Back to Journals » Journal of Inflammation Research » Volume 14
Prognostic Value of the Preoperative Lymphocyte-C-Reactive Protein Ratio in Hepatocellular Carcinoma Patients Treated with Curative Intent: A Large-Scale Multicentre Study
Authors Zhang YF , Lu LH, Zhong C , Chen MS, Guo RP , Wang L
Received 20 March 2021
Accepted for publication 20 May 2021
Published 10 June 2021 Volume 2021:14 Pages 2483—2495
DOI https://doi.org/10.2147/JIR.S311994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yong-Fa Zhang,1,2,* Liang-He Lu,3,4,* Chong Zhong,5,* Min-Shan Chen,3,4 Rong-Ping Guo,3,4 Lu Wang1,2
1Department of Hepatic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, People’s Republic of China; 3Department of Hepatobiliary Oncology of Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 4State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, People’s Republic of China; 5Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, 510405, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Wang
Department of Hepatic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China
Tel/Fax +8620-64175590
Email [email protected]
Rong-Ping Guo
Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China
Tel/Fax +8620-87342266
Email [email protected]
Purpose: This study aimed to evaluate the prognostic value of the lymphocyte-C-reactive protein ratio (LCR) score, a novel inflammation-based score based on lymphocytes and C-reactive protein, in hepatocellular carcinoma (HCC) patients treated with curative intent.
Patients and Methods: A total of 1158 HCC patients undergoing surgical resection or radiofrequency ablation with curative intent were recruited from 3 different centres and divided into a primary cohort (n=716) and a validation cohort (n=442). Univariate and multivariate analyses were performed to identify variables associated with overall survival (OS). The discriminatory accuracy of seven inflammation-based scores was compared by using the concordance index (C-index).
Results: The LCR score differentiated HCC patients into two groups with distinct prognoses (1-, 3-, and 5-year OS rates and median OS: 92.9%, 81.9%, 73.3% and 99.2 months and 79.8%, 56.6%, 49.7% and 69.1 months; P< 0.001). Multivariate analysis showed that LCR score, AFP, ALBI score, tumour size, and TNM stage were independently associated with OS. When patients were stratified according to different disease states, the LCR score could still differentiate HCC patients into two groups with distinct prognoses (all P< 0.005). The LCR score demonstrated a markedly superior C-index of 0.621 compared with the other inflammation-based scores (0.503– 0.590). These findings were supported by the validation cohort.
Conclusion: The preoperative LCR score is a novel, stable, and clinically feasible prognostic marker for patients with HCC, independent of liver function, tumour characteristics, and treatment allocation and is superior to other inflammation-based scores in terms of its prognostic ability.
Keywords: hepatocellular carcinoma, resection, radiofrequency ablation, prognosis, LCR, inflammation-based score
Introduction
Hepatocellular carcinoma (HCC) is a major health issue and is the third leading cause of death from cancer worldwide.1 Because of the scarcity of donor organs, surgical resection and radiofrequency ablation (RFA) are the mainstay curative treatment options; globally, three-quarters of all new patients undergo these treatments.2,3 However, a serious disadvantage of local treatment with regard to achieving cure and long-term survival is the high rate of recurrence, which exceeds 60% at 5 years even in patients with small tumours.4,5 In addition, the reported long-term survival of these patients varies.5–7 There is obvious heterogeneity in patients who are classified by the commonly used staging systems as having early HCC. Therefore, it is reasonable to seek inexpensive, readily available, simplified, and objective approaches to inform clinical decision-making and stratify patients into different risk groups.
Systemic inflammation via host-tumour interactions is currently recognized as the seventh hallmark of cancer8 and is intimately involved in tumour development and metastasis in various malignancies.9–12 Based on this knowledge, haematological components of the systemic inflammatory response have been combined to develop inflammation-based prognostic scores, including the Glasgow prognostic score (GPS),13 the modified Glasgow prognostic score (mGPS),14 the neutrophil-lymphocyte ratio (NLR),15 the platelet-lymphocyte ratio (PLR),16 the prognostic index (PI),17 and the prognostic nutritional index (PNI).18 Many studies have shown that these scores are associated with the survival of cancer patients, and they have been used clinically as useful prognostic indicators for cancers, including HCC.19,20 Recently, a novel prognostic score, the lymphocyte-C-reactive protein ratio (LCR), based on the preoperative lymphocyte count and C-reactive protein (CRP), was reported to be a powerful prognostic marker for colorectal cancer,21 gastric cancer and cholangiocarcinoma.22,23 However, whether the LCR can predict the prognosis of HCC patients and its superiority to conventional inflammation-based scores remain unclear.
Therefore, this study aimed to evaluate the prognostic value of the LCR score in patients with HCC with various disease stages and liver functional statuses and to conduct a direct comparison of various inflammation-based scores in a large-scale multicentre cohort.
Patients and Methods
Patients
Consecutive patients who underwent curative hepatic resection (HR) or RFA at the Sun Yat-sen University Cancer Center between January 2010 and December 2015 were enrolled in this study. The diagnosis of HCC was confirmed pathologically or according to the European Association for the Study of the Liver (EASL) diagnostic criteria.24 Only patients who met all of the following criteria were enrolled in the study: (a) age 18–75 years; (b) Child-Pugh class A or B cirrhosis; (c) absence of extrahepatic metastasis; (d) no evidence of hepatic decompensation including ascites refractory to diuretics, oesophageal or gastric variceal bleeding, or hepatic encephalopathy; and (e) no other anticancer treatments before surgical resection or RFA. Patients were excluded from the study if one or more of the following conditions were met: (a) patients were lost to follow-up; (b) patients had missing data for classification based on any of the seven inflammation-based scores; and (c) patients had other concurrent malignancies. Finally, 716 HCC patients were enrolled in the primary cohort of this study.
In addition, we evaluated the significance of the inflammation-based scores in an independent validation cohort of HCC patients treated with curative intent from Fudan University Shanghai Cancer Center and the First Affiliated Hospital of Traditional Chinese Medicine between January 2013 and December 2016 using the same inclusion and exclusion criteria. A total of 442 HCC patients were enrolled in the validation cohort.
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, Fudan University Shanghai Cancer Center and the First Affiliated Hospital of Traditional Chinese Medicine, and complied with the standards of the Declaration of Helsinki and current ethical guidelines. Written informed consent was obtained from each participant.
Laboratory Measurements of Inflammation-Related Factors in Routine Blood Tests
All blood values recorded in this study, including CRP, bilirubin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and α-fetoprotein (AFP) levels and blood cell, neutrophil, lymphocyte, and platelet (PLT) counts, were determined within 5–7 days prior to treatment. Serum CRP levels were measured using latex-enhanced nephelometry (N-Latex CRP II; Siemens Healthcare Diagnostics, Tokyo, Japan). The LCR was calculated as follows: lymphocyte count (number/mL)/CRP level (mg/dL).21 The GPS,13 modified GPS,14 NLR,15 PLR,16 PI17 and PNI18 were constructed as described in Supplementary Table 1. The albumin-bilirubin (ALBI) score was recorded to describe liver function. Tumour stage was recorded according to the 7th edition of The American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) tumour-node-metastasis (TNM) classification.
Treatment Protocols
Hepatic resection was performed using techniques as described previously.25,26 Resectable disease was defined according to previously reported criteria as the possibility to completely remove all tumours while retaining a sufficient liver remnant to maintain postoperative liver function, as assessed by the surgical team.26 Pringle’s manoeuvre was routinely used with a clamp/unclamp time of 10 min/5 min. RFA was performed using a previously described technique under real-time ultrasound guidance.27 For patients with multiple tumours, all lesions were treated in one single session. The evaluation of the complete ablation of lesions after RFA was performed by dynamic computed tomography (CT) at 4 weeks after treatment. Complete ablation was diagnosed when a low-density area in both the arterial and portal venous phases was observed and the size of the area was larger than the lesion before treatment.28
Follow-Up
Patients were followed carefully after the initial treatment. Follow-up examinations included laboratory tests (including serum AFP, liver function, and blood tests), abdominal ultrasonography, and contrast-enhanced CT every 3 months for the first 2 years and every 6 months thereafter. All patients with HBV-related HCC who were prepared for treatment for their HCC were counselled by a hepatologist for antiviral therapy regardless of the serum HBV DNA result.29 The follow-up start date was the date of the initial diagnosis of HCC. The end of follow-up was the time of the last follow-up (December 2020) or death.
Statistical Analysis
Overall survival (OS) was calculated from the date of the initial diagnosis of HCC until death or the end of the follow-up period. Disease-free survival (DFS) was defined as the interval between the operation and the date of diagnosis of the first recurrence or the last follow-up. Comparisons were made using unpaired Student’s t-test for continuous variables and the chi-square test for categorical variables. Survival was calculated using the Kaplan-Meier method, and comparisons between groups were made using the Log rank test. The Cox regression model was used to identify independent predictors of survival. The concordance index (C-index) method was used to rank the different inflammation-based scores based on their capacity to discriminate patients according to the outcome. To avoid overoptimistic results, the prognostic performance of all inflammation-based scores was further validated in an independent external validation cohort. The statistical analysis was performed using IBM SPSS v.24.0 (SPSS, Armonk, NY, USA) and R 2.13.2 (http://www.r-project.org/). All statistical tests were 2-tailed, and a p value less than 0.05 was considered significant.
Results
Patient Characteristics
In the primary cohort, a total of 533 (74.4%) patients underwent hepatic resection, and 183 (25.6%) patients underwent RFA. In the validation cohort, a total of 315 (71.3%) patients underwent hepatic resection, and 127 (28.7%) patients underwent RFA. For the primary cohort, 149 (20.8%) patients had an elevated CRP level (>10 mg/L), and 27 (3.8%) patients had hypoalbuminemia (<35g/L). A minority of patients were allocated to GPS 2 (5.9%), mGPS 2 (2.1%), NLR 1 (3.6%), PLR 2 (1.3%), PI 2 (1.8%), and PNI 1 (9.2%). A total of 409 (57.1%) patients were allocated to LCR 0, and 307 (42.9%) patients were allocated to LCR 1. The baseline characteristics of the primary cohort and the validation cohort are provided in Table 1. In addition, more surgery-related information for the primary cohort and the validation cohort are provided in Supplementary Table 2.
 |
Table 1 Baseline Characteristics of the Patients in the Primary and Validation Cohorts |
OS of the Primary and Validation Cohorts
The median OS times for the primary and validation cohorts were 87.4 months (range, 3.2–128.2 months) and 68.9 months (range, 3.0–97.3 months), respectively. For the primary cohort, the 1-, 3-, and 5-year OS rates were 87.3%, 70.6%, and 63.5%, respectively. For the validation cohort, the 1-, 3- and 5-year OS rates were 89.1%, 71.1%, and 64.0%, respectively. The relationships between the inflammation-based scores and OS in the primary cohort are shown in Figure 1A–G. Elevated GPS, mGPS, PLR, PI, PNI, and LCR were associated with significantly reduced OS in the primary cohort (all P<0.05; except for NLR, P=0.244). However, some overlapping curves were still observed between GPS 1 and GPS 2, mGPS 1 and mGPS 2, PLR 1 and PLR 2, and PI 1 and PI 2 (Figure 1). Interestingly, the LCR score identified two groups of patients with significantly different OS rates in the primary cohort (1-, 3- and 5-year OS rates and median OS: 92.9%, 81.9%, 73.3% and 99.2 months) for LCR score =0 (n=409) and (1-, 3- and 5-year OS rates and median OS: 79.8%, 56.6%, 49.7% and 69.1 months; P<0.001) for LCR score =1 (n=307; Figure 1G). Importantly, these findings were supported by the validation cohort (Figure 2).
 |
Figure 1 The relationship between the inflammation-based scores and overall survival in HCC patients in the primary cohort: (A) GPS, (B) mGPS, (C) NLR, (D) PLR, (E) PI, (F) PNI and (G) LCR. |
 |
Figure 2 The relationship between the inflammation-based scores and overall survival in HCC patients in the validation cohort: (A) GPS, (B) mGPS, (C) NLR, (D) PLR, (E) PI, (F) PNI and (G) LCR. |
Prognostic Factors in the Primary and Validation Cohorts
For the primary cohort, univariate analysis showed that white blood cell (WBC) count, albumin, CRP, AFP, ALBI score, tumour size, tumour number, macrovascular invasion, treatment, TNM stage, GPS, mGPS, PLR, PI, PNI, and LCR score were associated with OS (Table 2). Multivariate analysis showed that only AFP (hazard ratio (HR) 1.53, 95% confidence interval (CI): 1.19–1.96; P=0.001), ALBI score (HR 1.62, 95 CI: 1.24–2.10; P<0.001), tumour size (HR 1.36, 95% CI: 1.01–1.85; P=0.044), TNM stage (HR 2.42, 95% CI: 1.83–3.20; P<0.001), and LCR score (HR 1.63, 95% CI: 1.25–2.13; P<0.0001) were independently associated with OS (Table 2). For the validation cohort, the twelve factors correlated with survival in univariate analysis are reported in Table 3. The independent prognostic factors identified by multivariate analysis were AFP (HR 1.72, 95% CI: 1.25–2.37; P=0.001), tumour size (HR 1.56, 95% CI: 1.06–2.28; P=0.023), TNM stage (HR 1.98, 95% CI: 1.39–2.82; P<0.001), and LCR score (HR 1.89, 95% CI: 1.34–2.67; P<0.0001).
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factors for OS in the Primary Cohort |
 |
Table 3 Univariate and Multivariate Analyses of Prognostic Factors for OS in the Validation Cohort |
The LCR Score Predicts Prognosis in Subgroups of HCC Patients with Different Disease States
We further verified the predictive ability of the LCR score in HCC patients with different disease states. Kaplan–Meier curves were generated for the LCR score in the primary cohort for HCC patients with different treatment allocations (resection or RFA), TNM stages, ALBI grades, aetiologies (HBV/non-HBV), AFP levels (≤200 ng/mL/>200 ng/mL), and tumour sizes (≤5 cm/>5 cm) (Table 4). When the patients were stratified according to different disease states, the LCR score could still differentiate patients into two groups with distinct OS (all P<0.005, Table 4 and Figure 3). In addition, these results were confirmed in the validation cohort (Table 4 and Figure 4). Next, we further verify the LCR score’s ability to predict DFS. The LCR score could still differentiate patients into two groups with distinct DFS in HCC patients with different disease states (Supplementary Figure 1). These results were also confirmed in the validation cohort (Supplementary Figure 2).
 |
Table 4 LCR Score Predicts OS in Subgroups of HCC Patients with Different Disease States |
Comparison of the LCR Score with Other Commonly Used Inflammation-Based Scores
We next used the C-index to determine which inflammation-based scores performed best in predicting the survival of HCC patients treated with curative intent. The LCR scores consistently had higher C-index values (0.621) than the other scoring systems (0.503–0.590) for HCC in the primary cohort (Table 5). We found similar results in the validation cohort (Table 5).
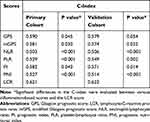 |
Table 5 Concordance Index for the Comparison of Different Inflammation-Based Scores in the Primary and Validation Cohorts |
Discussion
The identification of a simple and useful scoring system that is predictive of the prognosis of patients with HCC before treatment is an important objective. Our study is the first to report and validate that the preoperative LCR score was an independent prognostic risk factor for HCC patients treated with curative intent, independent of liver function, tumour characteristics, and treatment allocation. All findings were reproducible in a second independent validation cohort. These results will facilitate further clinical research on the importance of systemic inflammation for the prognosis of HCC patients.
HCC is biologically very heterogeneous.30,31 It has also been shown that even for patients in early stages, those who undergo radical treatment have different outcomes.4–6 To date, a few studies have used highly sophisticated gene expression analyses to analyse the complex molecular signatures of HCC to distinguish its heterogeneity and prognosis.32,33 However, these analyses are currently expensive and have unreliable repeatability; thus, they have not been widely used in clinical practice. Therefore, there is an urgent need for an easily determinable, simple, widely applicable, low-tech, and inexpensive marker from blood that can identify patients with very dismal prognostic features despite treatment.34 In this study, subgroup analyses with respect to TNM stage, treatment allocation, and ALBI grade supported the prognostic relevance of LCR independent of the disease state. In fact, the present study showed that TNM stage II patients with low LCR scores had a similar median OS as TNM stage I patients with high LCR scores (Table 4). Similar results were found in comparing TNM stage III/IV patients with low LCR scores and TNM stage II patients with high LCR scores. Even more surprisingly, ALBI grade I/II patients who had low LCR scores virtually had better OS rates than patients with ALBI grade I who had high LCR scores (Table 4). These findings are of key clinical relevance since the LCR score identified subgroups with different prognoses within a defined TNM stage or ALBI grade and different treatment allocations. Moreover, lymphocyte and CRP determination are inexpensive, reproducible, objective, widely available, and routinely performed in clinical practice and does not rely on invasive tissue collection. The reproducibility of our results was verified in two independent data sets, further supporting the reliability of the LCR score as a prognostic marker for HCC patients. In addition, a recent meta-analysis data provided evidence that transarterial chemoembolization+RFA offer comparable oncologic outcomes in patients with HCC as compared with resection and with added benefit of lower morbidity.35 Whether the LCR score have a predictive value in combination therapy needs further investigation.
Accumulating studies have demonstrated the potential of various types of systemic inflammatory factors as prognostic markers for determining oncological outcomes in human malignancies.19,20,36 Notably, there is evidence that the inflammatory field effect, reflected by elevated CRP, may be directly involved in tumour progression, which could explain its prognostic significance in HCC.37–39 Several risk factors are currently known be associated with HCC recurrence, including inflammation-related factors.7 However, the question of whether aggressive tumour behaviour prompts a prognostically detrimental inflammatory reaction or whether inflammation per se drives tumour progression remains to be elucidated.9,12 Our study revealed that high LCR score is significantly associated with aggressive and invasive factors (AFP, tumor size, tumor number, macrovascular invasion) and with high early recurrence of HCC patients as well (Supplementary Table 3). For all this, further preclinical studies in HCC are needed to elucidate the causal mechanisms of LCR in HCC progression.
There were a few potential limitations in this study. First, although we evaluated the preoperative LCR score in a large, multicentre cohort of patients with HCC, this was a retrospective study. Furthermore, another major limitation was that our three data sets included patients with HCC in an HBV-endemic area. It will certainly be necessary to validate the LCR score in other geographic regions to extend our results to patients with HCC of various aetiologies.
In conclusion, our study identified the LCR score as a novel, non-invasive, inexpensive, objective, available, and widely applicable prognostic marker for patients with HCC, irrespective of tumour stage, liver function and treatment allocation, and the LCR score was superior to other inflammation-based scores in terms of its prognostic ability. The LCR score may help surgeons determine surgical risk and oncological risk, thus facilitating the appropriate perioperative and postoperative management of patients with HCC.
Funding
This study was supported by grants from the Shanghai Sailing Program (No. 18YF1404900) and Clinical Research Plan of SHDC (SHDC2020CR4018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
4. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi:10.1097/00000658-200203000-00009
5. Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. doi:10.1097/SLA.0000000000000747
6. Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi:10.1016/j.jhep.2018.08.027
7. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi:10.1016/S0168-8278(02)00360-4
8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
9. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
10. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
11. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
12. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
13. Shedda S, Kosmider S, Faragher I, Jones I, Gibbs P. A critical review of the Glasgow prognostic score for colorectal cancer. Ann Surg. 2008;247(6):1087–1088; author reply 1088. doi:10.1097/SLA.0b013e3181758df1
14. Hirashima K, Watanabe M, Shigaki H, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49(6):1040–1046. doi:10.1007/s00535-013-0855-5
15. Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–151. doi:10.1097/SLA.0b013e3181a77e59
16. Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. doi:10.1038/bjc.2015.183
17. Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17(4):52–58. doi:10.3747/co.v17i4.567
18. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106(8):1439–1445. doi:10.1038/bjc.2012.92
19. Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi:10.1016/j.ejca.2011.03.028
20. Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107(6):988–993. doi:10.1038/bjc.2012.354
21. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2019.
22. Lu LH, Zhong C, Wei W, et al. Lymphocyte-C-reactive protein ratio as a novel prognostic index in intrahepatic cholangiocarcinoma: a Multicentre Cohort Study. Liver Int. 2021;41(2):378–387. doi:10.1111/liv.14567
23. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2019;39(4):1209–1217. doi:10.1016/j.clnu.2019.05.009
24. European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018.
25. Zhang YF, Zhou J, Wei W, et al. Intermediate-stage hepatocellular carcinoma treated with hepatic resection: the NSP score as an aid to decision-making. Br J Cancer. 2016;115(9):1039–1047. doi:10.1038/bjc.2016.301
26. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71
27. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432. doi:10.1200/JCO.2012.42.9936
28. Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221(2):447–454. doi:10.1148/radiol.2212010446
29. Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33(10):1104–1112. doi:10.1111/j.1365-2036.2011.04634.x
30. Villanueva A, Llovet JM, Groszmann RJ, Iwakiri Y, Taddei TH. Impact of intra-individual molecular heterogeneity in personalized treatment of hepatocellular carcinoma. Hepatology. 2012;56(6):2416–2419. doi:10.1002/hep.26124
31. Kim DY, Han KH. Staging for hepatocellular carcinoma in light of tumor heterogeneity: time to change or update? Hepatology. 2018;67(6):2076–2078. doi:10.1002/hep.29748
32. Nault JC, De Reynies A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145(1):176–187. doi:10.1053/j.gastro.2013.03.051
33. Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori M. Molecular signatures of noncancerous liver tissue can predict the risk for late recurrence of hepatocellular carcinoma. J Gastroenterol. 2010;45(2):146–152. doi:10.1007/s00535-009-0164-1
34. Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8(15):912. doi:10.21037/atm-2020-90
35. Gui CH, Baey S, D’Cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46(5):763–771. doi:10.1016/j.ejso.2020.01.004
36. Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi:10.1002/hep.26057
37. Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–1864. doi:10.1002/cncr.20976
38. Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12(3):252–265. doi:10.1016/j.ccr.2007.08.008
39. She S, Jiang L, Zhang Z, et al. Identification of the C-reactive protein interaction network using a bioinformatics approach provides insights into the molecular pathogenesis of hepatocellular carcinoma. Cell Physiol Biochem. 2018;48(2):741–752. doi:10.1159/000491903
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


