Back to Journals » Journal of Pain Research » Volume 13
Prophylactic Electroacupuncture on the Upper Cervical Segments Decreases Neuronal Discharges of the Trigeminocervical Complex in Migraine-Affected Rats: An in vivo Extracellular Electrophysiological Experiment
Authors Qu Z , Liu L, Zhao L , Xu X, Li Z, Zhu Y, Zhang C, Jing X, Wang X, Li B, Zhang CS, Fisher M, Wang L
Received 12 August 2019
Accepted for publication 24 December 2019
Published 10 January 2020 Volume 2020:13 Pages 25—37
DOI https://doi.org/10.2147/JPR.S226922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Zhengyang Qu, 1 Lu Liu, 1, 2 Luopeng Zhao, 1, 3 Xiaobai Xu, 1 Zhijuan Li, 1 Yupu Zhu, 1 Chen Zhang, 4 Xianghong Jing, 2 Xiaoyu Wang, 2 Bin Li, 1 Claire Suiqing Zhang, 5 Marc Fisher, 6 Linpeng Wang 1
1Acupuncture and Moxibustion Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, Beijing, People’s Republic of China; 2Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 3Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Institute of Traditional Chinese Medicine, Beijing, People’s Republic of China; 4Acupuncture and Moxibustion Department, Beijing Massage Hospital, Beijing, People’s Republic of China; 5School of Health and Biomedical Sciences, RMIT University, Melbourne, Victoria, Australia; 6Division of Stroke and Cerebrovascular Diseases, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA
Correspondence: Linpeng Wang
Acupuncture and Moxibustion Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, No. 23 Meishuguanhou Street, Dongcheng District, Beijing 100010, People’s Republic of China
Tel +86 1052176644
Email [email protected]
Purpose: This rat experiment aims to demonstrate the efficacy of electrical acupuncture in preventing migraine attacks by stimulating the acupoint GB20.
Introduction: Migraine, which takes 2ed at level four causes of GBD’s disease hierarchy, becomes a public health issue. It is important for physicians to supplement their knowledge of its treatment and consider alternative methods of therapy, such as acupuncture. However, the neurobiological and pathophysiological mechanisms of this prophylactic effect were unclear. The trigeminocervical complex is thought to be an important relay station in migraine pathophysiology as the key nuclei of the trigeminovascular system and the brainstem descending pain modulation system.
Methods: There were six groups involved in this study: control, model, electroacupuncture, non-acupoint electroacupuncture, saline+electroacupuncture and saline+non-acupoint electroacupuncture. We injected saline or inflammatory soup into dura mater to induce control or migraine in the rats. The mechanical pain threshold and the single-cell extraneural neurophysiology of the C1 spinal dorsal horn neurons in the trigeminocervical complex were detected.
Results: Pre-electroacupuncture could significantly increase the mechanical pain threshold of the periorbital region receptive field of the trigeminal nerve and decrease the discharges of neurons in the trigeminocervical complex. Acupuncture also reversed the abnormal skin pain response of the periorbital region receptive field of the trigeminal nerve caused by low-intensity stimulation.
Discussion: We believe that our study makes a significant contribution to the literature because it is the first of its kind to use GB20 to provide relief from migraine attacks and mechanical cephalic cutaneous hypersensitivity by regulating the neuronal discharge from trigeminocervical complex.
Keywords: acupuncture, headache prevention, hypersensitivity, electrophysiology, the trigeminocervical complex, inflammatory soup
Introduction
Migraine is a neurological condition that manifests as an episodic, recurrent, and often severe headache. It has an incidence rate of 12% worldwide1 and according to the GBD (the Global Burden of Disease) study (2016), migraine takes second place at level four causes of GBD’s disease hierarchy, which is responsible for 5.6% of all YLDs (years lived with disability) in the world. In the age group 15–49 years, migraine is the top cause of YLDs.2 The approximate cost of direct treatment and indirect economic losses (lost productivity) for a migraine patient anywhere in the world is about $1533 per year, and this cost is projected to increase to $4144 per year as soon as the patient develops chronic migraine.3,4 This is an important public health issue and it is the responsibility of the scientific community to develop cost-effective complementary and alternative therapies for migraine patients.
Acupuncture has a long history of use in traditional Chinese medicine and a broad range of applications for the treatment of mental and physical disorders. It has gained worldwide recognition for its effectiveness over the past few decades.5 Recent studies have confirmed the clinical prophylactic effects of acupuncture on migraines: a reduction in the degree of pain,6,7 reduction in the number of migraine attacks,8–11 and improvement in the quality of life of the patients.12–14 However, in spite of its positive effects, the mechanism of action of acupuncture is unclear.
Considerable research has proven that the increase of neuronal discharges15–17 and neurotransmitters such as glutamate,18 neuropeptide Y,19 C-fos,20 and CGRP (Calcitonin gene-related peptide)21 from the dorsal spinal cord neurons in TCC make a substantial contribution to attacks and mechanical CCH in migraine-affected rats. Otherwise, a dural inflammatory soup (IS) injection is used to initiate migraine attacks and induce hyperalgesia and allodynia in the receptive field of the periorbital region of trigeminal nerve, using mechanical pain threshold test with a Von-Frey filament.15,22–25 Mechanical CCH may develop solely within the periorbital region and may spread throughout the face, scalp, limbs, and body. In addition to this, mechanical stimulation can induce skin pain, including hyperresponsiveness and hypersensitivity, which is either accompanied by a migraine attack or is interictal to it.26,27 It has been reported that mechanical CCH is a common problem in 50–70% of migraine patients,26,28 and frequent occurrence of these episodic migraine attacks can lead to chronic migraine.26,29,30
We hypothesized that prophylactic acupuncture at fengchi (GB20) can prevent migraine and mechanical CCH by regulating the discharge rate of neurons in the TCC. In this study, we injected IS into the dura mater to induce migraine in rats. To evaluate the success of the model, and to explore the neurobiological mechanism of acupuncture for migraine, we obtained the mechanical pain threshold of the receptive field of the periorbital region of the trigeminal nerve and described the single-cell extra-neural neurophysiology of C1 spinal dorsal horn neurons in the TCC.
Materials and Methods
Animals and Ethical Statement
All experiments were conducted after receiving a license from the Beijing Animal Experimental Institution Review Committee (using the Animal Experiment Ethics Committee of Beijing Capital Medical University, Ref. No.: Aeei 2015–075). Male Sprague Dawley rats (age: 12 weeks, weight: 250–300 g, n=48, Chinese Academy of Military Science, certificate number: 0017617) were trained in controlled temperature (24±0.5°C), 12-hr light, and 12-hr dark cycles (light 08:00–20:00; darkness 20:00–08:00), with free access to food and tap water. All animal care and experiments were proved by the Institutional Animal Care and Use Committee of the Capital Medical University and conformed to the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
We used isoflurane (3% induction, 1.5% maintenance) mixed with oxygen to induce gas anesthesia. The animal was placed in an induction anesthesia box with a gas flow of 0.6 L/mins. When the rat was completely limp, it was fixed to a brain stereotactic (Shenzhen Life Technology Co., Ltd., Model: 68505) with an anesthesia adapter, to ensure continuous anesthesia. A heated (38°C) electric blanket was put under the animal during this time and its breathing, heartbeat, and the color of the skin under the hair and four claws were simultaneously monitored. The eyes of the rat were protected with an erythromycin ointment.
Experimental Design
After the pre-baseline text of mechanical pain threshold of the periorbital region receptive field of the trigeminal nerve (Hereinafter referred to as “the cutaneous receptive field pain threshold”), we started the surgical operation. A plastic cap with a stainless-steel cannula (26 gauge, Plastics One Inc., Roanoke, VA, USA) was secured to the skull with small screws and dental cement. For 7 days after the surgery, the test of the cutaneous receptive field pain threshold was done every day to eliminate the rats who could not return to pre-surgery MPTPRT levels. The behavioral data recorded on last day of this period (Day 0) was used as the baseline.
The rats were randomly divided into six groups by the random table method: control group (C) (20 μL of 0.9% sterile saline injected, no acupuncture operation, n=8); model group (M) (20 μL of IS injected, no acupuncture operation, n=8); electroacupuncture group (EA) (20 μL of IS injected, acupuncture performed bilaterally at GB20 with electric stimulation, n=8); non-acupoint electroacupuncture group (NA) (similar to the EA group, with the needle applied at 2 mm outside the GB20 and at a 1 mm depth into the skin, n=8); saline+electroacupuncture group (SEA) (20 μL 0.9% sterile saline injected, acupuncture performed bilaterally at GB20 with electric stimulation, n=8); and saline+non-acupoint electroacupuncture group (SNA) (similar to the SEA group, with the needle applied at 2 mm outside the GB20 and at a 1 mm depth into the skin, SNA, n=8).
On Days 1–3, all rats underwent electroacupuncture intervention, injection, and the cutaneous receptive field pain threshold successively on each day, except the C and M rats, which did not undergo electroacupuncture. On third day, the electrophysiological signals were recorded after pre-treatment, modeling and the cutaneous receptive field pain threshold testing, following which the histological experiment was performed after euthanasia. The IS was formulated with histamine, serotonin, and bradykinin of 2 mM each, and prostaglandin E2 (PGE2) of 0.2 mM in 0.9% sterile saline, with a pH of 7.0 (Figure 1).31–33 A total of six researchers participated in the experiment procedure and were denoted as follows: 1) person in charge of grouping; animal feeder; animal, saline and IS transmitter; 2) electroacupuncture executor; 3) modelmaker; 4) behavioral text; 5) electrophysiological recorder and experienced histopathologist; and 6) data analyst and statistician. Researchers 3, 4, 5, and 6 were blinded to the grouping situation of the rats.
Surgical Preparation
To gain access to the dura mater, the skull was exposed at a site 1 mm anterior to the fontanelle and 1 mm left of the midline: craniotomy was then performed using saline-cooled drilling with the hole covered by mineral oil. The injection seat was placed into the hole, and the device was fastened to the skull with a screw; it was then affixed with a powerful adhesive and a dental cement. Subsequently, gentamicin (0.04 million IU/100 g, 1 mL/100 g) intramuscular injection was administered to prevent infection. The rats were then allowed a 7-day convalescence period, during which an intraperitoneal injection of buprenorphine (30 μg dissolved in 0.3 mL of sterile saline) was administered daily for the first 3 days to maintain analgesia.
Electroacupuncture Intervention
The acupuncture operation was performed at 8 am. First, the rat was fastened to the shelf with an elastic cloth to ensure that the procedure was performed in the rat’s awake state. After fixation, the acupuncture sites were scrubbed with a 75% alcohol disinfectant and acupuncture was performed using 0.25×25 mm acupuncture needles. The location was the bilateral FengChi acupoints (GB20) located 3 mm lateral to the center of a line joining the two ears at the back of the head. Rats undergoing EA and SEA had the acupuncture needle inserted to a depth of 8 mm, while the NA and SNA groups received needles at a depth of 2 mm. All the needle handles were held with an electroacupuncture instrument (model: HANS-200, Nanjing Saiseikai Medical Technology Co., Ltd.). The rats received 15 mins of electrical stimulation with the parameters of 0.5~1 mA and 2~15 Hz of interrupted wave current. The insertions of the EA/SEA groups and the NA/SNA groups were kept different to identify the presence or absence of “De Qi” (strong perception of needles by the receiver). When the needle is inserted into the muscle and energized, the muscle exhibits the same beat as the electrical stimulation parameter rhythm; this is considered as De Qi. Conversely, the muscles do not twitch when the skin is broken.
IS or Saline Injection
The IS injection was administered immediately after completing the acupuncture operation. Following the placement of a 30-cm-long PE-10 tube into the opening of the drug injecting device, a bubble was created by first inhaling 1–2 μL of air, followed by inhalation of 20 μL of IS (saline for group C, SEA, and SNA). The bubble was detected as a marker during the injection. The parenteral solution was slowly pumped into the injection seat using a micro-injection pump for 5 mins at a rate of 4 μL/minute. A minute later, the tube was moved away, and the cap of the injection seat was covered.
Behavioral Study of the Cutaneous Receptive Field Pain Threshold
The test was performed between 8 am and 12 am. The rats were placed in the face-pain-detection tube (20×30 cm pedestal, 10 cm in diameter and 30 cm in length, Beijing Golden Crown Zhuo Yi Trading Co., Ltd. Supplementary Figure 1) to adapt for 30 mins until they quieted down. We used Von-Frey filaments (0.08 g, 0.16 g, 0.4 g, 0.6 g, 1 g, 2 g, 4 g, 6 g, 8 g, 15 g, 26 g, 60 g, and 100 g) (North Coast Medical, Inc., Morgan Hill, CA, USA) to test for the cutaneous receptive field pain threshold. The skin between the eyes and ears was tested (Figure 2A). When the wire was curved into an S-shape, the head-back reaction of the rat was observed. Starting from 2 g, the duration of each stimulation was controlled at about 30 s. When the rats showed a head-back reaction, the Von-frey filament was changed to a smaller wire, and if there was no reaction, the wire size was increased. The 50% head mechanical withdraw threshold value (MWT) was calculated using the Dixon up-down method.15,34
Electrophysiology Recording
After anesthesia and fixation, the skin and muscles in the midline of the rear neck were cut and stripped, and a left atlantoaxial vertebral plate resection was performed to expose the dura. A skull perforation was made at the top of the left occipital lobe (0.5*0.5 mm) for the reference electrode, and the left frontal and occipital lobes were exposed to accommodate the grounding electrodes.
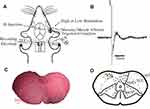
After peeling off the left atlantoaxial dura mater, the recording electrode (a concentric dipole tungsten wire recording electrode with a diameter of 75 μm, tip diameter of 3–4 μm, and an impedance of 10–15 kΩ, TM33CCINS, World Precision Instruments Shanghai Trading Co., Ltd. China; Supplementary Figure 2) was carefully implanted into a depth of 0.5–1.5 mm using a microelectrode manipulator (SR-1N, NARISHIGE Co., Ltd. Japan). It was then allowed to move up and down within 50 μm. Subsequently, the electrophysiological recording electrode was connected to a microelectrode AC amplifier (AM1800, A-M SYSTEMS, LLC. USA), a CED data acquisition and analysis system (Micro1401-3, A-M SYSTEMS, LLC. USA), and a PC computer (Spike 2 version 7.03a software, CED, Cambridge, UK). The signal was amplified 1000 times. The spontaneous discharges and induced activities of the neurons were sampled and analyzed at 100 Hz–50 kHz. In order to find the nerve cells, the receptive field of ophthalmic branch of trigeminal nerve was identified by high-intensity stimulation (pinched with forceps) and the recording electrodes were moved slowly and carefully.15 The discharge voltage was then observed the on the oscilloscope to determine neuronal activation. The signal was stabilized for 30 mins after finding the activated neurons, following which they were subjected to a 180-s baseline record. High and low (brushing with a soft brush) intensity stimulation was used to stimulate the rat’s ocular receptive field of the trigeminal nerve 3 thrice. Artificial cerebrospinal fluid was continuously dripped throughout the process to ensure a moist operative field (Figure 3A).
Neurons were found on the cutaneous periorbital region receptive field of the trigeminal nerve. The cutaneous receptive field was identified when the recording electrode was implanted in the spinal cord. The receptive field was verified for non-noxious inputs with low-intensity stimulation and for noxious inputs with high-intensity stimulation. When a neuron sensitive to the stimulation of the cutaneous periorbital region receptive field (V) of the trigeminal nerve was identified, it was tested for convergent input from the dura mater.
The Spike2 v7.03a software was used to analyze the data. The action potential was determined by matching the standard action potential waveform template, where each action potential represented the discharge activity of a single cell. In this study, the difference between groups was analyzed by studying the number of discharge activities of a unit of neurons in a specific time period. After a 300-s duration of firing rates, the discharge was recorded with high and low-intensity stimulation. The data in the 2 s before and after the stimulation marks, which was a total of 4 s of data, were divided into 20 parts with a 0.2-s time period. The changes in the firing rates in these continuous time periods were analyzed.
Histological Experiment
At the end of the experiment, a lesion-creating device (53500, Ugo Basile Biological Research Apparatus Co., Italy) was used to connect the recording electrodes with the parameters of 1 mA, 1 Hz, and 20 s, causing the neurons in the brain region where the electrodes were inserted to be burnt. Animals were anesthetized with pentobarbital sodium perfused with phosphate-buffered saline (PBS; pH 7.4), and fixed by perfusion with 500 mL of 4% paraformaldehyde in PBS, using standard immunohistochemical methods. The intact medulla oblongata was removed and soaked in 10% formalin solution for 48 hrs. Subsequently, it was dehydrated, paraffin-embedded, sliced with a thickness of 20 μm, and HE-stained. The insert position of the recording electrode was confirmed under a microscope to ensure that it could be drawn at the correct position on a map of the rat’s brain.
Statistical Analysis
Statistical Packages for the Social Sciences Software (SPSS) v.12.0 (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. A Dunnett test was used to compare the baseline data. The discharge number between groups was compared with a one-way analysis of variance followed by the Student-Newman-Keuls post hoc test. We used the LSD method for data analysis when any two groups were compared. The difference of discharge times of the 20 time periods of 2 s each (before and after the time point of Von-Frey) was analyzed by a variance analysis of repeated measurements. Two-tailed P-values <0.05 were considered statistically significant. Data are expressed as the mean±SD.
Results
A total of 48 rats were included in the experiment, 5 of which were eliminated because of paralgesia (based on the baseline value of behavioristics; we eliminated rats with too high or low pain thresholds). One rat died during the surgery, and we eventually included 42 rats in total with 7 rats in each group.
Trend of the Cutaneous Receptive Field Pain Threshold in the Course of the Experiment in Each Group
The cutaneous receptive field pain threshold was recorded on Day 0 and the baseline value was not statistically different between the groups. On Day 1, the cutaneous receptive field pain threshold of groups C, SEA, and SNA did not change significantly. When compared with the baseline, group M, EA, and NA showed a significant decrease. On Day 2, the cutaneous receptive field pain threshold of EA improved from the previous day, while the measurements of the other five groups did not change significantly as compared to the previous day. On Day 3, the cutaneous receptive field pain threshold of the right facial EA group continued to improve. However, that of the left facial group decreased as compared to Day 2 but was still better than Day 1. The other five groups showed no significant changes on Day 3 as compared to Day 1 and Day 2 (Figure 2B).
Comparison of the Cutaneous Receptive Field Pain Threshold Between Left and Right Facial Side
We made a comparison of the cutaneous receptive field pain threshold values of the left and right facial acupuncture sides from Day 0 to Day 4 in each group. The results were not statistically different, and the cutaneous receptive field pain threshold between the left face and the right facial sides of each group was almost equal during this period. We concluded that the migraine attack caused by the dural administration of IS was bilateral, not unilateral (Figure 2C).
Discharge Waveform of Evoked Neurons and the Recording Sites in TCC
We recorded 92 neurons in total. Our targets were the neurons with an average latency of about 10 ms, which was characteristic of Aδ nerve fibers. Only the neurons with a voltage greater than 40 μV were analyzed. With these standards, 42 neurons were recognized as Aδ fibers. They responded to the stimulation of the receptive field with an average latency of 11.2±0.3 ms (an example of evoked neuronal firing can be seen in Figure 3B).
The concentric, dipole, tungsten-wire recording electrode can not only be used as a recording electrode but also as a stimulation electrode when it is connected to the power supply of a positive and negative pole. While the lesion-creating device was energized, the nerve tissue at the tip of the electrode burned. After pathological sectioning and HE staining, hollow circular marks with blackened edges appeared (Figure 3C). All the neurons were located within the deep laminae (IV-VI) of TCC (Figure 3D).
Base Discharge Rate of Neurons in TCC
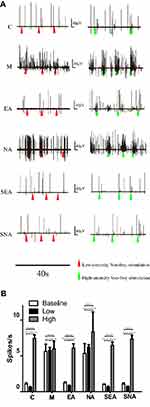
Each rat had a base discharge period of 180 s. In group C, an average of 193.86±30.21 discharges were recorded, of which the frequency of discharges was no more than 2 Hz. Similarly, in groups EA, SEA, and SNA, the discharges were recorded as 225.43±17.39, 182.72±12.48, and 193.29±30.94 times, respectively. The base discharge rate of the M and NA groups was much higher than the above-mentioned groups; an average number of 1014.14±182.12 discharges were recorded in group M, and the frequency of discharge was 5.63±1.01 Hz. The corresponding data of the NA group were 960.57±245.55 and 5.34±1.36 Hz (Figure 4A and B).
Changes in TCC Neuron Discharge Before and After High and Low-Intensity Stimulation on the Trigeminal Nerve Receptive Field
During electrophysiological recording, neither low nor high stimulation showed any obvious change in terms of discharge frequency in the M (Baseline: 5.63±1.01; Low stimuli: 5.76±0.43; High stimuli: 5.98±1.07) and NA (Baseline: 5.34±1.36; Low stimuli: 6.23±0.35; High stimuli: 8.37±2.76) groups. For groups C (Baseline: 1.08±0.17; Low stimuli: 0.60±0.12; High stimuli: 7.45±0.46), EA (Baseline: 1.25±0.10; Low stimuli: 0.83±0.09; High stimuli: 6.07±0.61), SEA (Baseline: 1.02±0.07; Low stimuli: 0.67±0.12; High stimuli: 6.48±0.43), and SNA (Baseline: 1.07±0.17; Low stimuli: 0.71±0.07; High stimuli: 7.36±0.48), there was no change in the discharge frequency before and after low-intensity stimulation. The discharges of TCC neurons of groups C, EA, SEA, and SNA increased after receiving high-intensity stimulation (Figure 5A).
The 2-s time periods before and after the stimulus point were stimulating. The M and NA groups showed no differences when comparing the frequency of discharges (Hz) between low and high-intensity stimulation periods. While the data in groups C, EA, SEA, and SNA showed no difference between the non-stimulating period and the low-intensity stimulation period, these two periods had much lower stimulation than that in the strong stimulation period (Figure 5B).
Effect of Electroacupuncture Intervention on High and Low-Intensity Stimulation Tolerance of Migraine-Affected Model Rats
For high-intensity stimulation, the responses of the six groups were as follows: (C: 7.45±0.46; M: 5.98±1.07; EA: 6.07±0.61; NA: 8.37±2.76; SEA: 6.48±0.43; SNA: 7.36±0.48); these were mostly similar among the groups. There was no significant difference in the average frequency of discharges (Hz) before and after stimulation (Figure 6A and B).
 |
Figure 6 Instantaneous discharge diagram of a TCC neuron stimulated by a high-intensity stimulation. (A) Discharges wave chart of 2 s before and after the moment of strong stimulation. The 0.0 of the abscissa means the moment that the stimulus was implemented. (B) Average discharge times/0.2 s in 2 s before and after the moment of high-intensity stimulation Grouping and quantity of each group are the same as Figure 2. There was no statistical significance between each group. (C) Instantaneous discharge diagram of a TCC neuron stimulated by a low-intensity stimulation. (A) Discharges wave chart of 2 s before and after the moment of weak stimulation. The 0.0 of the abscissa means the moment that the stimulus was implemented. (D) Average discharge times/0.2 s in 2 s before and after the moment of low-intensity stimulation. #P<0.05 compares with M (C:P=0.004; EA:P=0.013; SEA:P=0.017; SNA:P=0.044); *P<0.05 compares with NA (C:P=0.001; EA:P=0.006; SEA: P=0.006; SNA: P=0.018); There was no statistical significance between groups C, EA, SEA, and SNA. There was no statistical significance between groups M and NA. N=7/group. Abbreviations: C, control group; M, model group; EA, electroacupuncture group; NA, non-acupoint electroacupuncture group; SEA, saline+electroacupuncture group; SNA, saline+non-acupoint electroacupuncture group. |
For low-intensity stimulation, the reaction of the M (5.76±0.43) and NA (6.23±0.35) groups was similar to that shown during high-intensity stimulation. The average frequency of discharge (Hz) 2 s before and after stimulation in groups C (0.60±0.12), EA (0.83±0.09), SEA (0.67±0.12), and SNA (0.71±0.07) was considerably lower than that of groups M and NA (Figure 6C and D).
Discussion
Our experiments provide the first evidence that prophylactic intervention with electroacupuncture at fengchi (GB20) can prevent migraine and associated mechanical CCH by regulating and decreasing the discharge rate of neurons in the TCC. We demonstrated that acupuncture can achieve its preventive effect on mechanical CCH in the trigeminal nerve receptive field by regulating the activity state of TCC neurons. Moreover, this effect could improve the tolerance of the patient to low-intensity harmless stimuli, without reversing the normal pain sensation caused by high-intensity harmful stimuli. Conversely, non-acupoint electroacupuncture could not achieve these effects.
The migraine rat model in our study was similar to that employed in previous studies,15–17,22–25 where IS was applied to produce a significant and stable reduction of the cutaneous receptive field pain threshold by activating the trigeminovascular system. Interestingly, this effect shows no bilateral facial difference. Additionally, there was no significant increase in the cutaneous receptive field pain threshold on Days 1–3 as compared to that on Day 0 in group C. This may be related to the tolerance of rats to stimulation with Von-Frey filaments. Extracellular recordings also showed the successful establishment of the migraine model, where the discharges of TCC in groups M and NA appeared to be more prominent than that in other groups. This phenomenon occurred for at least 180 s. Such a model accurately mimics the development of central sensitization, trigeminospinal hypersensitivity, and cutaneous allodynia seen in patients with recurrent headaches.
In our previous trials, GB20 was shown to be a specific site for the clinical acupuncture treatment of migraine in contrast to other acupoints.10,14 Considering the specificity of GB20’s anatomical position, the traditional twisting and turning technique of insertion for De Qi can easily lead to spinal cord damage if applied carelessly. To resolve this issue, electrical stimulation was used to achieve De Qi. It is noteworthy that prophylactic electroacupuncture can reverse the decrease of the cutaneous receptive field pain threshold after IS injection. Interestingly, with the increase in the intervention frequency, this effect was enhanced to a certain extent.
The barrage of incoming signals with electrophysiological monitoring demonstrated that prophylactic GB20 electroacupuncture may have a preventive effect on migraine by reducing the activity of TCC neurons. Considering this connection between the neuronal activity of the TCC and the mechanical CCH, we could speculate from the electrophysiological data that the results were consistent with behavioral performance; groups M and NA had a significantly elevated base discharge rate of TCC neurons, while the rate in other groups was approximately 4 times lower. Excessive excitation in the TCC caused by external stimulation is an important factor resulting in a migraine attack. Thus, electroacupuncture therapy can reduce the active state of TCC neurons while preventing the onset of migraine. Additionally, the special anatomical location of GB20 allows for the electroacupuncture to stimulate and activate the periorbital region receptive field of the trigeminal nerve to some degree, regulate the TCC in a reverse direction along the trigeminal nerve, contribute to the diffuse noxious inhibitory control (DNIC),35,36 slumber the neurons, and have only an immediate effect and not a prophylactic function.37,38 Moreover, its mechanism is different from that of the greater occipital nerve block.
We also found that prophylactic treatment of migraine with GB20 electroacupuncture can significantly alleviate the abnormal pain caused by non-nociceptive stimulation. For rats in group C, the TCC neurons maintained a more moderate discharge rate when the trigeminal nerve’s receptive field either received weak mechanical stimulation or no stimulation. Once the rats received a stronger stimulation, their discharge rate surged, and the same situation happened with group EA. However, for rats with migraine episodes and those treated with non-acupoints, the neurons in TCC maintained a high-discharge frequency state with both strong and weak stimuli. Although the data showed that the firing rate decreased progressively in the strong-stimulating, weak-stimulating, and non-stimulating periods, there was no statistically significant correlation between them.
Interestingly, the SEA and SNA groups were similar to group C with respect to all the outcomes of the entire experiment. This indicates that electroacupuncture at GB20 was safe and harmless for neurons and nerves that generated inputs to TCC. Electroacupuncture induced moderate stimulation in the muscles and nerves; notably, the base discharge rate of the SEA group was 5.7% lower than that of the C group, but that in the SNA group was only 0.3% lower than that in the C group. To a certain extent, this indicates that EA may achieve its prevention by regulating TCC neurons in an unexposed state.
It is estimated that more than 90% of migraine sufferers have been using some form of medication.39 Hence, the primary goal of acute treatment for migraines is to reduce dysfunction by reducing the time of onset and reducing the degree of pain. The American Headache Association40 encourages the use of rapid treatment and discourages the use of spare and first aid medications as treatment for acute migraine. Our data suggest that EA may have a preventive effect on migraine attacks; hence, EA can be used as an important supplemental and replacement therapy for migraine attacks. The pharmacological management of migraine during pregnancy, lactation, and postpartum period is challenging due to the potential adverse effects of migraine drugs on the fetus and newborn.41 Acupuncture can be considered as a safe intervention for pregnant women as long as the abdominal acupuncture points are not used.42
A limitation of this study was that the recording sites of TCC neurons were concentrated; hence, they could not fully reflect the TCC or show a wide range of discharge activities. The study was further limited by the removal range of the C1 laminectomy, which allowed the electrophysiological recording to take place only in the medial region, rather than in the lateral area where it is easy to touch the bone tissue and damage the tips of the recording electrode.
Conclusions
In conclusion, our research shows that the prophylactic electroacupuncture at GB20 can prevent migraine attacks and associated mechanical CCH. This effect is achieved by reducing the discharge frequency of neurons in the TCC. Our findings provide theoretical support for the development of a neurobiological mechanism to prevent migraine through acupuncture. It also lays a solid foundation for further studies to be conducted on the topic.
Abbreviations
TCC, trigeminocervical complex; CCH, cephalic cutaneous hypersensitivity; IS, inflammatory soup; MWT, 50% Head mechanical withdraw threshold value; CGRP, calcitonin gene-related peptide.
Ethics Approval
All experiments were conducted under a license of the Beijing Animal Experimental Institution Review Committee (using the Animal Experiment Ethics Committee of Beijing Capital Medical University, Ref. No.: Aeei 2015-075).
Data Sharing Statement
All data about this study can be Shared with readers via contact corresponding author by e-mail.
Acknowledgments
The authors would like to thank Jiatong Hu, Xiaoning Zhang, Xiaoqin Lv, Yi Ming for their assistance. We would like to thank Editage for English language editing.
Author Contributions
ZYQ contributed as first authors by designing and carrying out studies, acquiring and analyzing data, and drafting the manuscript. LPW made final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. LL, LPZ, XBX, ZJL, YPZ, CZ made assistant to the experiment procedure and made acquisition, analysis, and interpretation of data. XHJ, XYW, BL provided technical support and revised it critically for important intellectual content for this study. CSZ, MF revised it critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20:22–33. doi:10.18553/jmcp.2014.20.1.22
2. Vos T, Abajobir AA, Abate KH et al,. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
3. Lanteri-Minet M. Economic burden and costs of chronic migraine. Curr Pain Headache Rep. 2014;18:385. doi:10.1007/s11916-013-0385-0
4. Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the united states: a web-based survey. Headache. 2016;56:306–322. doi:10.1111/head.12755
5. Liu R, Yu S, He M, et al. Health-care utilization for primary headache disorders in China: a population-based door-to-door survey. J Headache Pain. 2013;14(1):1–8. doi:10.1186/1129-2377-14-1
6. Li Y, Liang F, Yang X, Tian X, Yan J, Sun G. Acupuncture for treating acute attacks of migraine: a randomized controlled trial. Headache. 2009;49(6):805–816. doi:10.1111/hed.2009.49.issue-6
7. Melchart D, Thormaehlen J, Hager S, Liao J, Linde K, Weidenhammer W. Acupuncture versus placebo versus sumatriptan for early treatment of migraine attacks: a randomized controlled trial. J Intern Med. 2003;253(2):181–188. doi:10.1046/j.1365-2796.2003.01081.x
8. Musil F, Pokladnikova J, Pavelek Z, Wang B, Guan X, Valis M. Acupuncture in migraine prophylaxis in Czech patients: an open-label randomized controlled trial. Neuropsychiatr Dis Treat. 2018;14:1221–1228. doi:10.2147/NDT
9. Zhao L, Chen J
10. Wang LP, Zhang XZ, Guo J, et al. Efficacy of acupuncture for migraine prophylaxis: a single-blinded, double-dummy, randomized controlled trial. Pain. 2011;152(8):1864–1871.
11. Alecrim-Andrade J, Maciel-Júnior JA, Carnè X, Severino Vasconcelos GM, Correa-Filho HR. Acupuncture in migraine prevention: a randomized sham controlled study with 6-months posttreatment follow-up. Clin J Pain. 2008;24(2):98–105. doi:10.1097/AJP.0b013e3181590d66
12. Yang CP, Chang MH, Liu PE, et al. Acupuncture versus topiramate in chronic migraine prophylaxis: a randomized clinical trial. Cephalalgia. 2011;31(15):1510–1521. doi:10.1177/0333102411420585
13. Facco E, Liguori A, Petti F, et al. Traditional acupuncture in migraine: a controlled, randomized study. Headache. 2008;48(3):398–407. doi:10.1111/hed.2008.48.issue-3
14. Wang LP, Zhang XZ, Guo J, et al. Efficacy of acupuncture for acute migraine attack: a multicenter single blinded, randomized controlled trial. Pain Med. 2012;13(5):623–630. doi:10.1111/j.1526-4637.2012.01376.x
15. Boyer N, Dallel R
16. Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(s1):S39–S44. doi:10.1111/hed.2006.46.issue-s1
17. Takehana S, Kubota Y, Uotsu N, Yui K, Shimazu Y, Takeda M. Acute intravenous administration of dietary constituent theanine suppresses noxious neuronal transmission of trigeminal spinal nucleus caudalis in rats. Brain Res Bull. 2017;131:70–77. doi:10.1016/j.brainresbull.2017.03.004
18. Laursen JC, Cairns BE, Dong XD, et al. Glutamate dysregulation in the trigeminal ganglion: A novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience. 2014;256:23–35. doi:10.1016/j.neuroscience.2013.10.009
19. Martins-Oliveira M, Akerman S, Tavares I, Goadsby PJ. Neuropeptide Y inhibits the trigeminovascular pathway through NPY Y1 receptor. Pain. 2016;157(8):1666–1673. doi:10.1097/j.pain.0000000000000571
20. Pei P, Liu L, Zhao L, Cui Y, Qu Z, Wang L. Effect of electroacupuncture pretreatment at GB20 on behaviour and the descending pain modulatory system in a rat model of migraine. Acupunct Med. 2016;34(2):127–135. doi:10.1136/acupmed-2015-010840
21. Zhao LP, Liu L, Pei P, Qu ZY, Zhu YP, Wang LP. Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine. Neural Regen Res. 2017;12(5):804–811. doi:10.4103/1673-5374.206652
22. Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47(7):1026–1036. doi:10.1111/hed.2007.47.issue-7
23. Wang S, Wu BX, Liu CY, et al. Expression of ASIC3 in the trigeminal nucleus caudalis plays a role in a rat model of recurrent migraine. J Mol Neurosci. 2018;66(1):44–52. doi:10.1007/s12031-018-1113-3
24. Wang XY, Zhou HR, Wang S, et al. NR2B-Tyr phosphorylation regulates synaptic plasticity in central sensitization in a chronic migraine rat model. J Headache Pain. 2018;19(1):102. doi:10.1186/s10194-018-0935-2
25. Baixue W, Wang S, Chen L, et al. Protein kinase C γ contributes to central sensitization in a rat model of chronic migraine. J Mol Neurosci. 2017;63(2):131–141. doi:10.1007/s12031-017-0960-7
26. Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. 2013;136(Pt 11):3489–3496. doi:10.1093/brain/awt251
27. Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81–91. doi:10.1002/ana.v68:1
28. Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70(17):1525–1533. doi:10.1212/01.wnl.0000310645.31020.b1
29. Chen N, Zhang J, Wang P, Guo J, Zhou M, He L. Functional alterations of pain processing pathway in migraine patients with cutaneous allodynia. Pain Med. 2015;16(6):1211–1220. doi:10.1111/pme.12690
30. Burstein R
31. Fried NT, Maxwell CR, Elliott MB, Oshinsky ML. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia. 2018;38(4):674–689. doi:10.1177/0333102417703764
32. Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi:10.1002/ana.21537
33. Kayama Y, Shibata M, Takizawa T, et al. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: relevance to migraine pathophysiology. Cephalalgia. 2018;38(5):833–845. doi:10.1177/0333102417712719
34. Sumizono M, Sakakima H, Otsuka S, et al. The effect of exercise frequency on neuropathic pain and pain-related cellular reactions in the spinal cord and midbrain in a rat sciatic nerve injury model. J Pain Res. 2018;7(11):281–291. doi:10.2147/JPR.S156326
35. Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls (DNIC) in dura-sensitive medullary dorsal horn neurons. J Neurosci. 2009;29(50):15828–15835. doi:10.1523/JNEUROSCI.3623-09.2009
36. Murase K, Kawakita K. Diffuse noxious inhibitory controls in anti-nociception produced by acupuncture and moxibustion on trigeminal caudalis neurons in rats. Jpn J Physiol. 2000;50(1):133–140. doi:10.2170/jjphysiol.50.133
37. Tang Y, Kang J, Zhang Y, Zhang X. Influence of greater occipital nerve block on pain severity in migraine patients: a systematic review and meta-analysis. Am J Emerg Med. 2017;35(11):1750–1754. doi:10.1016/j.ajem.2017.08.027
38. Kinfe TM, Schuss P, Vatter H. Occipital nerve block prior to occipital nerve stimulation for refractory chronic migraine and chronic cluster headache: myth or prediction? Cephalalgia. 2015;35(4):359–362. doi:10.1177/0333102414541685
39. Cooke LJ, Becker WJ. Migraine prevalence, treatment and impact: the canadian women and migraine study. Can J Neurol Sci. 2010;37(5):580–587. doi:10.1017/S0317167100010738
40. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754–762. doi:10.1212/WNL.55.6.754
41. Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):274–290. doi:10.1007/s13311-017-0592-1
42. Park J, Sohn Y, White AR, Lee H. The safety of acupuncture during pregnancy: a systematic review. Acupunct Med. 2014;32(3):257–266. doi:10.1136/acupmed-2013-010480
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.