Back to Journals » Clinical Epidemiology » Volume 15
Prognostic Value of Post-Operative C-Reactive Protein-Based Inflammatory Biomarkers in Colorectal Cancer Patients: Systematic Review and Meta-Analysis
Authors Gwenzi T , Zhu A, Schrotz-King P , Schöttker B , Hoffmeister M , Edelmann D, Brenner H
Received 28 April 2023
Accepted for publication 9 June 2023
Published 27 June 2023 Volume 2023:15 Pages 795—809
DOI https://doi.org/10.2147/CLEP.S415171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Henrik Sørensen
Tafirenyika Gwenzi,1,2 Anna Zhu,2,3 Petra Schrotz-King,1 Ben Schöttker,3 Michael Hoffmeister,3 Dominic Edelmann,4 Hermann Brenner1,3,5,6
1Division of Preventive Oncology, German Cancer Research Center (DKFZ) and National Center for Tumor Diseases (NCT), Heidelberg, 69120, Germany; 2Medical Faculty Heidelberg, Heidelberg University, Heidelberg, 69120, Germany; 3Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, 69120, Germany; 4Division of Biostatistics, German Cancer Research Center (DKFZ), Heidelberg, 69120, Germany; 5Network Aging Research, Heidelberg University, Heidelberg, 69115, Germany; 6German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, 69120, Germany
Correspondence: Hermann Brenner, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 581, Heidelberg, 69120, Germany, Tel +49 6221 42 1300, Fax +49-6221 42 1302, Email [email protected]
Abstract: Post-operative inflammation in cancer patients can be modulated by drugs and diets, but evidence on its prognostic role, which would be crucial for personalized treatment and surveillance schemes, remains rather limited. We aimed to systematically review and meta-analyse studies on the prognostic value of post-operative C-reactive protein (CRP)-based inflammatory biomarkers among patients with colorectal cancer (CRC) (PROSPERO#: CRD42022293832). PubMed, Web of Science and Cochrane databases were searched until February 2023. Studies reporting associations between post-operative CRP, Glasgow Prognostic Score (GPS) or modified Glasgow Prognostic Score (mGPS) with overall survival (OS), CRC-specific survival (CSS) and recurrence-free survival (RFS) were included. Hazard ratios (HRs) with 95% confidence intervals (CIs) for the predictor-outcome associations were pooled using R-software, version 4.2. Sixteen studies (n = 6079) were included in the meta-analyses. Elevated post-operative CRP was a predictor of poor OS, CSS and RFS compared with low CRP levels [HR (95% CI): 1.72 (1.32– 2.25); 1.63 (1.30– 2.05); 2.23 (1.44– 3.47), respectively]. A unit increase in post-operative GPS predicted poor OS [HR (95% Cl): 1.31 (1.14– 1.51)]. Moreover, a unit increase in post-operative mGPS was associated with poor OS and CSS [HR (95% Cl): 1.93 (1.37– 2.72); 3.16 (1.48– 6.76), respectively]. Post-operative CRP-based inflammatory biomarkers have a significant prognostic role for patients with CRC. Prognostic value of these easy-to-obtain routine measurements thereby seems to outperform most of the much more complex blood- or tissue-based predictors in the current focus of multi-omics-based research. Future studies should validate our findings, establish optimal time for biomarker assessment and determine clinically useful cut-off values of these biomarkers for post-operative risk-stratification and treatment-response monitoring.
Keywords: C-reactive protein, survival, assess, risk-stratification, treatment-response
Introduction
Colorectal cancer (CRC) remains the second most common cause of cancer death worldwide despite improvements in early detection, surgical techniques, and chemoradiotherapy.1 Prognosis most strongly depends on stage at diagnosis, with 5-year relative survival ranging from above 90% for stage I to less than 20% for stage IV cancers.2 However, evidence on prognostic factors besides and within stages, which would be crucial for personalized treatment and surveillance schemes, remains rather limited.
CRC prognosis is strongly linked to host inflammatory response at both tumor micro-environment and systemic levels.3,4 In recent years, an increasing number of studies have reported post-treatment inflammatory blood biomarkers to be predictive of CRC outcomes.5–12 While inflammation can be reduced by anti-inflammatory drugs13–15 and diets with low dietary inflammation scores,16–18 comparative and integrative evaluation of clinical relevance of post-operative inflammatory response is limited due to heterogeneity in key design features of pertinent studies, such as selection of inflammatory markers, definition of cut-offs, timing of blood draw, length of follow-up, specific survival endpoints assessed and covariates adjusted for.
The most widely reported inflammatory marker in cancer research is C-reactive protein (CRP), whose analysis in cancer monitoring is routine, relatively cheap, and simple.19 Serum CRP has been reported as a useful CRC prognosticator, either alone or in combination with albumin as is the case with the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS).20 High GPS and mGPS are reflective of an elevated inflammatory response and poor nutritional state.21 In this systematic review and meta-analysis, we aim to evaluate and summarize the evidence on the prognostic value of post-operative serum CRP-based parameters (CRP, GPS and mGPS) among CRC patients with respect to various survival outcomes.
Materials and Methods
The protocol of this systematic review was registered in the international prospective register of systematic reviews (PROSPERO, registration no. CRD42022293832). For the systematic review, the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed and presented in the Supplementary Material “Prisma Checklist”.22 For meta-analysis, the Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines were followed.23
Search Strategy and Data Extraction
The targets for this review were original observational studies focusing on post-operative serum CRP-based parameters (CRP, GPS and mGPS) as predictors of survival outcomes among patients with CRC. The outcomes of interest were overall survival (OS), CRC-specific survival (CSS) and recurrence-free survival (RFS). Unpublished studies, abstracts, reviews, dissertations, theses, editorials, study protocols, clinical guidelines, commentaries, and letters were excluded. We further excluded studies that did not have details of CRP-based biomarker measurement times with respect to surgery, reported exposure-outcome associations based on pre-operative CRP-based biomarkers, did not report on any of the survival outcomes of interest, or had patients who did not undergo surgical treatment for CRC. Studies were included in a meta-analysis if they had reported hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between post-operative serum CRP-based parameters and any of the listed CRC outcomes of interest.
Systematic searches were conducted using Medline (PubMed interface), the Cochrane Central Register of Controlled Trials (CENTRAL), and ISI Web of Science databases from inception until February 2023. Non-English publications were excluded. The EndNote software version 9 was used for reference management. Two researchers (T.G and A.Z) with the help of a librarian conducted the searches and screened studies for review inclusion. The PRISMA study flow diagram is provided in Figure 1 and the search strings are given in Supplementary Table 1. For database searches, we used medical subject headings (MeSH), free-text words, synonyms, and related terms for the concepts “colorectal neoplasm”, “serum inflammatory marker”, “post-operative”, “prognosis” and “survival”.
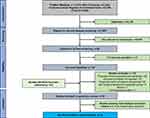 |
Figure 1 PRISMA flow chart. |
From included studies, two researchers (T.G, A.Z) independently extracted the following data: first author, publication year, country, number of participants, cancer stage, cancer site, sex, mean/median age, time of blood sample collection, predictor serum marker, CRC prognostic outcomes, hazard ratios (HRs) and 95% confidence intervals for the predictor-outcome associations. When possible, the most adjusted estimates of HRs were extracted. Disagreements arising after independent data extraction were resolved by further review and discussion in consultation with all authors. For studies that did not report on any of the predefined data domains, contacts were made to request for the additional details from the corresponding authors.
Assessment of Study Quality
The Quality In Prognosis Studies (QUIPS) tool was independently used by two researchers (T.G, A.Z) to assess the risk of bias for individual studies.24,25 Studies were judged as of low, moderate or high risk of bias based on six domains of study participation, attrition, prognostic factor measurement, outcome measurement, confounding, and statistical analysis and reporting. In cases of critical point disagreements between the two researchers, consensus was reached by further discussion among all authors.
Statistical Analyses
For a given predictor, meta-analysis was conducted if at least two studies reported on the predictor-outcome association. Random effects meta-analysis was applied on the corresponding HRs using the inverse variance method for pooling. For the meta-analyses, the values of the predictor variables were categorized as shown in Supplementary Table 2. The HRs of the individual studies and the pooled HR were visualised in forest plots. The restricted maximum likelihood estimator was used for estimation of the between-study variance τ2. The I² statistic, expressing the percentage of total variability due to study heterogeneity, was calculated. Testing for heterogeneity was performed using Cochran’s Q-test. Where possible, publication bias was assessed using Egger`s linear regression test of funnel plot asymmetry. In addition, sensitivity analyses were conducted by assessing the effect of time of blood collection on prognostic associations and excluding studies that had not adjusted for key covariates (age and stage). All statistical analyses were conducted with R-software 4.2 using the package “meta”, version 5.5.26 All p-values were two-sided, and significance level was set at 0.05.
Results
Literature Search
The PRISMA flow diagram for study selection is depicted in Figure 1. A total number of 9242 individual studies were reviewed, and of those, 89 were considered in the full-text screening. In addition, by examining the reference lists of included studies, we identified two additional studies that were not captured through the initial search strategy. A list of studies excluded in the full-text selection and the respective exclusion criteria can be found in Supplementary Table 3.
Study Characteristics
General information about the included studies is provided in Table 1. A total of 19 studies were included in the systematic review, with sample sizes ranging from 147 to 813 and a total number of 6895 patients. Eight studies were conducted in the United Kingdom (UK),27–34 six in Asia,21,35–39 one in the United States40 and the rest originated from the European region.41–44 Twelve studies used serum CRP levels as a prognostic predictor, five used GPS and four used mGPS for prognostication. In six studies, blood samples for laboratory analysis were collected within a week after surgery, while in ten studies blood samples were collected between one and six months after surgery. For prognostic analysis, most of the studies where blood samples were taken one or more months after surgery used cut-off points of 10 mg/L and 35 g/L for CRP and albumin, respectively. Much higher cut-offs for CRP (mostly >150 mg/L, range 150 to 170 mg/L) and a lower cut-off for albumin (25 g/L) were used in the studies collecting blood samples within a week after surgery. Eleven studies adjusted for at least age and cancer stage (TNM or Duke`s). Supplementary Table 4 shows other covariates adjusted for in the included studies such as sex, comorbidities, surgical procedure, use of neoadjuvant/adjuvant therapy, and surgical complications. Quality assessment results based on QUIPS are reported in Supplementary Table 5 and presented in Figure 2. High risk of bias was observed for the domain of study confounding in five studies.21,33,35,36,39
 |
Table 1 Characteristics of Included Studies |
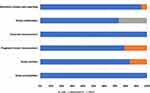 |
Figure 2 Graphical presentation of quality assessment of 19 studies included in the systematic review (QUIPS risk of bias assessment). |
C-Reactive Protein and CRC Prognostic Outcomes
Results of studies investigating the association between post-operative CRP and survival showed that elevated CRP was a significant predictor of poor OS, CSS and RFS compared with low CRP levels [HR (95% CI): 1.72 (1.32–2.25); 1.63 (1.30–2.05); 2.23 (1.44–3.47), respectively] (Figure 3). Substantial heterogeneity was observed in the meta-analysis of nine studies on the association between CRP and OS (I2 = 70%, p < 0.01) (Figure 3A). Therefore, sensitivity analyses were conducted to investigate the sources of heterogeneity. The pooled result of five studies28,40–43 investigating the relationship between CRP assessed within four weeks to four months post-operatively (cut-off = 10mg/L) and OS showed more than 2-fold hazard ratio for those above the cut-off (HR, 95% CI: 2.24, 1.45–3.44) (Figure 4A). Considerable heterogeneity (I2 = 69%, p < 0.01) was observed for this meta-analysis. When restricting the analysis to the three studies that had adjusted for age and cancer stage, an even stronger association with no heterogeneity was observed (adjusted HR, 95% CI: 2.40, 1.60–3.61) (Figure 4B). Meta-analysis for three studies on the association between CRP assessed within a week after surgery (cut-off range = 150–170mg/L) and OS also showed a significant albeit weaker association (HR, 95% CI: 1.50, 1.12–2.01) (Figure 4C). For this meta-analysis, the percentage of between-study heterogeneity was 39% and only one study adjusted for age and cancer stage.32 Notably, the association was much stronger in that single study (adjusted HR, 95% CI: 2.14, 1.34–3.41) than in the two studies without such adjustment.
The pooled result of two studies showed approximately 2-fold higher risk for poor CSS among patients whose CRP assessed at four months post-operatively was above 10mg/L (HR, 95% CI: 1.93, 1.34–2.79) (Figure 4D). A weaker but significant relationship between CRP and CSS was also seen in the meta-analysis of four studies that assessed CRP within one week post-operatively (cut-off range = 150–170mg/L) (HR, 95% CI: 1.52, 1.16–1.97) (Figure 4E). One of these studies by McSorley et al adjusted for multiple covariates including sex, tumor-node-metastasis (TNM) stage and surgical complications.32
Glasgow Prognostic Score and CRC Prognostic Outcomes
Meta-analysis of two studies33,34 investigating the relationship between GPS determined within a week post-operatively and OS showed approximately 30% higher risk for poor OS for each unit increase in GPS (HR, 95% CI: 1.31, 1.14–1.51) (Figure 5A). Two other studies investigating the association between GPS measured four weeks after surgery suggested a stronger association with OS, but results could not be combined in a meta-analysis because of different coding of GPS in the analysis. One study37 reported an adjusted HR (95% CI) of 1.66 (0.98–2.80) per unit increase in GPS while the other one35 reported a HR (95% CI) of 1.98 (0.93–4.21) comparing combined GPS 1 and 2 with GPS 0 without controlling for any covariates.
Modified Glasgow Prognostic Score and CRC Prognostic Outcomes
Meta-analysis of two studies30,37 investigating the relationship between mGPS determined ≥ four weeks post-operatively and OS showed approximately 2-fold higher risk for poor OS for each unit increase in mGPS among patients with CRC (adjusted HR, 95% CI: 1.93, 1.37–2.72) (Figure 5B). Pooled results of two studies30,31 investigating the relationship between mGPS assessed ≥ four weeks after surgery and CSS showed a particularly strong association per unit increase in mGPS [HR (95% CI): 3.16, 1.48–6.76] (Figure 5C). Both studies were from the UK but one study30 adjusted for at least age and cancer stage.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis evaluating the prognostic value of CRP-based post-operative inflammatory response and CRC survival. Our results show substantially increased risk for poor survival among patients with elevated CRP, GPS and mGPS measured four or more weeks after surgery.
Epidemiological studies have recently shown that CRP, GPS and mGPS are significant predictors of survival in different cancers including CRC.45,46 Although the exact mechanisms on how CRP and albumin are linked to CRC prognosis after surgery are elusive, post-operative tissue injury, infection, or residual tumor cells have been reported to induce immune-cell-mediated acute or chronic inflammation.47,48 Activation of immune cells promotes release of several pro-inflammatory cytokines, such as interleukin-6 (IL-6), IL-1, and tumor necrosis factor α (TNF-α).49 In addition, pro-inflammatory diets may increase serum levels of IL-6, CRP and TNF-α particularly for CRC patients with a low count of tumor-infiltrating lymphocytes.18 In liver hepatocytes, pro-inflammatory cytokines induce CRP production while suppressing albumin production. Elevated CRP increases expression of oncogenes resulting in DNA damage, impaired immune function, cell proliferation, angiogenesis, apoptotic resistance, tumor growth, invasion, and progression.50 On the other hand, hypoalbuminemia may reduce tolerance to anticancer drugs and therefore increase therapeutic toxicity.51 Given this background, it seems plausible that post-operative serum CRP and albumin may be valuable prognosticators for CRC patients undergoing potentially curative resection.
Our meta-analyses suggest that CRC patients with elevated serum CRP levels (≥10mg/L) four or more weeks after surgery have an increased risk for poor prognosis compared with patients who have normal CRP levels (<10 mg/L). CRP assessments done within a week after surgery also showed associations with survival, but such associations were reported for much higher CRP elevations (≥150 mg/L) and summary estimates of HRs from meta-analyses were lower than those for CRP measurements done four or more weeks after surgery. Higher CRP levels within a week after surgery may predominantly reflect consequences of surgical stress and wound healing, which may be overcome to a large extent after four weeks. Consequently, inflammatory biomarkers assessed within four weeks after surgery may be less predictive of long-term prognosis than CRP elevations beyond this period.
GPS and mGPS are metrics based on a combination of the acute phase proteins CRP and albumin, which are reflective of an individual’s immune and nutritional status. Elevated GPS and mGPS reflect abnormally high CRP and/or hypoalbuminemia. According to our meta-analysis, elevated GPS and mGPS assessed four weeks to six months following surgery are strongly associated with worse overall survival of CRC patients. Furthermore, a unit increase in mGPS assessed four weeks after surgery was associated with about three times higher risk for poor CRC-specific survival. These findings are consistent with previous studies that have reported a significant independent predictive role of CRP/Albumin for recurrence of other cancers such as renal cell carcinoma,52–54 esophageal cancer,55,56 hepatocellular carcinoma,57 cholangiocarcinoma,58 and nasopharyngeal cancer.59
In addition to the already discussed role of elevated CRP in CRC prognosis, hypoalbuminemia may account for poor prognosis observed in patients with elevated GPS or mGPS. In a previous systematic review and meta-analysis of studies with over 1.49 million participants with various disease conditions, high serum albumin levels (>35g/L) were strongly associated with better overall survival (HR, 95% CI = 0.66, 0.55–0.77) and cancer-specific survival (HR, 95% CI = 0.65, 0.48–0.88).45 Other studies have also reported poor overall survival for patients with low serum albumin levels in various conditions including diabetes,46 cardiovascular disease,60,61 COVID-19,62–66 kidney disease,67 and liver cirrhosis.68–71 Thus, dysregulation of albumin in CRC patients may be predictive of poor prognosis possibly reflecting an impaired immune and liver function, poor nutritional status or a compromised therapeutic tolerance.72
Potential Clinical Implications and Need for Further Research
How to best monitor course of disease of CRC patients, enable personalized judgment of prognosis and make best possible personalized post-operative treatment decisions is subject to intensive ongoing research. However, most biomarkers are strongly related to stage at diagnosis and provide rather limited prognostic value beyond tumor stage. This particularly applies to biomarkers that are either up- or downregulated prior to surgery. In addition, biomarkers measured shortly after surgery, for example during the hospital stay following surgery, may primarily reflect surgical stress and post-surgical wound healing and be less relevant for judgment of long-term prognosis. Our results suggest that measurement of CRP, GPS and mGPS in blood samples taken four weeks to six months after surgery could be more helpful in this respect. Given that they can be easily measured at low cost in almost any standard laboratory, their measurement could be easily implemented in routine clinical care. Further research is needed, however, to evaluate the use of their prognostic information for enhanced, personalized post-operative care and surveillance. If and to what extent these biomarkers may also be helpful for evaluating efficacy of further (post-operative) treatments or measures of tertiary prevention, should also be addressed in future research. While a significant number of individual studies (7 in total) included patients with stage IV cancer, we could not conduct subgroup analyses by stage because studies did not report stage-stratified results (probably because of small sample sizes). We suggest future studies to be designed with this in mind and ascertain if these prognostic biomarkers may be useful in palliative care settings.
Strengths and Limitations
Most studies included in our review were of high quality for the various domains assessed for quality. However, despite the comprehensive literature search, we cannot rule out having missed non-English studies that were excluded because of inadequate resources. Furthermore, there was heterogeneity in the degree of adjustment for covariates. In particular, some studies did not adjust for any covariates. This would be a major concern if the aim was to assess the causal role of inflammation. However, our focus was on the use of the inflammation-related markers for prognostication. Interestingly, associations for CRP and mGPS with overall survival persisted or increased rather than decreased when meta-analyses were restricted to studies with covariate adjustment. These patterns suggest that these biomarkers may be useful to refine assessment of survival perspectives beyond the established prognostic criteria such as stage at diagnosis. Carefully planned future studies should provide associations with different levels of adjustment to better delineate the prognostic value of CRP-based markers beyond established prognostic criteria. Future studies should also address potential differences in the prognostic value of CRP-based blood markers between men and women.
Variations in adjuvant therapies, lifestyle, environmental and genetic factors across patient cohorts from different countries may also be a source of heterogeneity. While there is limited evidence on ethnic variations of CRP/albumin serum levels, a previous study has reported significant variations in a leucocyte-based inflammatory response parameter among Europeans, East Asians, Black Americans, Hispanic Americans, and White Americans.73 Nevertheless, the consistency of our results across study populations suggests that the prognostic role of CRP-based biomarkers may hold in a wide range of different populations. However, the small number of studies identified for each association hindered to address this in more detail and also hindered meaningful analysis of publication bias. Furthermore, many included studies did not provide details of technologies and methods of serum assays which may vary widely.
Conclusions
This systematic review and meta-analysis of patient cohort studies suggests that serum CRP-based biomarker levels, measured four weeks or more weeks after surgery, could be an easy-to-determine highly informative prognostic marker for CRC patients. In particular, they could provide important incremental prognostic information that is independent of tumor stage, age, and other established prognostic markers. Potential applications, to be corroborated, validated and expanded in further research may be possible use of CRP-based biomarkers for long-term monitoring of the course of the disease, risk stratification for treatment decisions, and evaluation of the efficacy of post-operative treatment and tertiary prevention measures.
Abbreviations
CRC, Colorectal cancer; CRP, C-reactive protein; CSS, Colorectal cancer-specific survival; GPS, Glasgow Prognostic Score; HR, Hazard Ratio; IL, Interleukin; mGPS, Modified Glasgow Prognostic Score; MOOSE, Meta-analyses of observational studies in epidemiology; OS, Overall survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QUIPS, Quality In Prognosis Studies; RFS, Recurrence-free survival; TNF-α, Tumor necrosis factor-alpha; TNM, Tumor node metastasis; 95% CI, 95% confidence interval.
Data Sharing Statement
All data generated or analysed during this study are included in this published article [and its Supplementary Information Files].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Dominic Edelmann reports Lecture fees from Pfizer, outside the submitted work. Prof. Dr. Hermann Brenner reports grants from German Federal Ministry of Education and Research, during the conduct of the study. The authors declare that they have no other financial or non-financial competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. CancerNet. Colorectal cancer statistics; 2022. Available from: https://www.cancer.net/cancer-types/colorectal-cancer/statistics.
3. Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi:10.1016/j.critrevonc.2017.06.002
4. Rossi S, Basso M, Strippoli A, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16(4):264–274. doi:10.1016/j.clcc.2017.03.015
5. Haruki K, Shiba H, Fujiwara Y, et al. Perioperative change in peripheral blood monocyte count may predict prognosis in patients with colorectal liver metastasis after hepatic resection. J Surg Oncol. 2012;106(1):31–35. doi:10.1002/jso.23033
6. Chan JCY, Diakos CI, Chan DLH, et al. A longitudinal investigation of inflammatory markers in colorectal cancer patients perioperatively demonstrates benefit in serial remeasurement. Ann Surg. 2018;267(6):1119–1125. doi:10.1097/SLA.0000000000002251
7. Li Z, Zhao R, Cui Y, Zhou Y, Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci Rep. 2018;8(1):9453. doi:10.1038/s41598-018-27896-y
8. Matsuoka H, Ando K, Hu Q, et al. Postoperative C-reactive protein/albumin ratio is a biomarker of risk of recurrence and need for adjuvant chemotherapy for stage III colorectal cancer. Int J Clin Oncol. 2020;25(7):1318–1326. doi:10.1007/s10147-020-01672-3
9. Lin JX, Wang ZK, Huang YQ, et al. Dynamic changes in pre- and postoperative levels of inflammatory markers and their effects on the prognosis of patients with gastric cancer. J Gastrointest Surg. 2021;25(2):387–396. doi:10.1007/s11605-020-04523-8
10. Tan JW, Thiagarajan S, Zhou S, et al. ASO author reflections: postoperative inflammatory markers as a surveillance tool in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2021;28(11):6636–6637. doi:10.1245/s10434-021-09733-1
11. Thiagarajan S, Tan JW, Zhou S, et al. Postoperative inflammatory marker surveillance in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2021;28(11):6625–6635. doi:10.1245/s10434-020-09544-w
12. Yasui K, Shida D, Nakamura Y, Ahiko Y, Tsukamoto S, Kanemitsu Y. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer. 2021;124(5):933–941. doi:10.1038/s41416-020-01189-6
13. Hiľovská L, Jendželovský R, Fedoročko P. Potency of non-steroidal anti-inflammatory drugs in chemotherapy. Mol Clin Oncol. 2015;3(1):3–12. doi:10.3892/mco.2014.446
14. Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing Drugs in Oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
15. Zhang Z, Chen F, Shang L. Advances in antitumor effects of NSAIDs. Cancer Manag Res. 2018;10:4631–4640. doi:10.2147/CMAR.S175212
16. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. doi:10.1038/ni.3754
17. Kaluza J, Håkansson N, Harris HR, Orsini N, Michaëlsson K, Wolk A. Influence of anti-inflammatory diet and smoking on mortality and survival in men and women: two prospective cohort studies. J Intern Med. 2019;285(1):75–91. doi:10.1111/joim.12823
18. Ugai T, Liu L, Tabung FK, et al. Prognostic role of inflammatory diets in colorectal cancer overall and in strata of tumor-infiltrating lymphocyte levels. Clin Transl Med. 2022;12(11):e1114. doi:10.1002/ctm2.1114
19. Johnson TV, Abbasi A, Owen-Smith A, et al. Postoperative better than preoperative C-reactive protein at predicting outcome after potentially curative nephrectomy for renal cell carcinoma. Urology. 2010;76(3):766.e761–765. doi:10.1016/j.urology.2010.01.052
20. Toiyama Y, Miki C, Inoue Y, Tanaka K, Mohri Y, Kusunoki M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011;2(1):95–101. doi:10.3892/etm.2010.175
21. Son W, Shin SJ, Park SH, et al. Clinical impact of combined modified Glasgow prognostic score and c-reactive protein/albumin ratio in patients with colorectal cancer. Diagnostics. 2020;10(11):859. doi:10.3390/diagnostics10110859
22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi:10.1186/s13643-021-01626-4
23. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi:10.1001/jama.283.15.2008
24. Moons KGM, Hooft L, Williams K, Hayden JA, Damen J, Riley RD. Implementing systematic reviews of prognosis studies in Cochrane. Cochrane Database Syst Rev. 2018;(10). doi:10.1002/14651858.ED000129
25. Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi:10.1136/bmj.k4597
26. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi:10.1136/ebmental-2019-300117
27. Wigmore SJ, McMahon AJ, Sturgeon CM, Fearon KC. Acute-phase protein response, survival and tumour recurrence in patients with colorectal cancer. Br J Surg. 2001;88(2):255–260. doi:10.1046/j.1365-2168.2001.01669.x
28. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90(2):215–219. doi:10.1002/bjs.4038
29. Crozier JE, McKee RF, McArdle CS, et al. Preoperative but not postoperative systemic inflammatory response correlates with survival in colorectal cancer. Br J Surg. 2007;94(8):1028–1032. doi:10.1002/bjs.5706
30. Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97(9):1266–1270. doi:10.1038/sj.bjc.6604027
31. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109(1):24–28. doi:10.1038/bjc.2013.330
32. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832–2840. doi:10.1245/s10434-016-5204-5
33. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100–1109. doi:10.1245/s10434-016-5659-4
34. McSorley ST, Tham A, Dolan RD, et al. Perioperative blood transfusion is associated with postoperative systemic inflammatory response and poorer outcomes following surgery for colorectal cancer. Ann Surg Oncol. 2020;27(3):833–843. doi:10.1245/s10434-019-07984-7
35. Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol. 2015;13(1):194. doi:10.1186/s12957-015-0609-3
36. Yamamoto M, Saito H, Uejima C, et al. Prognostic value of the combination of pre- and postoperative C-reactive protein in colorectal cancer patients. Surg Today. 2018;48(11):986–993. doi:10.1007/s00595-018-1689-9
37. Zhou ZQ, Pang S, Yu XC, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798–808. doi:10.1007/s11596-018-1946-6
38. Matsubara D, Arita T, Nakanishi M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2019;25(4):602–613. doi:10.1007/s10147-019-01580-1
39. Li JJ, Zhang YW, Xu Q, et al. Systemic inflammatory markers of resectable colorectal cancer patients with different mismatch repair gene status. Cancer Manag Res. 2021;13:2925–2935. doi:10.2147/CMAR.S298885
40. Hua XW, Kratz M, Malen RC, et al. Association between post-treatment circulating biomarkers of inflammation and survival among stage II-III colorectal cancer patients. Br J Cancer. 2021;125(6):806–815. doi:10.1038/s41416-021-01458-y
41. Hermunen K, Soveri LM, Boisen MK, et al. Postoperative serum CA19-9, YKL-40, CRP and IL-6 in combination with CEA as prognostic markers for recurrence and survival in colorectal cancer. Acta Oncol. 2020;59(12):1416–1423. doi:10.1080/0284186X.2020.1800086
42. Osterman E, Mezheyeuski A, Sjoblom T, Glimelius B. Beyond the NCCN risk factors in colon cancer: an evaluation in a Swedish population-based cohort. Ann Surg Onc. 2020;27(4):1036–1045. doi:10.1245/s10434-019-08148-3
43. Lehtomaki K, Mustonen H, Kellokumpu-Lehtinen PL, et al. Lead time and prognostic role of serum CEA, CA19-9, IL-6, CRP, and YKL-40 after adjuvant chemotherapy in colorectal cancer. Cancers. 2021;13(15):3892. doi:10.3390/cancers13153892
44. Wesselink E, Balvers MGJ, Kok DE, et al. Levels of inflammation markers are associated with the risk of recurrence and all-cause mortality in patients with colorectal cancer. Cancer Epidem Biomar. 2021;30(6):1089–1099. doi:10.1158/1055-9965.EPI-20-1752
45. Seidu S, Kunutsor SK, Khunti K. Serum albumin, cardiometabolic and other adverse outcomes: systematic review and meta-analyses of 48 published observational cohort studies involving 1,492,237 participants. Scand Cardiovasc J. 2020;54(5):280–293. doi:10.1080/14017431.2020.1762918
46. Marques P, de Vries F, Dekkers OM, Korbonits M, Biermasz NR, Pereira AM. Serum inflammation-based scores in endocrine tumors. J Clin Endocrinol Metab. 2021;106(10):3796–3819. doi:10.1210/clinem/dgab238
47. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
48. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
49. Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. doi:10.1053/j.gastro.2010.01.058
50. Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-reactive protein and cancer—diagnostic and therapeutic insights. Front Immunol. 2020;11(1):595835. doi:10.3389/fimmu.2020.595835
51. Murdock JL, Duco MR, Reeves DJ. Tolerability of highly protein bound targeted oral oncolytic drugs in patients with hypoalbuminemia: a retrospective analysis. Ann Pharmacother. 2021;55(2):165–173. doi:10.1177/1060028020942485
52. Cho DS, Kim SI, Choo SH, Jang SH, Ahn HS, Kim SJ. Prognostic significance of modified Glasgow Prognostic Score in patients with non-metastatic clear cell renal cell carcinoma. Scand J Urol. 2016;50(3):186–191. doi:10.3109/21681805.2015.1136677
53. Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis. Cancer Manag Res. 2019;11(1):6163–6173. doi:10.2147/CMAR.S208839
54. Tsujino T, Komura K, Hashimoto T, et al. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma - a data from multi-institutional study in Japan. Urol Oncol. 2019;37(11):812.e811–812.e818. doi:10.1016/j.urolonc.2019.04.002
55. Matsuda S, Niihara M, Tsubosa Y, et al. Clinical significance of postoperative recovery of serum albumin levels in patients with esophageal cancer who underwent transthoracic esophagectomy. Surg Today. 2016;46(10):1138–1145. doi:10.1007/s00595-015-1300-6
56. Matsunaga T, Miyata H, Sugimura K, et al. Prognostic significance of C-reactive protein-to-prealbumin ratio in patients with esophageal cancer. Yonago Acta Med. 2020;63(1):8–19. doi:10.33160/yam.2020.02.002
57. Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–1864. doi:10.1002/cncr.20976
58. Ito T, Shinkawa H, Takemura S, et al. Impact of the preoperative C-reactive protein to albumin ratio on the long-term outcomes of hepatic resection for intrahepatic cholangiocarcinoma. Asian Pac J Cancer Prev. 2020;21(8):2373–2379. doi:10.31557/APJCP.2020.21.8.2373
59. He S, Wang Y, Chen H, et al. C-Reactive Protein/Albumin Ratio (CAR) as a prognostic factor in patients with non-metastatic nasopharyngeal carcinoma. J Cancer. 2016;7(15):2360–2366. doi:10.7150/jca.16443
60. El Iskandarani M, El Kurdi B, Murtaza G, Paul TK, Refaat MM. Prognostic role of albumin level in heart failure: a systematic review and meta-analysis. Medicine. 2021;100(10):e24785. doi:10.1097/MD.0000000000024785
61. Hong Z, Jiang Y, Liu P, Zhang L. Association of microalbuminuria and adverse outcomes in hypertensive patients: a meta-analysis. Int Urol Nephrol. 2021;53(11):2311–2319. doi:10.1007/s11255-021-02795-w
62. Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11):e241955. doi:10.1371/journal.pone.0241955
63. Soetedjo NNM, Iryaningrum MR, Damara FA, et al. Prognostic properties of hypoalbuminemia in COVID-19 patients: a systematic review and diagnostic meta-analysis. Clin Nutr ESPEN. 2021;45:120–126. doi:10.1016/j.clnesp.2021.07.003
64. Wang F, Ao G, Wang Y, et al. Risk factors for mortality in hemodialysis patients with COVID-19: a systematic review and meta-analysis. Ren Fail. 2021;43(1):1394–1407. doi:10.1080/0886022X.2021.1986408
65. Wu Y, Li H, Zhang Z, et al. Risk factors for mortality of coronavirus disease 2019 (COVID-19) patients during the early outbreak of COVID-19: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(5):5069–5083. doi:10.21037/apm-20-2557
66. Zinellu A, Mangoni AA. Serum complement C3 and C4 and COVID-19 severity and mortality: a systematic review and meta-analysis with meta-regression. Front Immunol. 2021;12(1):696085. doi:10.3389/fimmu.2021.696085
67. Ma L, Zhao S. Risk factors for mortality in patients undergoing hemodialysis: a systematic review and meta-analysis. Int J Cardiol. 2017;238(1):151–158. doi:10.1016/j.ijcard.2017.02.095
68. Ashour AA, Atta MA, Sadek KW, et al. Albumin administration in patients with decompensated liver cirrhosis: a meta-analytic update. Eur J Gastroenterol Hepatol. 2021;33(4):479–486. doi:10.1097/MEG.0000000000001932
69. Is B, Bombassaro IZ, Tovo CV, et al. Albumin in the management of hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol. 2021;26(1):100541. doi:10.1016/j.aohep.2021.100541
70. Shrestha DB, Budhathoki P, Sedhai YR, et al. Safety and efficacy of human serum albumin treatment in patients with cirrhotic ascites undergoing paracentesis: a systematic review and meta-analysis. Ann Hepatol. 2021;26(1):100547. doi:10.1016/j.aohep.2021.100547
71. Teh KB, Loo JH, Tam YC, Wong YJ. Efficacy and safety of albumin infusion for overt hepatic encephalopathy: a systematic review and meta-analysis. Dig Liver Dis. 2021;53(7):817–823. doi:10.1016/j.dld.2021.04.030
72. Kudarha RR, Sawant KK. Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater Sci Eng C. 2017;81(1):607–626. doi:10.1016/j.msec.2017.08.004
73. Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. doi:10.1371/journal.pone.0112361
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



