Back to Journals » Patient Related Outcome Measures » Volume 9
Psychological treatments for the management of postsurgical pain: a systematic review of randomized controlled trials
Authors Nicholls JL, Azam MA , Burns LC, Englesakis M , Sutherland AM, Weinrib AZ, Katz J , Clarke H
Received 24 June 2017
Accepted for publication 28 November 2017
Published 19 January 2018 Volume 2018:9 Pages 49—64
DOI https://doi.org/10.2147/PROM.S121251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Howland
Judith L Nicholls,1 Muhammad A Azam,1,2 Lindsay C Burns,1,2 Marina Englesakis,3 Ainsley M Sutherland,1 Aliza Z Weinrib,1,2 Joel Katz,1,2,4 Hance Clarke1,4
1Pain Research Unit, Department of Anesthesia and Pain Medicine, Toronto General Hospital, 2Department of Psychology, York University, 3Library and Information Services, University Health Network, 4Department of Anesthesia, University of Toronto, Toronto, ON, Canada
Background: Inadequately managed pain is a risk factor for chronic postsurgical pain (CPSP), a growing public health challenge. Multidisciplinary pain-management programs with psychological approaches, including cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT), and mindfulness-based psychotherapy, have shown efficacy as treatments for chronic pain, and show promise as timely interventions in the pre/perioperative periods for the management of PSP. We reviewed the literature to identify randomized controlled trials evaluating the efficacy of these psychotherapy approaches on pain-related surgical outcomes.
Materials and methods: We searched Medline, Medline-In-Process, Embase and Embase Classic, and PsycInfo to identify studies meeting our search criteria. After title and abstract review, selected articles were rated for risk of bias.
Results: Six papers based on five trials (four back surgery, one cardiac surgery) met our inclusion criteria. Four papers employed CBT and two CBT-physiotherapy variant; no ACT or mindfulness-based studies were identified. Considerable heterogeneity was observed in the timing and delivery of psychological interventions and length of follow-up (1 week to 2–3 years). Whereas pain-intensity reporting varied widely, pain disability was reported using consistent methods across papers. The majority of papers (four of six) reported reduced pain intensity, and all relevant papers (five of five) found improvements in pain disability. General limitations included lack of large-scale data and difficulties with blinding.
Conclusion: This systematic review provides preliminary evidence that CBT-based psychological interventions reduce PSP intensity and disability. Future research should further clarify the efficacy and optimal delivery of CBT and newer psychological approaches to PSP.
Keywords: postsurgical pain, CBT, acute pain, chronic pain, chronic postsurgical pain, multidisciplinary pain management
Introduction
Over the years, there have been significant developments in knowledge of and approach to pain management. Particularly as related to the treatment of chronic pain, it has been well established and documented in the literature that a multidisciplinary approach with an emphasis on psychosocial and movement interventions is associated with improved outcomes.1,2
Acute pain in the postoperative period presents a significant challenge, with potentially devastating long-term effects if pain is poorly managed. Severe postoperative pain can lead to decreased alveolar ventilation, atelectasis, and possible pulmonary consolidation.3 In addition, there are other systemic adverse effects, including tachycardia, hypertension, insomnia, and impaired wound healing.3 Poorly treated acute postoperative pain also increases the risk of developing chronic pain after surgery.4
Chronic postsurgical pain (CPSP) is defined as pain experienced due to a surgical procedure that persists beyond the expected time frame of recovery and cannot be explained by other biological causes, including a preexisting chronic pain disorder.5,6 CPSP develops much more commonly than expected, and its incidence has been reported to be between 10% and 70% of patients depending on surgery type.4,7 Many types of intraoperative and biopsychosocial factors play a role in the development of CPSP, including the type of surgery performed. Tissue or nerve damage as a result of surgical incisions is often unavoidable in most major surgical procedures, but a significant proportion of these cases do not heal within the time that acute PSP is expected to resolve (2–3 months postsurgery). In the aftermath of surgery, pain that was once a symptom of inflammation, neuropathy, or tissue healing becomes unremitting and pathological. Genetic predisposition8 and insidious processes, such as peripheral and central sensitization,9 constitute the biological mechanisms by which CPSP is thought to manifest. Other risk factors that can explain the transition from acute PSP to CPSP include psychological, environmental, and social factors. Specifically, such characteristics as fear-avoidance beliefs and catastrophic thinking, as well as psychiatric symptoms, including depression, anxiety, and posttraumatic stress, have been increasingly linked to the development of CPSP.4,10,11 The impact of CPSP is pervasive, causing significant suffering, global distress, and physical disability, which only add to the growing health care burden.
In the area of chronic pain management, psychological interventions have been shown to be effective in reducing psychological symptoms, disability, and even pain. Over time, there has been extensive research on hypnosis as a complementary technique in the management of PSP. The most recent meta-analysis in 2013 revealed positive treatment effects for pain, emotional distress, medication consumption, and recovery.12 For this reason, we endeavored to focus our systematic search on standardized psychotherapy and mindfulness-based protocols, as these have been less commonly explored in the context of PSP. Cognitive behavioral therapy (CBT) is one of the most common psychological interventions for pain management, and utilizes the concept that thoughts/cognitive processes, emotions, and behaviors are interconnected, and adaptive ways of thinking, feeling, and behaving can be achieved to help patients cope with chronic pain.13 CBT is linked with not only improvement in pain intensity but also mood and catastrophic thinking.14 Moreover, there is evidence for the effectiveness of CBT for specific pain conditions, including back pain, headache, and fibromyalgia.15 A recent meta-analysis demonstrated that mindfulness-meditation-based interventions are associated with decreases in pain intensity.2 Acceptance and commitment therapy (ACT) is a relatively newer psychological intervention being implemented in the chronic pain health care setting. ACT is based on behavioral principles and the psychological flexibility model,16 and unlike CBT, it does not emphasize the restructuring of distorted or catastrophic cognitions. When used as an adjunctive therapy in pain management, ACT fosters the possibility of improved pain acceptance,17 which can have important implications for adaptive recovery in postsurgical patients.
It is becoming more evident that behavioral interventions can serve as adjuncts to medical strategies to support the overall well-being of postsurgical patients.18 Given what is known about the development of CPSP and the promising evidence for psychological interventions in the treatment of chronic pain, there is likely a role for psychological interventions in preparation for surgery or during the recovery phase, when pain has yet to become pathological.18,19
The literature on the effectiveness of perioperative psychosocial interventions is quite limited. Additionally, it is unclear from the literature whether patients with specific characteristics and risk factors or undergoing specific surgeries may benefit more from early psychosocial intervention than in other situations. This systematic review seeks to assess and summarize the available evidence for psychological interventions on pain-related outcomes of surgery.
Materials and methods
Search strategy
Based on PRISMA guidelines,20 we searched the Medline (1946–February 24, 2017), Medline in Process (February 24, 2017), Embase and Embase Classic (1947–February 24, 2017), and PsycInfo (1806–February 24, 2017) databases using a combination of mapped medical subject-heading (MeSH) terms and keywords to identify all randomized controlled trials (RCTs) that evaluated the impact of specific psychological interventions on PSP outcomes. Our search terms focused on constructs of psychological interventions (eg, cognitive therapy, mindfulness, and ACT), pain (eg, chronic pain, back pain, central nervous system, and pain), and the postsurgical period (eg, postoperative). Our search strategy (Table S1) was designed in collaboration with an experienced research librarian (ME). Additional articles were identified through a hand search of relevant bibliographies.
Selection of papers
After removal of duplicates, two authors (JN and MAA) independently reviewed all titles and abstracts to identify papers that met our systematic review eligibility criteria: psychological intervention of CBT, ACT, or mindfulness-based psychotherapy; intervention initiating either prior to surgery or up to 2 months postsurgery; outcome of acute or CPSP; adult (aged ≥18 years) population; RCT study design; original research study; and published in English language. We excluded all secondary literature (eg, reviews and commentaries), non-peer-reviewed articles (eg, theses), and conference proceedings. All discrepancies were resolved via consensus. Following a manual search of relevant bibliographies to identify additional references that met our inclusion criteria, all remaining abstracts were forwarded for full-text review. Two authors (JN and MAA) independently reviewed full-text articles for inclusion on the basis of selection criteria, and remaining discrepancies were resolved via consensus.
Bias-risk assessment
To assess the risk of bias in each study that met inclusion criteria, two raters (MAA and LCB) independently employed the Cochrane risk-of-bias assessment tool21 to assess the degree of selection bias at the study level and performance and detection bias, attrition bias, and reporting bias at the outcome level. Each component was evaluated as indicating high, low, or unclear risk of bias according to Cochrane criteria.21 Any discrepancies in bias-risk ratings were resolved by an independent third rater (JN). An overview of bias risk across included papers is presented in Table 1.
  | Table 1 Risk-of-bias assessment Note: Used Cochrane risk-of-bias assessment tool. |
Data abstraction and synthesis
We used standardized data-abstraction forms to capture consistent data among raters. Three authors (JN, MAA, and LCB) independently abstracted data from each article, and any discrepancies in data capture were resolved via consensus. For all included papers, we extracted summary data on study characteristics, including citation, country, surgical population, sample size, age, sex, intervention and comparison arms, outcome definitions (Table 2), and specific results data, including analysis type (intent to treat [ITT] vs completers), timing of intervention, statistical analyses used, adjusted covariates, assessment time points, and key results (Table 3). Due to heterogeneity in interventions, statistical analyses, follow-up periods, and outcomes assessed, we were unable to undertake a quantitative meta-analysis.
Results
Literature-search results
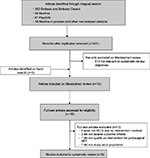
We identified 521 nonduplicate citations from our mapped search and an additional five citations through bibliography mining (Figure 1). Following title and abstract review, 510 articles were assessed and excluded for failure to meet eligibility criteria. Of the 16 articles forwarded for full-text review, an additional ten were excluded: five were not RCTs, one was not an intervention study, two failed to assess pain-related outcomes, one did not qualify as an intervention for PSP, and one was not based on an adult population. Overall, five studies met our inclusion criteria and were synthesized in this review. Risk-of-bias ratings for each study are presented in Table 1.
Overview of papers
We identified six papers22–27 based on five RCTs that assessed the impact of perioperative psychological interventions on PSP outcomes. Characteristics of included papers are summarized in Table 2 and extracted results displayed in Table 3.
Risk-of-bias assessment
Consensus ratings for risk of bias according to Cochrane criteria are shown in Table 1. In general, randomization and concealment of allocation were conducted well across trials, but the obvious nature of receiving psychological interventions posed a methodological challenge that precluded participant blinding to study arms across trials (T1–T6) and limited researcher blinding to study arms in five of six papers (T1 and T3–T6). The self-report nature of most outcomes in these papers made detection bias a related and inherent challenge. One paper (T4) partially overcame these expectation-related challenges by informing participants that they would be allocated to one of two common rehabilitation approaches whose efficacy had not yet been established. It is unclear whether researchers from other papers described their studies as superiority trials. Risks of bias in attrition in reporting were generally low across papers, with balanced losses to follow-up between study arms (in terms of both numbers and reasons for attrition) and comprehensive reporting of both significant and nonsignificant findings among all included papers. Published protocols were available for two of the five trials (T2 on ClinicalTrials.gov; T5/T6 on the ISRCTN registry), allowing verification of an a priori approach to data analysis and outcome reporting. Based on these registry data, it was apparent that T5 (a secondary analysis of T6) misreported their secondary outcome of acute postoperative pain as a primary outcome. Given that we did not require pain-related outcomes to be primary, this finding did not affect the study’s inclusion in the systematic review.
Context
Included trials were conducted in Sweden (T1), the US (T2 and T3), Italy (T4), and Denmark (T5 and T6), with sample sizes of 53–130 and males comprising 19%–83% of study samples. In terms of surgical populations, five studies included back-surgery patients (T1, T2, and T4–T6). Specifically, T1 and T4–T6 included patients who underwent spinal fusion and T2 included patients who underwent laminectomy surgery. One study included cardiac-surgery patients with comorbid major or minor depression (T3) (Table 2). Papers T1 and T2 specifically included (as part of the inclusion criteria) patients who experienced preexisting back pain or lower-extremity pain (sciatica). Papers T3, T5, and T6 included patients with spinal pathology whether or not there was preexisting back or lower-extremity pain and commented on the incidence of these in their study populations. T3 made no comment on the presence of preexisting chronic pain.
Interventions
For psychological interventions, four papers based on three RCTs used a CBT approach (T3–T6) and two used CBT integrated with physical therapy (T1 and T2). No RCTs that assessed ACT or mindfulness-based interventions on pain-related outcomes were found. Interventions were delivered postoperatively in four of the six studies (T1–T4), preoperatively in one study (T5), and both pre- and postoperatively in another research article based on the same RCT study (T6). Duration and delivery of psychotherapy interventions varied substantially among studies, ranging from three to eight sessions, from 30 minutes to 3 hours per session, delivered in person or by phone, in individual or group format, and were led by a nurse, physiotherapist, or psychologist (Table 2).
Outcomes
Pain interference and/or severity were primary outcomes in one study (T2) and secondary outcomes in five studies (T1, T3, T4, T5, and T6), whereas functional outcomes were primary in four studies (T1, T2, T4, T6) and secondary in one study (T5). All six studies used well-validated questionnaire-based outcome measures for pain, including the visual analog scale (VAS; T1), 11-point numeric rating scale (NRS; T4 and T5), the Brief Pain Inventory (BPI; T2 and T3), Short Form 36 – body pain scale (SF-36-BP; T4), and the Low-Back Pain Rating Scale (T6), and one study additionally measured pain indirectly based on postoperative consumption of rescue analgesics (T5). According to the IMMPACT core outcome measures for chronic pain, clinical trials T1–T5 utilized recommended measures for both pain and physical functioning.28 It is important to note that T6 was aimed more at evaluation of pain in the acute postoperative period. Five of the six studies reported on functional outcomes, including disability using the Oswestry Disability Index (ODI; T1, T2, T4, and T6), ambulation using the Cumulated Ambulation Score (T5), and mobility using the five-chair-stand test (T2), timed up-and-go (TUG) test (T2), and 10 m walk test (T2) (Table 2). One study (T1) included detailed information regarding participant recruitment and progress throughout the trial according to the CONSORT guidelines.29 This represents another core outcome measure in the IMMPACT recommendations. Additionally, only one study (T3) included a measure of emotional functioning: the Beck Depression Inventory.28 Timing of assessments for pain-related outcomes included the first postoperative week (acute recovery period) in one study (T5) and longer-term follow-up (chronic period) in the remaining studies, ranging from 2 months to 2–3 years (T1–T4 and T6) (Table 2).
Analyses
Five of the six included studies analyzed data according to ITT principles (T1, T2, and T4–T6), whereas one study limited their analysis to completers (ie, those for whom complete follow-up data were available; T3). Of these five studies that employed ITT, only T1 reported their method of imputing missing data. The T1 authors reported that they varied their imputation method according to reason for dropout; specifically, when dropout was unrelated to allocation, the mean value for their group was used, and when dropout was related to either increased pain or an absence of pain, the 10th and 90th percentile score was used, respectively. Parametric analyses were employed in four studies (T1–T4) and nonparametric analyses employed in two (T5, T6). Three of the six studies statistically adjusted for demographics, clinical variables, and pretreatment outcome measures (T1–T3). Among the three remaining studies with unadjusted analyses, all employed change in scores from baseline as outcome measurements, partially controlling for spurious imbalances in group allocation of pretreatment outcome scores (Tables 2 and 3).
Effects of CBT integrated with physiotherapy on pain-related outcomes
Two RCTs (T1 and T2; Table 2) evaluated the impact of postoperative CBT integrated with physiotherapy on pain-related outcomes among back-surgery patients. Of these, a Swedish study (T1, n=107) employed an intervention known as psychomotor therapy and an American study (T2, n=86) used an intervention known as cognitive behavior-based physical therapy (CBPT). These interventions appeared similar in content, with both involving elements of CBT, including behavioral self-management, cognitive restructuring, and relaxation training, integrated into their physiotherapy regimens. Treatment arms were comparable between studies, with both involving education and exercise support. In terms of delivery, both interventions were led by physiotherapists, but T1 offered three 90-minute in-person sessions at 3, 6, and 9 weeks postoperatively, whereas T2 offered six weekly 30-minute telephone sessions beginning 6 weeks after surgery. T1 outcomes were assessed at 3 months, 6 months, 1 year, and 2–3 years postsurgery with analyses adjusted for age and sex, whereas T2 assessed outcomes only up to 3 months after surgery and controlled for age, education level, comorbidities, physical therapy utilization, and pretreatment outcome scores. Data from both studies were analyzed according to ITT principles. Both RCTs included outcome measures for pain and function, the results of which are described in the following sections.
Pain outcomes
In the Swedish trial (T1), pain outcomes were measured on a VAS for back pain and between-group differences in pain-intensity reductions analyzed using repeated-measures (RM) analysis of covariance (ANCOVA). The authors found significantly greater pain reductions in the intervention arm at 3 and 6 months that became nonsignificant at 1 year, but again reached marginal significance at 2–3 years postsurgery. Absolute decreases in pain from baseline and difference in change in scores between groups were 29.9 mm (difference of 11.7 mm, effect sizes d=1.45 for psychomotor therapy vs d=0.98 for exercise therapy) at 3 months, 35.9 mm (difference of 9.9 mm, d=1.7 for psychomotor therapy vs d=1.29 for exercise therapy) at 6 months, and 39.2 mm (difference of 9.8 mm, d=1.34 for psychomotor therapy vs d=1.29 for exercise therapy) at 2–3 years. According to research examining cutoff values for clinical significance, the absolute decreases in VAS (≥35) at 6 months and 2–3 years would be considered clinically meaningful outcomes.30,31 RM ANOVA for between-group differences in the overall trend of pain reduction was also significant at the P<0.01 level (Table 3).
In the US trial (T2), pain outcomes were measured using the BPI subscales for back-pain intensity, leg-pain intensity, and pain-related interference with activities of daily living, and between-group differences in score reductions across measures were analyzed using RM ANOVA and multivariable linear regression. The authors found significantly greater reductions in all pain measures in the intervention arms at 3-month follow-up after surgery. In unadjusted analyses (RM ANOVA), absolute decreases in pain from baseline and differences in change in scores (on a 0–10 scale) between groups were 1.1 (difference of 0.88) for back-pain intensity (d=0.62), 1.3 (difference of 1.2) for leg-pain intensity (d=0.62), and 1.7 (difference of 1.5) for pain interference (d=0.72).
Similar results were obtained after multivariable adjustment, with effects remaining significant at the P<0.01 level and effect sizes of R2=0.64 for back pain, R2=0.45 for leg pain, and R2=0.49 for pain interference (Table 3). Generally, a 2-point reduction on the NRS is usually regarded as a clinically meaningful outcome.32
Functional outcomes
In the Swedish trial (T1), disability was measured using the ODI, and between-group differences in disability reductions were analyzed using RM ANCOVA. Between-group differences in the overall trend of disability reduction were significant. The authors found significantly greater reductions in disability scores in the intervention arm at all time points (ie, 3 months, 6 months, 1 year, and 2–3 years postsurgery) (Table 3). Absolute decreases in disability from baseline and differences in change in scores (0–100 ODI scale) between groups were 19.9 (difference of 9.7, d=1.2) at 3 months, 23.8 (difference of 10.7, d=1.32) at 6 months, 25.5 (difference of 11.1, d=1.39) at 1 year, and 24.9 (difference of 9.8, d=1.43) at 2–3 years.
The US trial (T2) also measured disability outcomes using the ODI in addition to performance-based mobility outcomes, including the five-chair-stand test, TUG test, and 10-m walk test. Between-group differences in score reductions across measures were analyzed. The authors found significantly greater reductions in ODI scores, five-chair-stand test, and marginally greater reductions in TUG and 10-m walk test scores in the intervention arm at 3 months postsurgery. In unadjusted analyses (RM ANOVA), absolute decreases in ODI and performance-based measures from baseline and differences in change in scores between groups were 17.3 (difference of 9.8 points, d=0.79) for ODI, 11.6 seconds (difference of 7 seconds, d=0.49) for the five-chair-stand test, 2.1 seconds (difference of 1.6 seconds, d=0.41) for the TUG test, and 0.20 m/s (difference of 0.1 m/s, d=0.41–0.49) for the 10 m walk test, with the intervention accounting for a substantial proportion of observed effect sizes. Similar results were obtained after multivariable adjustment, with all effects remaining significant at the P<0.05 level and effect sizes of R2=0.59 for the ODI, R2=0.52 for the five-chair-stand test, R2=0.62 for the TUG test, and R2=0.33 for the 10 m walk test (Table 3).
Effects of CBT on pain-related outcomes
Four studies based on three RCTs (T3–T6; Table 2) evaluated the impact of CBT on pain-related outcomes among cardiac (T3) and back (T4–T6)-surgery patients. Of these, a US study (T3, n=53) and an Italian study (T4, n=130) delivered eight 1-hour individual sessions in the postoperative period, led by a nurse and psychologist, respectively. The other two studies (T5 and T6) were based on a single Danish RCT (n=96) that delivered four 3-hour sessions of group-based CBT preoperatively and two sessions postoperatively. These sessions were led by a psychologist, occupational therapist, physiotherapist, social worker, spine surgeon, and former patient. T5 outcomes were assessed at 3 months, 6 months, and 1 year postoperatively, whereas T6 was a secondary analysis of T5, with outcomes assessed in the acute postoperative period (days 1–7 following surgery). As such, T5 analyses reflect outcomes of the preoperative intervention only, whereas T6 analyses reflect outcomes of the full pre- and postoperative CBT-intervention protocol.
Comparison arms varied among studies, with T3 employing usual care, T4 offering exercise support, T5 providing education, and T6 providing both education and exercise support. Three studies assessed outcomes of surgery at 2 months and beyond, with T3 measuring outcomes at 2 months, T4 at 1 year, and T6 at 3 months, 6 months, and 1 year postoperatively, whereas one study (T5) assessed outcomes during the first postoperative week. Only one study (T3) adjusted for covariates, including age, sex, marital status, minority status, employment, body-mass index, mental health, medications, sleep quality, postoperative complications, and baseline pain scores. Although the other studies did not adjust for covariates, they employed changes in scores from baseline as their outcome measures, thereby attempting to control for imbalances in pretreatment outcome scores between study arms. It should be noted that changes in scores have received significant criticism in pain research, with IMMPACT guidelines noting the fact that changes in pain scores are not equivalent from patient to patient (eg, changes in severe pain-intensity range compared to changes in mild pain-intensity range).28,30
Data from T4–T6 were analyzed according to ITT principles and reported on measures of both pain and function, whereas T3 analyses were limited to completers and reported on pain outcomes only, the results of which are described in the following sections.
Pain outcomes
In the US trial (T3), pain outcomes were measured using the BPI subscales for pain severity and pain-related interference with activities of daily living, and between-group differences in score reductions across measures were analyzed. The authors found significantly greater reductions in pain severity and interference in the intervention arm at 2-month follow-up (P<0.05 for both) in univariate analyses although the trend for pain severity lost significance upon multivariable adjustment. Regression analyses revealed a relatively large effect for CBT over usual care, conferring an additional 3.4-point reduction (95% CI –4.84 to –0.58) in pain interference (on a 0–10 scale), representing a clinically meaningful change in outcome. We conducted an analysis using Cohen’s d, which showed a large effect size of 0.83 (Table 3).
In the Italian trial (T4), pain outcomes were measured using the 11-point NRS for back and leg pain and on the SF-36-BP for body pain. Group × time interaction effects were significant for all outcomes at the P<0.001 level on linear mixed-model analyses, favoring CBT over exercise support (Table 3). Cohen’s d calculation revealed medium effect sizes when the NRS and SF-36 were evaluated and large effect sizes for back pain and disability.
In the Danish trial with 1-year follow-up (T6), the PRS was used to assess pain at 3 months, 6 months, and 1 year postsurgery. Unadjusted nonparametric analyses revealed no significant improvements in pain outcomes associated with group CBT over the follow-up period (Table 3). In the secondary analysis of acute surgical outcomes (T5) based on the same Danish trial, median pain intensity over the first postoperative week was measured using a 0–10 NRS and indirectly through consumption of rescue (ie, breakthrough) analgesics. Unadjusted nonparametric analysis similarly did not detect significant improvements in acute pain by either measure based on preoperative CBT-group involvement (Table 3). T5 and T6 used nonparametric tests and provided insufficient data to facilitate manual calculations for effect sizes.
Functional outcomes
In the Italian trial (T4), disability was measured using the ODI at 2 months postsurgery. Group × time interaction effects were significant in favor of CBT intervention over exercise support (F=20.37, P<0.001) in linear mixed-model analyses (F=20.37, P<0.001) (Table 3). Disability was also measured using the ODI in the Danish trial with long-term follow-up (T6). Unadjusted nonparametric analyses revealed significant improvements in disability outcomes at 3 months postsurgery (P=0.003) and marginally significant improvements at 6 months (P=0.056) and 1 year postsurgery (P=0.082) (Table 3). In the secondary analysis of acute surgical outcomes (T5) based on the same Danish trial, measures of mobility were employed, including timed tests for walking, rising and sitting from a chair, and getting in and out of bed. Unadjusted nonparametric analysis identified significant improvements in walking speed (P=0.02) and marginally significant improvements in speed of rising and sitting from a chair (P=0.056) and getting in and out of bed (P=0.082) associated with preoperative CBT-group involvement (Table 3).
Discussion
The objective of this systematic review was to provide a synthesis of the evidence from RCTs on perioperative, psychological interventions to manage PSP. Despite growing interest in extending the utility of psychological interventions for pain management to surgical settings, this study highlights the relative sparsity of clinical trials on the efficacy of perioperative psychological interventions for pain-related outcomes. Nonetheless, the data synthesized from preliminary RCTs identified in this systematic review generally supported the efficacy of psychological interventions to reduce pain disability and intensity after surgery. Given the tremendous individual and societal burden of CPSP, these findings speak to the potential value of integrating psychological interventions into standard perioperative care, and to the need for future research on these approaches.
Overall, the studies reviewed demonstrated that psychological interventions in the perioperative period yielded significant improvements in multiple pain-related outcomes. All of the included studies employed effective randomization and moderate sample sizes. There was also consistent use of well-validated outcome measures for pain (VAS, NRS, McGill Pain Questionnaire, BPI). Most papers, however, did not include all of the core recommendations according to the IMMPACT guidelines.
While all papers included the recommended core outcome measures for pain and physical functioning, only one paper (T3) included an assessment of emotional functioning and only one (T1) outlined detailed information according to the CONSORT protocol.28 Despite the well-validated outcome measures for disability, some useful outcome data that may have added quality to the papers may have been missed.
There was considerable heterogeneity in the CBT protocols used across the studies. We also identified significant variation in study design, analysis, timing and duration of intervention, degree of multivariate adjustment, follow-up intervals, and outcome reporting. Therefore, we were able to make some general conclusions regarding the effectiveness of psychological interventions, but the data required to make specific recommendations (types, timing, duration, delivery methods, and specific high-yield populations) were insufficient. There was also inadequate blinding in several of the studies, and this may have contributed to bias. It should be noted that blinding in psychological interventions is practically unfeasible.
Despite newer approaches employed in the treatment of chronic pain, including ACT and mindfulness-based psychotherapy,33,34 our analysis indicated that there is a lack of data on these approaches, as they were not employed in any of the identified studies.19
For five of the six studies identified, the intervention was not delivered by experienced therapists, which may have resulted in smaller effect sizes and decreased validity. T4 was the only study that had psychologist-delivered CBT. Follow-up interventions should place a higher priority on the training, experience, and expertise of the therapists delivering the interventions to optimize treatment delivery.
In terms of heterogeneity with respect to surgical procedures, five of the six studies evaluated outcomes for spinal surgery (including lumbar fusion and laminectomy), while one study assessed outcomes following cardiac surgery. While we were able to draw some comparisons, the studies included did not sufficiently represent the variety of major surgeries that are most common, and particularly those associated with a higher risk of CPSP (thoracic procedures, amputation, hernia repair, and breast surgery).35 This made it difficult to come to strong conclusions and make procedure-specific recommendations. Future research on the potential differential efficacy of interventions according to treatment-provider type and surgery type would be valuable.
Overall, four of the six studies identified significant reductions in pain. Additionally, studies T1–T4 demonstrated moderate–large effect sizes for the intervention group. Of note, the Rolving et al26,27 studies (T5 and T6; two analyses of one RCT) were the only ones that reported no significant differences in pain scores, and we were unable to analyze effect sizes. Despite wide variability in studies, we were able to identify some characteristics in these studies that may have contributed to this. In the other studies, the psychotherapy was administered on an individual basis, while the intervention in T5/T6 was administered in a group setting. In the T6 analysis, the majority of the sessions were in the preoperative period, and in the T5 analysis, all of the sessions were done in the preoperative period. To complement existing literature, future research should investigate whether pain outcomes may be best targeted when the psychological intervention is delivered primarily in the postoperative period. The T5 and T6 analyses were also unique in that nonparametric analyses were used and the results were not adjusted.
It is difficult to determine whether the presence of preexisting chronic pain had some bearing on the outcomes noted, as two of the studies included all patients with preexisting chronic pain (T1 and T2). In the studies that incorporated some patients with preexisting chronic pain (T4–T6), no comments or analyses were made on the specific outcomes of those with preoperative chronic pain and whether this made a significant difference in outcomes or incidence of postoperative pain and disability.
According to the literature, very few of the studies reported clinically meaningful outcomes30,32 when using standardized, validated methods. However, the majority of these studies demonstrated medium–large effect sizes and statistically significant results. This may suggest that continued research should focus on production of newer outcome measures that may allow for better correlation with statistical analyses.
All of the papers that measured disability (five of six) used the same standardized, validated measure (ODI). It is important to note that all of these studies noted significant improvements in disability (with moderate–large effect sizes noted in T1, T2, and T4) after the intervention was performed and regardless of the protocol for the CBT intervention. However, for the T5 analysis, significance was noted only at 3-month follow-up and the effects lost at 6-month and 1-year follow-up. In all of the other studies that assessed outcomes after spinal surgery beyond the 3-month mark, the improvements in ODI remained significant. This may be an indicator that psychology-based individual consultations are superior to group-based interventions, and calls for further clarification. Additionally, despite large effect sizes and statistically significant outcomes, correlating these measures to achieve a minimal clinically important difference has proven to be challenging according to the literature. Several minimal clinically important differences have been advocated in the past, and it has been determined that there is extreme variability in predictive modeling. These are generally affected by baseline characteristics, and clinicians must be aware of this when incorporating into clinical practice.36
Another consideration was the manner in which CBT was implemented. Two of the studies (T1 and T2) used CBPT in the postoperative period. These were both studies with a low risk of bias. While there were notable differences in the studies, it is interesting to note that CBPT was superior to the controls (education program in T2 and exercise alone in T1) in both analyses. In the T1 study, the sessions were fewer but longer and were also in person vs telephone as in T2. It is interesting to note that both interventions produced meaningful and sustained outcomes, suggesting that the length of each interventional session should be an important consideration for effective delivery. This could aid in establishing time-sensitive and cost-effective protocols. However, it is difficult to make a concrete recommendation based on these two papers alone, especially considering the fact that T2 ceased follow-up after 3 months. Therefore, replication and similar designs in future studies are needed.
Further research is also needed to determine the optimal number of hours of treatment required in order to achieve clinically meaningful effects. However, some preliminary observations were made from our review. T1 and T2 both required less than 5 hours total to deliver the interventions, while T3 and T4 both used a total of 8 hours of intervention time. These papers all had significant results for similar outcomes, despite different intervals, methods of intervention delivery, and surgical populations. Of these interventions, it would be useful to explore whether a CBPT intervention enables the most efficient perioperative pain-intervention design, considering it encompasses physical and psychological treatment methods over a short period. Both T5 and T6 involved longer-term interventions and more of hours of therapy (12 hours for the 2016 analysis and 18 hours for the 2015 analysis). A detailed cost-effectiveness analysis would be beneficial in determining whether shorter interventions can improve outcomes and cost-effectiveness.
Strengths and limitations of our study warrant consideration. This review complements and extends research for other specific pain-management techniques, such as hypnosis and relaxation, to include psychotherapy regimens with demonstrated effectiveness in a general pain setting. A major strength was our adherence to PRISMA20 guidelines, including a comprehensive and replicable research strategy with two independent raters and risk of bias assessment by three independent raters. Lastly, we undertook a systematic approach to data extraction and evidence synthesis. Regarding strengths of included RCTs, it is noteworthy that all but one of the trials applied ITT principles, although only one study clearly reported the methods used to impute missing data. These reporting omissions are relevant, because differential approaches to missing-data imputation can affect estimations of differences between study groups.37 Regardless of approach, however, ITT is generally recommended over completer (ie, per protocol) analyses, due to its several strengths, including preservation of the integrity of the randomization and more realistic estimations of average treatment effects.38 In terms of limitations, while we included three well-known psychological interventions in our search strategy (CBT, ACT, and mindfulness), we only found papers based on one of the psychological intervention approaches – CBT. In addition, identification and selection of relevant articles may have been influenced by publication bias. Finally, significant heterogeneity in papers reviewed meant that a quantitative meta-analysis was not possible.
Pain is a multifactorial biological process, and several psychological factors are known to modulate individual differences in pain perception39 and experiences. Psychological risk factors for CPSP include preoperative depression,40 anxiety,10 pain catastrophizing,10,41 posttraumatic stress-disorder symptoms,42 and fear of surgery,43 among others. Moreover, the general effectiveness of psychological interventions for chronic pain management has been well established in recent years,44,45 lending credence to the prospect of incorporating psychological approaches in the perioperative setting to manage PSP.
The perioperative period represents a critical window of intervention in which effective pain management may reduce the likelihood of progression from acute to debilitating and costly chronic pain. Aside from the small number of RCTs of psychological treatments for PSP included in this review, all of which evaluated CBT-related interventions, there is emerging evidence for mind–body interventions46 and hypnosis47 in improving pain and disability in the postoperative setting. In addition, a recent, uncontrolled pilot study of a single-session pain-psychology class targeting pain catastrophizing has demonstrated large treatment effects in reducing catastrophizing in chronic pain outpatients after 4 weeks.48
ACT is another psychological treatment with known efficacy for chronic pain that has shown initial support in surgical settings, based on emerging observational research19 from the Toronto General Hospital’s Transitional Pain Service (TPS). At the TPS, high-risk surgical patients are seen prior to surgery and followed up for 6 months postsurgery as outpatients with the option to receive psychological services grounded in ACT and mindfulness training to assist in managing PSP, disability, and opioid tapering.11 Despite the practical and logistical challenges in integrating psychological interventions in the perioperative-care pathway, the need for services similar to the TPS has arguably never been greater, given the burgeoning public health problems tied to CPSP and long-term, dose-escalating opioid use.49 In light of this, greater emphasis must be placed on rigorous research studies that report and describe their interventions with careful detail, including information related to the timing, structure, and content.
This systematic review illustrates that clinical trial research on perioperative, psychological interventions for PSP remains in the early stages. Nonetheless, evidence from the initial RCTs supports the clinical utility of psychological interventions in the perioperative period to reduce the risk of long-term pain outcomes. These findings are in keeping with the continually growing body of empirical evidence for the role of modifiable psychological risk factors in the transition from acute PSP to CPSP.4,35,50 Given escalating rates of major and minor surgical procedures, psychological interventions hold the potential to reduce debilitating and costly pain outcomes for a growing number of people. As such, these findings are of high clinical relevance to patients, clinicians, and policy makers, and call urgently for confirmation. Future observational and RCT research is necessary to validate and improve the specificity of findings, including the scope and differential effectiveness of various psychological interventions, optimal timing, dosage, and delivery of services, cost-effectiveness studies, and efficacy studies for postsurgical opioid weaning, potentially setting the stage for large-scale implementation.
Disclosure
Joel Katz is supported by Canadian Institutes of Health Research Canada Research Chair in Health Psychology at York University. Hance Clarke is supported by a Merit Award from the Department of Anesthesia, University of Toronto. The authors report no other conflicts of interest in this work.
References
Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. | ||
Hilton L, Hempel S, Ewing B, et al. Mindfulness meditation for chronic pain: a systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213. | ||
Harsoor S. Emerging concepts in post-operative pain management. Indian J Anaesth. 2011;55(2):101–103. | ||
Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–744. | ||
Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86. | ||
Werner MU, Kongsgaard UE. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113(1):1–4. | ||
Wicksell RK, Olsson GL. Predicting and preventing chronic postsurgical pain and disability. Anesthesiology. 2010;113(6):1260–1261. | ||
Stamer UM, Stüber, F. Genetic factors in pain and its treatment. Curr Opin Anesthesiol. 2007;20(5):478–484. | ||
Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77(2):362–379. | ||
Theunissen M, Peters M, Bruce J, Gramke H, Marcus M. Preoperative anxiety and catastrophizing; a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. 2012;28(9):819–841. | ||
Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695–702. | ||
Tefikow S, Barth J, Maichrowitz S, Beelmann A, Strauss B, Rosendahl J. Efficacy of hypnosis in adults undergoing surgery or medical procedures: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2013;33(5):623–636. | ||
Benzon H, Rathmell J, Wu C, Turk D, Argoff C. Pharmacological, psychological and physical modalities. In: Raj’s Practical Management of Pain. 4th ed. Philadelphia: Mosby; 2008. | ||
Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioural therapy for chronic pain. Pain. 2007;127(3):276–286. | ||
Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database of Systematic Reviews. 2010;(7):CD002014. | ||
Harrison AM, Scott W, Johns L, Morris EM, McCracken LM. Are we speaking the same language? Finding theoretical coherence and precision in “mindfulness-based mechanisms” in chronic pain. Pain Med. 2017;18(11):2138–2151. | ||
Hughes L, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552–568. | ||
Weinrib AZ, Burns LC, Mu A, et al. A case report on the treatment of complex chronic pain and opioid dependence by a multidisciplinary transitional pain service using the ACT matrix and buprenorphine/naloxone. J Pain Res. 2017;10:747–755. | ||
Azam MA, Weinrib A, Montbriand J, et al. Acceptance and commitment therapy to manage pain and opioid use after major surgery: preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain. 2017;1(1):37–49. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | ||
Higgins JP, Green S. Table 8.5.D: Criteria for judging risk of bias in the “risk of bias” assessment tool. 2014. Available from: http://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/MENO/A/MENO_22_4_2014_07_08_ISSA_MENO-D-14-00011_SDC2.pdf. Accessed December 1, 2017. | ||
Abbott AD, Tyni-Lenne R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine. 2010;35(8):848–857. | ||
Monticone M, Ferrante S, Teli M. Management of catastrophising and kinesiophobia improves rehabilitation after fusion for lumbar spondylolysthesis and stenosis. Eur Spine J. 2014;23(1):87–95. | ||
Archer KR, Devin CJ, Vanston SW, et al. Cognitive-behavioral-based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. 2016;17(1):76–89. | ||
Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs. 2016;15(6):417–424. | ||
Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Does a preoperative cognitive-behavioral intervention affect disability, pain behavior, pain, and return to work the first year after lumbar spinal fusion surgery? Spine (Phila Pa 1976). 2015;40(9):593–600. | ||
Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Preoperative cognitive-behavioural intervention improves in-hospital mobilisation and analgesic use for lumbar spinal fusion patients. BMC Musculoskelet Disord. 2016;17:217. | ||
Dworkin R, Turk D, Farrar J, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. | ||
Moher D, Hopewell S, Schulz K, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. | ||
Farrar J, Young JJ, LaMoreaux L, Werth J, Poole R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. | ||
Farrar J, Berlin J, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25(5):406–411. | ||
Dworkin R, Turk D, Wyrwich K, Beaton D, Cleeland C, Farrar J. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. | ||
McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: pilot outcome and process results. J Pain. 2013;14(11):1398–1406. | ||
Chiesa A, Serretti A. Mindfulness-based interventions for chronic pain: a systematic review of the evidence. J Altern Complement Med. 2011;17(1):83–93. | ||
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. | ||
Schwind J, Learman K, O’Halloran B, Showalter C, Cook C. Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Owestry disability index for the same sample of patients. J Man Manip Ther. 2013;21(2):71–78. | ||
Armijo-Olivo S, Warren S, Magee D. Intention to treat analysis, compliance, drop-outs and how to deal with missing data in clinical research: a review. Phys Ther Rev. 2009;14(1):36–49. | ||
Gupta S. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. | ||
Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. | ||
Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114(4):551–561. | ||
Pavlin DJ, Sullivan MJ, Freund PR, Roesen K. Catastrophizing: a risk factor for postsurgical pain. Clin J Pain. 2005;21(1):83–90. | ||
Kleiman V, Clarke H, Katz J. Sensitivity to pain traumatization: a higher-order factor underlying pain-related anxiety, pain catastrophizing and anxiety sensitivity among patients scheduled for major surgery. Pain Res Manag. 2011;16(3):169–177. | ||
Peters ML, Sommer M, de Rijke JM, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg. 2007;245(3):487–494. | ||
Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153–166. | ||
Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1–2):1–13. | ||
Vranceanu AM, Hageman M, Strooker J, ter Meulen D, Vrahas M, Ring D. A preliminary RCT of a mind body skills based intervention addressing mood and coping strategies in patients with acute orthopaedic trauma. Injury. 2015;46(4):552–557. | ||
Kendrick C, Sliwinski J, Yu Y, et al. Hypnosis for acute procedural pain: a critical review. Int J Clin Exp Hypn. 2016;64(1):75–115. | ||
Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC. From catastrophizing to recovery: a pilot study of a single-session treatment for pain catastrophizing. J Pain Res. 2014;2014(7):219–226. | ||
Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. | ||
Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP): a systematic review. Eur J Pain. 2009;13(7):719–730. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.