise) (Table 2). These phases enabled the development of representative pathways depicting the steps involved in COPD care and identifying the barriers to optimal care from the perspective of clinical experts in each country. The interviewing followed an iterative process in both phases, with questions amended (using field notes) to refine and validate the pathway and capture emerging themes.
 |
Table 2 Details on the Time Period and the Interviewer/Facilitator Involved in Each Phase |
Phase One
A global pathway framework for all countries was developed using the GOLD recommendations3 and national guidelines.14–17 Evidence was collated (June-August 2020) to identify published epidemiological, clinical and economic data specific to each country. PubMed and grey literature (conference abstracts, HTA reports, publicly available national databases reporting on COPD outcomes) were searched, and reference lists of relevant papers were reviewed (Appendix A). Country-specific COPD data were extracted. No formal assessment of data quality was undertaken, instead expert opinion was used to select the most appropriate and recent sources to quickly establish a framework for developing further understanding. Published data (eg, which HCPs patients were seeing and where), were used to adapt the GOLD pathway framework to create country-specific COPD pathways.
An interview discussion guide (Appendix B) was developed, informed by gaps in the quantitative data, to facilitate HCPs to critically review the pathways and the supporting quantitative data.
Phase Two – Validation Exercise
Phase two was used to validate the pathways developed and barriers identified in phase one, and identified further barriers. The discussion guide was refined from phase one interviews (Appendix C) to ensure additional questions were included.
Interview Process
Interviews were conducted and recorded in English using Microsoft Teams. Informed consent was assumed by participants’ agreement and completion of the interview. An interpreter was used when required (n=7). Across all interviews, two researchers and the participant were present plus an interpreter if required. Interviewees received an honorarium to compensate them for their time upon completion of the interview. No repeat interviews were conducted. The interviews were transcribed verbatim. Transcripts were not returned to participants for comment and/or correction. Where interpreters were involved in the interviews, only English translations were transcribed.
Data Analysis
A thematic analysis was conducted using NVivo (version 11; QRS International) following the approach recommended by Braun and Clarke.18 All interviews were analyzed. A deductive approach was taken, with the discussion guide and COPD care pathways providing predetermined themes expected to be reflected in the data, including pre-diagnosis, diagnosis, treatment and management, management of exacerbations and COVID-19 (Appendix D). Additional themes were generated through the coding process. AM and AR analyzed the transcripts. Transcripts were coded independently, with 20% of transcripts (N=5) double coded to ensure consistency. Any discrepancies between coders were resolved through comparison and discussion. Four participants provided feedback on the findings.
Ethics
Given the nature of the project, the work was considered a service evaluation, and no ethics approval or review was required. No patient-specific information was discussed or collected, and no medical intervention was undertaken. All information collected was handled in compliance with relevant European Union General Data Protection Regulation legislation; results were anonymized before reporting.
Results
Thirty-three HCPs were approached and 24 were interviewed, 9 in Phase 1 and 15 in Phase 2 (Appendix E). Interview length ranged from 82–112 minutes. Four HCPs declined due to COVID-19 workload and five did not provide a reason for non-participation. Six participants from Japan and one from Germany required an interpreter.
COPD Care Pathway Mapping
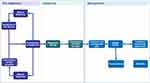
The COPD care pathway showed broad consistency across the countries (Figure 1). However, some differences were noted including variation in the specialty of HCPs via whom patients entered the pathway, diagnostic testing used in primary and secondary care, and length of hospital stay (Appendix F). Full details of each pathway and the supporting quantitative evidence are available in Appendix G–J.
Key Themes
Three themes emerged (Table 3), providing a view of common challenges to optimal COPD care in high-income countries:
- Journey to diagnosis
- Treatment and management
- Impact of COVID-19
 |
Table 3 The Themes and Sub-Themes That Describe the Barriers to Optimal COPD Care Across Japan, Canada, England, and Germany |
Structural Factors
Within these respective themes, common structural factors were observed to have an impact on the delivery of care across the pathway (supporting quotes found in Table 4; Appendix K). Insurance and reimbursement were considered a barrier to the provision of quality COPD care in Japan, Canada, and Germany, particularly affecting access to pharmacological medication.
 |
Table 4 Qualitative Evidence to Support Common Structural Differences |
In all four countries rural communities were described as experiencing challenges due to limited access to healthcare services. HCPs felt this impacted patients’ access to earlier diagnosis, regular monitoring, and urgent treatment of acute exacerbations.
Short appointment duration, especially in primary care, was felt to increase the difficulty of diagnosing complex chronic diseases such as COPD; limiting the amount of time for spirometry and adequate treatment reviews – including regular inhaler technique checks and assessment of COPD severity via questionnaires such as the COPD Assessment Test.19
Uniquely in this sample, in Germany, COPD patients can enroll in a disease management program (DMP) described as an “integral approach of disease management, stressing a general practitioner (GP) based coordination of care” for chronic conditions.20 However, there was a lack of consensus amongst HCPs on its effectiveness, while opinions appeared dependent on whether the HCP was enrolled in the DMP program or not.
Journey to Diagnosis
Patients not presenting to healthcare when they have symptoms, or being misdiagnosed due to poor-quality spirometry, pose challenges to patient diagnosis (Table 5; Appendix K).
 |
Table 5 Qualitative Evidence for the Subthemes Within the Theme: Journey to Diagnosis |
Low Consideration of COPD by Patients (HCP Reported)
HCPs believed that general awareness of COPD is poor. Several factors prevent patients from presenting to healthcare: 1) believing COPD symptoms are due to age rather than disease, 2) smoking stigma associated with COPD, and 3) believing COPD is untreatable. Reportedly, patients “adapt” to their disease until they cannot cope any longer often presenting with advanced COPD, the trigger being an exacerbation.
Low Consideration of COPD by HCPs
This sample believed that the wider HCP community lacked interest in chronic respiratory diseases, and by extension lacked interest in becoming respiratory-specialized HCPs. They described COPD as a lower priority compared to chronic conditions such as diabetes and heart disease which receive a higher media profile. It was thought that many HCPs do not identify COPD due to poor awareness and training. Limited awareness that the COPD disease trajectory can be improved with available treatment means that HCPs may not prioritize the importance of prompt diagnosis and proper management.
Misdiagnosis Due to Poor Quality Diagnosis
Even for specialists, COPD is “difficult” to differentiate from asthma, viral infections, or common comorbidities such as heart disease because there are similarities in common presenting symptoms, such as cough and breathlessness. Combined with poor-quality spirometry this means that undiagnosed or misdiagnosed COPD is common, with patients often seen multiple times before receiving a correct diagnosis. In Canada, a study found that underdiagnosis and overdiagnosis in COPD are 5 times more common than correct diagnosis21 and in England 85% of patients with COPD visited a primary care physician for a respiratory-related reason in the 5 years preceding their COPD diagnosis, suggesting a missed opportunity for diagnosis.22
Poor Utilization of Spirometry
Underuse and “poor” utilization and interpretation of spirometry were identified by HCPs as major barriers to COPD diagnosis. A lack of training in performing good-quality spirometry was reported as the primary reason, along with time constraints and inadequate financial reimbursement. In Canada, only 56% of physician diagnosed COPD was confirmed with spirometry in primary care.23 A previous study found that the most frequently reported reason why primary care HCPs did not have a spirometer in Japan was “I do not need it”.24 It was suggested that dedicated diagnostic hubs or spirometry nurses who regularly perform spirometry would improve the use of spirometry. Patient experience of the procedure was also reported as a barrier.
Treatment and Management
Following diagnosis, patients are often managed with sub-optimal pharmacological and non-pharmacological treatments (Table 6; Appendix K).
 |
Table 6 Qualitative Evidence for the Subthemes Within the Theme: Treatment and Management |
Poor Utilization of Non-Pharmacological Treatments
HCPs felt that non-pharmacological interventions are consistently “neglected” although vaccinations, smoking cessation counselling, exercise therapy, pulmonary rehabilitation, dietary advice, and mental health support were described as “really important”.
HCPs cited reasons hindering the uptake of smoking cessation counselling including low patient motivation, wide variation in the type and effectiveness of interventions offered, and lack of time available to HCPs to provide smoking cessation counselling. In Germany, only 24% of patients with COPD who were current/former smokers had participated in smoking cessation counselling.25
Pulmonary rehabilitation was seen as underused or difficult to access, with patients not understanding the importance of physical activity, long waiting lists, and limited availability and capacity of facilities providing pulmonary rehabilitation. In Japanese hospitals, a pulmonary rehabilitation program was often not provided primarily due to having an “inadequate workforce”.26
Pharmacological Treatment is Often Inadequate or Inappropriate
A lack of regular inhaler technique assessments was described as “one of the biggest problems” by HCPs, as the benefits of medication can be reduced by poor inhaler technique and medication adherence. This was a particular concern in elderly patients. In Canada, 78% of patients believed they have good inhaler technique, compared to 35% of specialists who thought their patients had good inhaler technique27 and in Germany, 12% of patients never received an explanation in how to correctly use their inhalers.28
Although the rapid increase of inhalers per therapeutic class was seen as good, poor knowledge and understanding by HCPs of the available inhaler options was raised as an issue. HCPs reported that some HCPs will persist with familiar inhalers they learnt about at university or were shown by drug representatives. Time constraints in HCP appointments and lack of resources to provide training for patients were also reported.
Exacerbations Remain Unreported and Unrecognized
Exacerbations are a major cause of hospitalization. COPD is the second most common cause of emergency admission in England29 and inpatient hospitalizations in Canada.30 HCPs felt that patients have difficulty recognizing when to present to healthcare settings depending on the symptoms and severity of their exacerbation, and noted that exacerbations were often not reported at all or, if they were, reporting was delayed until the next treatment review.
An HCP’s perception of a patient’s willingness and confidence to carry out an action plan, (written plan detailing how patients should self-manage a deterioration in symptoms) will dictate whether an action plan will be provided. Some HCPs report that increasing a patient’s confidence in being able to self-manage could lead to reductions in exacerbation duration and hospitalizations.
Impact of COVID-19
No published information was found on COVID-19 and the impact on the COPD pathway specific to these four countries. Interviewees reported that COVID-19 has impacted the COPD pathway, accelerating existing trends such as poor utilization of spirometry and reduced access to non-pharmacological interventions. However, the shift to telehealth necessitated by the pandemic has provided opportunities for improving parts of the care pathway (Table 7).
 |
Table 7 Qualitative Evidence for the Subthemes Within the Theme: Impact of COVID-19 |
Increased Use of Telehealth
HCPs, especially in England and Canada, reported a rapid adoption of telehealth for routine COPD care (using telephone and online video conferencing platforms) to protect both patients and HCPs during the pandemic. HCPs indicated that there were opportunities to retain virtual care post-COVID for very severe, but stable, patients as it eases HCP access. However, interviewees voiced concerns about possible misdiagnoses due to remote consultations. They believed HCPs may miss subtle signs otherwise identifiable if physically present, or patients may be less forthcoming with symptoms as face-to-face consultation time builds trust and openness.
Impacts on Patient Health
HCPs reported a reduction in exacerbation frequency due to patients acquiring fewer infections due to shielding. However, other HCPs described a reluctance to present to healthcare as patients were “terrified” of catching COVID. Negative impacts on the patient psychological and physical well-being were also described.
Impacts on Diagnosis and Treatment and Management
HCPs reported an intensification of the issue of identifying and diagnosing COPD due to further reduced access to spirometry and symptomatic overlaps with COVID-19. Reduced access to pulmonary rehabilitation and fewer treatment reviews were also reported.
Discussion
Main Findings
The COPD care pathway was mapped in four countries and the barriers to optimal COPD care were identified using data from published studies and qualitative data gathered from HCP interviews. This work provides a comprehensive visual representation of the COPD care pathway currently available in Japan, Canada, England, and Germany, covering pre-diagnosis through to acute care. The common barriers to optimal COPD care identified across the four studied countries highlight the continued need for improved care provision.
Interpretation of Findings in Relation to Previous Work
This study reinforces findings from the work by Kayyali et al10 who explored the current COPD care pathways in five countries and HCPs perception of barriers and issues. However, this study offers a more granular view of the care options available to patients and identify key points along the pathway where barriers emerge.
The available published data varied across the countries which made it difficult to report and compare COPD burden. A standardized reporting framework or global quality standards could make it easier for countries to measure progress. The collected data showed that length of hospital stay was higher in Japan, thought to be due to the absence of home-care provision after discharge eg, Japanese patients remain in hospital until fully recovered.
This study confirmed that smoking stigma, lack of awareness of risk factors and symptoms in both patients and HCPs,31 and underuse of spirometry prevent optimal COPD care.23 HCPs indicated that spirometry was sub-optimal due to lack of time, access, poor-quality interpretation and training. Together this highlights that barriers preventing earlier COPD diagnoses exist on both the patient and HCP side.
Our results demonstrate that there is substantial opportunity to improve access to non-pharmacological interventions, such as non-pharmacological rehabilitation32 and smoking cessation, to impact symptom control and reduce the risk of deterioration. Regularly reinforcing inhaler technique for patients,33 as well as improving HCPs education on the selection of an appropriate inhaler will ensure patients receive the correct medication dosage. Optimizing non-pharmacological and pharmacological interventions will prevent exacerbations, which cause poorer HRQoL, faster disease progression, and generate a higher financial burden on healthcare services.4,34
Although this study focuses on reporting the barriers to optimal COPD care, COPD care has advanced enormously and there has been a move towards new care models, particularly integrated disease management (IDM) programs, as a solution to some of these barriers.35 In a region of Canada, an IDM known as Best Care COPD has been shown to improve patient outcomes.36,37 In Switzerland it been suggested that advanced practice nurses may also be able to play a key role within a multi-professional team in future models of care to coordinate patient care across sectors.38 Increased use of telehealth technologies for pulmonary rehabilitation or remote consultations, likely to have been accelerated by the COVID-19 pandemic, are also providing additional options for care models.39,40 There is a need for a multidisciplinary approach across all stages of the pathway, there is no point in diagnosing patients earlier if they then cannot be appropriately treated or access effective non-pharmacological interventions.
COVID-19 substantively changed how COPD patients behaved (which was expected due to the recommendations given to vulnerable patient groups) and how they were treated during the pandemic, although there is little published data to date. Wu et al41 found that HCPs and patients agreed that the adoption of telehealth could be appropriate for certain activities such as smoking cessation counselling or initiation of rescue packs. The reported reduction in frequency of hospitalization for exacerbations aligns with Alqahtani et al42 who reported a 50% reduction in hospital admissions for exacerbations during the pandemic. This could be because patients avoided hospital due to fear of COVID-19, as reported by HCPs here, although Alqahtani et al believed the reduction was due to social distancing and masks. Interestingly, it seems this reduction in frequency of hospitalization was also seen in patients with cardiovascular diseases, although again, the extent to which this reduction was driven by the interventions introduced during the pandemic or alternative explanations is unclear.43,44
Potential Implications for HCPs or Policymakers
This work highlights the need for strategies to optimize COPD care from the patient, HCP, and healthcare system perspectives. As the burden of COPD continues to grow,1 evidence from current care pathways can inform policy reform at an international and national level. In particular, understanding the gaps between guidelines and/or best practice and what is happening currently can indicate where change is needed. Campaigns are vital: to raise awareness of COPD for the general population and HCPs, education and training for HCPs to perform spirometry, ensuring sufficient appointment duration, and improving acute and chronic disease management using pharmacological and non-pharmacological interventions. Addressing these gaps will be important to allow for the implementation of future innovations in the management of COPD.
Further Research
Further research is required to fully characterize the COPD care pathways in several areas. Additional research including the patient perspective is vital, and could help further prioritize key barriers. Second, many patients with COPD have extensive comorbidities.45 As there is an association between comorbidities and exacerbations,46 further research could explore how the care of comorbidities links to the COPD care pathway and outcomes. Third, the long-term impacts on the COPD pathway due to COVID-19 are not yet measured. Fourth, the lack of published data for COPD in Japan meant increased reliance on HCP input. This suggests that better epidemiological and healthcare data are required to show where care needs improving. This work highlighted common issues in high-income countries, but future work should focus on low- and middle-income countries where over 80% of deaths caused by COPD occur.2 There is also a need to study the pathway for different groups and settings within countries, as it may vary, for example, by urbanicity or socioeconomic status. Finally, these care pathways could form the basis for future modelling work to explore the long-term health and financial outcomes of policy changes in these four countries.
Strengths and Limitations
A key strength of this work was the combination of detailed quantitative data with qualitative interviews which provided the opportunity to generate pathways shaped by both published research and current, in-field experience to better understand the factors affecting COPD management. Despite differences in health system infrastructure across the four countries, findings remained broadly consistent.
While a diverse variety of HCPs were sought, they may not represent the complete spectrum of care in each nation. Although all participants were offered financial compensation in an effort to increase and broaden the available pool of participants, participating HCPs may have a special interest in COPD, so the pathway may represent better practice than on average. 75% of the sample size were respiratory specialists, so the primary care pathway may need further validation. Most HCPs interviewed were from urban practices so they may not reflect healthcare in rural areas. Additionally, although experienced interpreters were used for interviews, using translated transcripts for data analysis carries the risk that phrases/ideas do not reflect their actual meaning. Availability of published data varied between countries, with particularly limited data from Japan. The durability of the results may be impacted if COVID-19 or updated GOLD recommendations cause future changes in the care pathway.
Conclusion
Evidenced Care Pathways can highlight and support interventions and policies to improve care and outcomes for patients and reduce unnecessary resource use and associated costs for the healthcare provider/payer. Our findings add to the evidence that COPD is an important public health issue that needs urgent prioritization to reduce exacerbations and premature mortality, especially considering the additional challenges of COVID-19.
Acknowledgments
We would like to thank all participants who contributed valuable insight into care for people with COPD including Dr Shawn Aaron, Dr Mustafa Abdo, Dr Kazuhisa Asai, Dr Amy Dewar, Madonna Ferrone, Dr Thomas Hering, Dr Steve Holmes, Dr Takeo Horie, Dr Frank Kanniess, Dr Gunther Öhlschläger, Dr Katsuya Onishi, Dr Rakesh Patel, Dr Murugesan Raja, Terry Robinson, Dr Wan Tan, and Dr Athanasios Xanthopoulos. Many thanks to Tam de Lacey for his help with the literature search and conducting two interviews. Thank you also to Krishnan Puri Sudhir for helping conduct two of the interviews and Charlotte Hamlyn-Williams for advice on the analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This was work conducted by Aquarius Population Health which was funded by AstraZeneca; however, the design, results and interpretation were generated independently by the authors. AstraZeneca was given the opportunity to review the manuscript for medical and scientific accuracy.
Disclosure
AM, RGA, YM, AR (at the time of the project), GW (at the time of the project), and EA work at Aquarius Population Health and have received consultancy fees from other pharmaceutical and MedTech companies unrelated to this work. Outside of the submitted work, TW reports receiving consulting fees and/or fees for attending lectures, meetings and conferences and/or travel expenses and/or research grants from My mhealth, AstraZeneca, GlaxoSmithKline, Synairgen, Bergenbio, UCB, UKRI, NIHR, Valneva, OM Pharma, Boehringer Ingelheim, Roche, Chiesi, Teva and Nutricia. TW is a founder, director and shareholder of My mhealth. TW has served on monitoring boards for trials sponsored by Synairgen and Valneva, and has applied for patents with GlaxoSmithKline and My mhealth. HW reports receiving consulting fees and/or fees/honoraria for attending/presenting lectures, meetings and conferences and/or travel expenses and/or grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Bayer, GlaxoSmithKline and Verona Pharma. HW has served on monitoring boards for AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Bayer and GlaxoSmithKline. JS reports receiving consulting fees and/or fees/honoraria for attending/presenting lectures, meetings and conferences from AstraZeneca, Chiesi, Teva and Mundipharma. JS has received payment for expert testimony from Bush and Co Ltd. MB reports receiving consulting fees and/or fees/honoraria for attending/presenting lectures, meetings and conferences and/or travel expenses and/or grants from AstraZeneca, GlaxoSmithKline, Pfizer, Sanofi, Covis, Grifols, Boehringer Ingelheim, Valeo, The Lung Association of Saskatchewan, Alberta Lung and NWT, CIHR, Alberta Innovates, Novartis and Mereo. MB has a leadership or fiduciary role in the Canadian Thoracic Society and Alberta Health Services. GW reports receiving grants or contracts from Adelphi Values PROVE. MI reports no conflicts of interest. No other conflicts of interest were declared.
References
1. World Health Oraganization. The top 10 causes of death; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. World Health Organization. Chronic obstructive pulmonary disease (COPD) fact sheet; 2021. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-copd.
3. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2020 Report; 2020.
4. Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6. doi:10.1016/j.ejim.2019.12.014
5. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta–analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05-020415
6. Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi:10.2147/CEOR.S34321
7. Nishimura S, Zaher C. Cost impact of COPD in Japan: opportunities and challenges? Respirology. 2004;9(4):466–473. doi:10.1111/j.1440-1843.2004.00617.x
8. Boehm A, Pizzini A, Sonnweber T, et al. Assessing global COPD awareness with Google Trends. Eur Respir J. 2019;53(6):6. doi:10.1183/13993003.00351-2019
9. Williams S, Sheikh A, Campbell H, et al. Respiratory research funding is inadequate, inequitable, and a missed opportunity. Lancet Respir Med. 2020;8(8):e67–e68. doi:10.1016/S2213-2600(20)30329-5
10. Kayyali R, Odeh B, Frerichs I, et al. COPD care delivery pathways in five European Union countries: mapping and health care professionals’ perceptions. Int J Chron Obstruct Pulmon Dis. 2016;11:2831–2838. doi:10.2147/COPD.S104136
11. Utens C, Maarse J, Schayck O, Van, Maesen B, Mölken MR, Van, Smeenk F. Care delivery pathways for chronic obstructive pulmonary disease in England and the Netherlands: a comparative study. Int J Integr Care. 2012;12(2). doi:10.5334/ijic.811
12. Quint JK, O’Leary C, Venerus A, et al. Prescribing pathways to triple therapy: a multi-country, retrospective observational study of adult patients with chronic obstructive pulmonary disease. Pulm Ther. 2020;6(2):333–350. doi:10.1007/s41030-020-00132-7
13. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi:10.1093/intqhc/mzm042
14. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Mech Meas Manage. 2007;14:28.
15. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2018. Available from: https://www.nice.org.uk/guidance/ng115.
16. Japanese Respiratory Society. Guidelines for the diagnosis and treatment of COPD (Chronic Obstructive Pulmonary Disease); 2010. Available from: https://www.jrs.or.jp/modules/english/index.php?content_id=15.
17. Vogelmeier C, Buhl R, Burghuber O, et al. Leitlinie zur Diagnostik und Therapie von Patienten mit chronisch obstruktiver Bronchitis und Lungenemphysem (COPD): herausgegeben von der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e. V. und der Deutschen Atemwegsliga e. V., unter Beteiligung der Österreichischen Gesellschaft für Pneumologie. Pneumologie. 2018;72(4):253–308. doi:10.1055/s-0043-125031
18. Braun V, Clarke V. Using thematic analysis in psychology. Null. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
19. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Leidy NK. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
20. Achelrod D, Welte T, Schreyögg J, Stargardt T. Costs and outcomes of the German disease management programme (DMP) for chronic obstructive pulmonary disease (COPD)—A large population-based cohort study. Health Policy. 2016;120(9):1029–1039. doi:10.1016/j.healthpol.2016.08.002
21. Gershon AS, Thiruchelvam D, Chapman KR, et al. Health services burden of undiagnosed and overdiagnosed COPD. CHEST. 2018;153(6):1336–1346. doi:10.1016/j.chest.2018.01.038
22. Johnson KM, Khakban A, Bryan S, Sin DD, Sadatsafavi M. Healthcare system encounters before COPD diagnosis: a registry-based longitudinal cohort study. Thorax. 2020;75(2):108–115. doi:10.1136/thoraxjnl-2019-213554
23. Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–19. doi:10.1155/2008/173904
24. Ryujin Y, Ogawa E, Nagao T, et al. Behavioral changes in general practitioners towards chronic obstructive pulmonary disease over five years: an observational study. Inter Med. 2015;54(14):1705–1710. doi:10.2169/internalmedicine.54.4170
25. Lutter JI, Lukas M, Schwarzkopf L, et al. Utilization and determinants of use of non-pharmacological interventions in COPD: results of the COSYCONET cohort. Respir Med. 2020;171:106087. doi:10.1016/j.rmed.2020.106087
26. Motegi T, Yamada K, Ishii T, Gemma A, Kida K. Long-term management of chronic obstructive pulmonary disease: a survey of collaboration among physicians involved in pulmonary rehabilitation in Japan. Respir Investig. 2012;50(3):98–103. doi:10.1016/j.resinv.2012.06.004
27. Hernandez P, Balter MS, Bourbeau J, Chan CK, Marciniuk DD, Walker SL. Canadian practice assessment in chronic obstructive pulmonary disease: respiratory specialist physician perception versus patient reality. Can Respir J. 2013;20(2):97–105. doi:10.1155/2013/369019
28. Hämmerlein A, Müller U, Schulz M. Pharmacist-led intervention study to improve inhalation technique in asthma and COPD patients: improvement of inhalation technique. J Eval Clin Pract. 2011;17(1):61–70. doi:10.1111/j.1365-2753.2010.01369.x
29. Resource impact report: Chronic obstructive pulmonary disease in over 16s: diagnosis and management (update) (NG115). Tools and resources chronic obstructive pulmonary disease in over 16s: diagnosis and management guidance NICE. Available from: https://www.nice.org.uk/guidance/ng115/resources/resource-impact-report-pdf-6602803741.
30. Canadian Institute for Health Information. Hospital stays in Canada. Available from: https://www.cihi.ca/en/hospital-stays-in-canada.
31. Lippiett KA, Richardson A, Myall M, Cummings A, May CR. Patients and informal caregivers’ experiences of burden of treatment in lung cancer and chronic obstructive pulmonary disease (COPD): a systematic review and synthesis of qualitative research. BMJ Open. 2019;9(2):e020515. doi:10.1136/bmjopen-2017-020515
32. Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: a report from the Canadian thoracic society COPD clinical assembly. Can Respir J. 1900;22:369851. doi:10.1155/2015/369851
33. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005
34. Bollmeier SG, Hartmann AP. Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am J Health Syst Pharm. 2020;77(4):259–268. doi:10.1093/ajhp/zxz306
35. Poot CC, Meijer E, Kruis AL, Smidt N, Chavannes NH, Honkoop PJ. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;9:CD009437. doi:10.1002/14651858.CD009437.pub3
36. Ferrone M, Masciantonio MG, Malus N, et al. The impact of integrated disease management in high-risk COPD patients in primary care. NPJ Prim Care Respir Med. 2019;29(1):1–9. doi:10.1038/s41533-019-0119-9
37. Sibbald SL, Misra V, daSilva M, Licskai C. A framework to support the progressive implementation of integrated team-based care for the management of COPD: a collective case study. BMC Health Serv Res. 2022;22(1):420. doi:10.1186/s12913-022-07785-x
38. Schmid-Mohler G, Clarenbach C, Brenner G, et al. Advanced nursing practice in COPD exacerbations: the solution for a gap in Switzerland? ERJ Open Res. 2020;6(2):00354–2019. doi:10.1183/23120541.00354-2019
39. Janjua S, Carter D, Threapleton CJ, Prigmore S, Disler RT. Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2021;(7). doi:10.1002/14651858.CD013196.pub2
40. Michaelchuk W, Oliveira A, Marzolini S, et al. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: a systematic review and meta-analysis. Int J Med Inform. 2022;162:104754. doi:10.1016/j.ijmedinf.2022.104754
41. Wu F, Burt J, Chowdhury T, et al. Specialty COPD care during COVID-19: patient and clinician perspectives on remote delivery. BMJ Open Respir Res. 2021;8(1):e000817. doi:10.1136/bmjresp-2020-000817
42. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0255659. doi:10.1371/journal.pone.0255659
43. Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi:10.1016/S0140-6736(20)31356-8
44. Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes. 2021;7(1):18–27. doi:10.1093/ehjqcco/qcaa084
45. Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig. 2015;53(6):249–258. doi:10.1016/j.resinv.2015.02.004
46. Westerik JAM, Metting EI, van Boven JFM, Tiersma W, Kocks JWH, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31. doi:10.1186/s12931-017-0512-2
47. Igarashi A, Fukuchi Y, Hirata K, et al. COPD uncovered: a cross-sectional study to assess the socioeconomic burden of COPD in Japan. Int J Chron Obstruct Pulmon Dis. 2018;13:2629–2641. doi:10.2147/COPD.S167476
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.