Back to Journals » Cancer Management and Research » Volume 11
Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma
Authors Feng JF, Wang L, Yang X, Jiang YH
Received 20 March 2019
Accepted for publication 5 July 2019
Published 31 July 2019 Volume 2019:11 Pages 7243—7251
DOI https://doi.org/10.2147/CMAR.S208320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Ji-Feng Feng,1,2 Liang Wang,1 Xun Yang,1 You-Hua Jiang1,2
1Department of Thoracic Surgery, Institute of Cancer Research and Basic Medical Sciences of Chinese Academy of Sciences, Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou 310022, People’s Republic of China; 2Key Laboratory Diagnosis and Treatment Technology on Thoracic Oncology, Hangzhou, Zhejiang Province 310022, People’s Republic of China
Background: We firstly identified a combination of lactate dehydrogenase (LDH) along with albumin (ALB), which was defined as LAR (LDH/ALB ratio). The purpose of our study here was initially to explore the prognostic role of LAR in patients with esophageal squamous cell carcinoma (ESCC) undergoing esophagectomy.
Patients and methods: A retrospective study was conducted including 346 resectable ESCC patients. Patients who received curative surgery without any neoadjuvant therapy were included in the current study. The X-tile program was performed to calculate the optimal cut-off values for LDH, ALB and LAR. The Kaplan-Meier methods, Cox regression univariate and multivariate analyses were utilized to analyze the prognostic factors for cancer-specific survival (CSS).
Results: There were 76 (22.0%) women and 270 (78.0%) men in all 346 patients. The mean value for serum LDH, ALB and LAR were 180±62 U/L (range 28–473 U/L), 40.3±5.3 g/L (range 26.6–52.4 g/L) and 4.6±1.8 (range 0.64–14.97), respectively. According to the X-tile program, the optimum cut-off points were 220 (U/L), 40.5 (g/L), and 5.5 for LDH, ALB, and LAR, respectively. The 5-year CSS was 31.8%. Patients with a high level of LAR (>5.5) were associated with poor CSS (13.3% vs 38.3%, P<0.001). Multivariate analyses revealed that LAR was an independent predictor in resectable ESCC patients (P=0.038).
Conclusion: Our retrospective observations indicate that LAR is a useful potential prognostic biomarker in resectable ESCC patients who received curative surgery without any neoadjuvant therapy with the optimal cut-off value of 5.5.
Keywords: esophageal squamous cell carcinoma, lactate dehydrogenase, albumin, prognosis
Introduction
Esophageal cancer (EC) is the main cancer worldwide (the 8th incidence and the 6th cause of death). The incidences vary widely in different countries and regions. To date, approximately 53.8% and 51.9% of all ECs occurred and died in China.1 The major kind of pathological type (90%-95%) is esophageal squamous cell carcinoma (ESCC) in China.2,3
Lactate dehydrogenase (LDH) is an important enzyme in energy production in various cell types. Recent studies revealed that serum LDH is related to prognosis in a variety of malignancies, such as renal cell carcinoma, breast cancer, non-small cell lung cancer, pancreatic cancer, nasopharyngeal carcinoma and so on.4–8 In EC, however, the prognostic role for serum LDH remains controversial.9–11 It has been also reported that albumin (ALB) participate in systemic inflammatory response. Over the past few years, studies have revealed that ALB is still a controversial prognostic biomarker for prognosis, including EC.12–14
It is commonly recognized that LDH and ALB are both cheap and simple serum biomarker which could be conducted in daily clinical practices. In this study, we have firstly identified that a combination of LDH along with ALB, which defined as LAR (LDH/ALB ratio). To the best of our knowledge, no study regarding the prognostic role of LAR so far has assessed. Therefore, the purpose of our study here was initially to explore the prognostic role of LAR for predicting prognosis with the optimal cut-off value in resectable ESCC patients.
Patients and methods
Patients
From January 2007 to December 2010 in the Department of Thoracic Oncological Surgery, Zhejiang Cancer Hospital, a retrospective study was conducted including 346 resectable ESCC patients. The eligibility criteria were included: 1) ESCC was confirmed by histopathology; 2) surgery was performed with curative esophagectomy; and 3) preoperative levels of serum LDH and ALB were obtained before surgery within one week. The exclusion criteria were as follows: 1). patients who received preoperative neoadjuvant therapy; 2). patients who had any form of acute or chronic inflammatory diseases or infections; 3). patients who had any form of systemic diseases, and 4) those diagnosed with distant metastases. Written informed consent for the collection of specimen and other medical information were obtained from all patients before surgery. The current study was approved by the Ethics Committees of our hospital (IRB Approval No. IRB-2018–130).
Surgery and follow-up
All patients underwent curative esophagectomy. The standard esophagectomy includes the Ivor Lewis procedure (ESCC in the middle or lower third) and McKeown procedure (ESCC in the upper third). The two-field (thoracoabdominal lymphadenectomy) was the major method of lymphadenectomy. Follow-up was performed in regular intervals, and the follow-up results were obtained through reviews of the hospital records and outpatient visit.
Data collection
The main clinical characteristics, such as age, gender, tumor length and location, vessel invasion, differentiation, TNM stage, preoperative weight loss (WL), performance status (PS), serum levels of LDH and ALB, were extracted in retrospective medical records. The TNM in this study was accordance with the AJCC/UICC TNM staging system (the 7th edition).15 The PS was accordance with the criteria of Eastern Clinical Oncology Group (ECOG).16 Malnutrition was usually defined as a WL >5% of baseline.17,18 Preoperative WL in the current study was defined as unintentional WL during the 3 months before diagnosis.
Statistical analysis
The X-tile program was performed to calculate the optimal cut-off values for LDH, ALB and LAR.19 The chi-squared tests and an independent-sample t-test were used to compare the clinical characters grouped by LAR. The Kaplan-Meier methods, Cox regression univariate and multivariate analyses were utilized to analyze the prognostic factors for CSS. Statistical analyses were conducted with SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
There were 76 (22.0%) women and 270 (78.0%) men in all 346 patients. The mean value for serum LDH, ALB and LAR were 180±62 U/L (range 28–473 U/L), 40.3±5.3 g/L (range 26.6–52.4 g/L) and 4.6±1.8 (range 0.64–14.97), respectively. According to the X-tile program, the optimum cut-off points for LDH, ALB and LAR were 220 U/L, 40.5 g/L and 5.5, respectively (Figure 1). Patients then were divided into 2 groups for further analyses (LAR ≤5.5 and LAR >5.5).
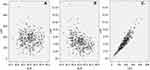
The clinicopathologic characters regarding LAR and other clinical characters were shown in Table 1. LAR was significantly associated with differentiation (P=0.033) and TNM stage (P=0.042). The levels of LAR were significantly correlated with the levels of ALB (P<0.001) and LDH (P<0.001). However, there were no significant differences between the LAR groups in terms of PS (P=0.329) and WL (P=0.383). In addition, no correlations were found between the levels of ALB and LDH (r=−0.086, P=0.109). However, LAR was significantly correlated with LDH (r=0.934, P<0.001) and ALB (r=−0.413, P<0.001) (Figure 2).
 |
Table 1 Comparison of baseline clinical characteristics based on LAR |
Cancer-specific survival analyses
A high LAR level (>5.5) was associated with poor CSS (P<0.001) in the CSS analyses by the Kaplan-Meier method. To be more specific, the 5-year CSS was 13.3% in patients with LAR >5.5 and 38.3% in those with LAR ≤5.5 (Figure 3A). In subgroup analyses based on TNM stage, we revealed that LAR was also significantly correlated with CSS (Figure 3B–D).
Cox regression univariate and multivariate analyses
In univariate analyses, several factors (tumor length, vessel invasion, LAR, LDH, ALB, TNM and WL) were significantly associated with CSS (Table 2). However, we found that LAR (>5.5 vs ≤5.5, P=0.038), but not for LDH (>220 vs ≤220, P=0.322) or ALB (>40.5 vs ≤40.5, P=0.300), was an independent predictor for CSS by multivariate analyses. In addition, we confirmed that TNM stage (P<0.001) and WL (P=0.012) were other significant prognostic variables by multivariate analyses (Table 2).
 |
Table 2 Univariate and multivariate analyses of CSS in ESCC patients |
Discussion
Our study demonstrated some important findings: (1) LAR was a strong predictor of CSS; (2) LAR, but not LDH or ALB, was a useful predictive indicator. In this study, we have firstly identified that a combination of LDH along with ALB, which defined as LAR (LDH to ALB ratio). It is generally recognized that LDH and ALB may be influenced by a variety of other non-cancer related conditions, the potential basis could be decreased by the LDH to ALB ratio (LAR). Therefore, the role of the LAR would be more reliable than the effect of either LDH or ALB.
It is demonstrated that various malignancies express a higher level of LDH than normal cells. Serum LDH was associated with systemic inflammatory response.7 Recent studies revealed that LDH is related to cancer prognosis.4–8 In EC, however, the prognostic role for serum LDH remains controversial. Furthermore, the optimum cut-off point for LDH to predict prognosis for EC is still controversial.9–11 Recent published studies have showed that ALB was a systemic inflammatory response-related factor, which reflected both nutritional and chronic inflammatory status. However, ALB is still a controversial prognostic biomarker for prognosis, including EC.12–14
LDH and ALB are both routinely tested serum enzymes in clinical practice, which makes them easily available. In this study, we have firstly identified that a combination of LDH along with ALB, which defined as LAR (LDH/ALB ratio). As everyone knows, LDH and ALB may be influenced by a variety of other non-cancer related conditions, the potential basis could be decreased by the LAR. In our study, patients with a high level of LAR (>5.5) were associated with poor CSS (13.3% vs 38.3%, P<0.001). We found that LAR (>5.5 vs ≤5.5, P=0.038), but not for LDH (>220 vs ≤220, P=0.322) and ALB (>40.5 vs ≤40.5, P=0.300), was an independent predictor for CSS by multivariate analyses.
Malnutrition is usually defined as a WL of more than 5% during the whole treatment.17,18 However, the prognostic role of preoperative WL in EC patients remains controversial.20,21 Van Der Schaaf et al.20 have reported that a >10% preoperative WL leads to decrease of 5-year overall survival in patients with EC, whereas Skipworth et al.21 have revealed that preoperative WL does not influence the perioperative mortality rate and prognosis in EC. To investigate the prognostic value of WL in patients with ESCC, patients were divided into two groups based on their rate of WL before surgery in the current study (WL ≤5.0% and WL >5.0%). Our results revealed that preoperative WL was an independent predictor for CSS by multivariate analyses (P=0.012).
Serum ALB is commonly used as an important marker of nutrition. However, the relation between ALB and WL remains controversial. Kuo et al.22 reported WL was significantly associated with ALB in colon cancer patients. However, Unal et al.23 revealed that there was no significant difference between the WL and ALB in patients with head and neck cancer. In the current study, we further explored the association between LAR and nutritional status. We found that it was not significantly associated with preoperative WL (P=0.383), although LAR reflected not only inflammation but also nutritional status of cancer patients. The results in our study concluded that systemic inflammatory response exerted more potent prognostic value than nutritional status. In addition, a study from Unal et al.23 revealed that if malnutrition develops in a short time, ALB is not a clinically relevant nutritional marker, which might also explain part of the reason.
The role of preoperative neoadjuvant chemotherapy and/or radiotherapy followed by surgery compared to surgery alone has been addressed by several randomized control trials.24–26 The most promising CROSS trial revealed that neoadjuvant chemoradiotherapy improved survival among patients with potentially curable EC.25 In contrast to the results of the CROSS trial, the French trial (FFCD 9901) reported that preoperative chemoradiotherapy did not improve survival in patients with stage I or II EC.26 Recently, two meta-analyses concluded a survival benefit of neoadjuvant chemoradiotherapy or chemotherapy compared to surgery alone in patients with EC.27,28 However, patients who received preoperative treatment were excluded in the current study because neoadjuvant treatment might have a side effect on LDH and ALB. Currently, there is no general consensus on the role of adjuvant treatment in patients with locally advanced EC. A phase III trial performed by Ando et al.29 (JCOG9204) revealed that patients with ESCC receiving surgery plus postoperative adjuvant chemotherapy with two courses of cisplatin and 5-fluorouracil showed superior 5-year disease-free survival than those receiving surgery alone. In another randomized trial (JCOG9907), moreover, they indicated that preoperative chemotherapy with cisplatin plus 5-fluorouracil can be regarded as standard treatment for patients with stage II/III ESCC.30 A meta-analysis of 11 randomized controlled trials and nonrandomized studies revealed that patients with stage III-IV ESCC are most likely to benefit from adjuvant chemotherapy.31 Furthermore, a network meta-analysis was performed to assess the efficacy of surgery with different adjuvant therapies for patients with EC.32 They concluded that patients with surgery plus adjuvant chemoradiotherapy have the best prognosis including long-term survival and low risk of recurrence compared to the other adjuvant therapies. In the current study, however, no significant differences were found regarding 5-year CSS in adjuvant therapy (32.4% vs 30.4%, P = 0.324). Three possible reasons were as follows: Firstly, the postoperative adjuvant therapy was not mandatory in our study. Secondly, ESCC with stage I-III was included in the current study while patients with stage I did not receive adjuvant therapy. Thirdly, although adjuvant therapy was followed by surgery for stage II-III, the survival of locally advanced ESCC was poor.
It is worth noting that our results can be considered relevant with potential applications in the clinical practice in the therapy of ESCC. With an elevated LAR, patients with early-stage ESCC may need closer follow-up, and those with local advanced ESCC may require more aggressive adjuvant chemotherapy and/or radiotherapy. However, the results of our study are expected more large-sample trials to confirm in future.
Limitations should be acknowledged in this study. The major limitations of this study are its retrospective character and the relatively small samples. Moreover, patients who received preoperative treatment, such as chemotherapy and/or radiotherapy were excluded, which might have influenced the result in this study. On the one hand, neoadjuvant treatment will have a side effect on LDH and ALB. On the other hand, neoadjuvant treatment can improve cancer survival for locally advanced ESCC, but not for early-stage ESCC.25,26,33 Therefore, the potential applications of these findings can be limited due to the fact that these patients were treated with surgery alone and, in many countries, the standard of treatment in these patients is preoperative chemoradiotherapy. However, the results of our study are expected more large-sample trials to confirm in future.
Conclusion
This study is the first for us to identify LAR and to determine its prognostic value in ESCC patients undergoing esophagectomy without any neoadjuvant therapy. LAR was an effective and independent predictor in resectable ESCC patients who received curative surgery without any neoadjuvant therapy with the optimum cut-off point of 5.5.
Acknowledgment
This study was funded by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (NO. 2018KY290, 2019RC129 to FJF; NO. 2019RC128 to WL).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233–242. doi:10.2188/jea.je20120162
4. Shen J, Chen Z, Zhuang Q, et al. Prognostic value of serum lactate dehydrogenase in renal cell carcinoma: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166482. doi:10.1371/journal.pone.0166482
5. Brown JE, Cook RJ, Lipton A, Coleman RE. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate treated patients. Clin Cancer Res. 2012;18(22):6348–6355. doi:10.1158/1078-0432.CCR-12-1397
6. Gong T, Liu J, Jiang J, et al. The role of lactate deshydrogenase levels on non-small cell lung cancer prognosis: a meta-analysis. Cell Mol Biol. 2019;65(1):89–93. doi:10.14715/cmb/2019.65.1.16
7. Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. doi:10.1038/srep45194
8. Wei Z, Zeng X, Xu J, Duan X, Xie Y. Prognostic value of pretreatment serum levels of lactate dehydrogenase in nonmetastatic nasopharyngeal carcinoma: singlesite analysis of 601 patients in a highly endemic area. Onco Targets Ther. 2014;7:739–749. doi:10.2147/OTT.S59804
9. Wei XL, Zhang DS, He MM, et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37(2):1879–1887. doi:10.1007/s13277-015-3851-y
10. Zhang P, Xi M, Li QQ, et al. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5(8):689–695. doi:10.7150/jca.9569
11. Huang H, Wang XP, Li XH, et al. Prognostic value of pretreatment alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio and gamma glutamyltransferase (GGT) in patients with esophageal squamous cell carcinoma. BMC Cancer. 2017;17:544. doi:10.1186/s12885-017-3523-y
12. Gao Q, Qiu JC, Huang XH, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. doi:10.1186/s12935-018-0648-2
13. Matsunaga T, Miyata H, Sugimura K, et al. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res. 2019;39(1):449–458. doi:10.21873/anticanres.13133
14. Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011;2(5):879–885. doi:10.3892/etm.2011.308
15. Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American joint committee on cancer/international union against cancer staging manuals. Cancer. 2010;116(16):3763–3773. doi:10.1002/cncr.25146
16. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655.
17. Shen LJ, Chen C, Li BF, et al. High weight loss during radiation treatment changes the prognosis in under-/normal weight nasopharyngeal carcinoma patients for the worse: a retrospective analysis of 2433 cases. PLoS One. 2013;8(7):e68660. doi:10.1371/journal.pone.0068660
18. Beaver ME, Matheny KE, Roberts DB, et al. Predictors of weight loss during radiation therapy. Otolaryngol Head Neck Surg. 2001;125(6):645–648. doi:10.1067/mhn.2001.120428
19. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
20. Van Der Schaaf MK, Tilanus HW, Van Lanschot JJ, et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg. 2014;147(1):490–495. doi:10.1016/j.jtcvs.2013.07.072
21. Skipworth J, Foster J, Raptis D, et al. The effect of preoperative weight loss and body mass index on postoperative outcome in patients with esophagogastric carcinoma. Dis Esophagus. 2009;22(7):559–563. doi:10.1111/j.1442-2050.2009.00939.x
22. Kuo YH, Shi CS, Huang CY, et al. Prognostic significance of unintentional body weight loss in colon cancer patients. Mol Clin Oncol. 2018;8(4):539–543.
23. Unal D, Orhan O, Eroglu C, et al. Prealbumin is a more sensitive marker than albumin to assess the nutritional status in patients undergoing radiotherapy for head and neck cancer. Contemp Oncol (Pozn). 2013;17(3):276–280. doi:10.5114/wo.2013.35281
24. Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. doi:10.1200/JCO.2009.22.2083
25. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088
26. Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–2422. doi:10.1200/JCO.2013.53.6532
27. Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi:10.1016/S1470-2045(11)70142-5
28. Lv J, Cao XF, Zhu B, et al. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15(39):4962–4968. doi:10.3748/wjg.15.4962
29. Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group study-JCOG9204. J Clin Oncol. 2003;21(24):4592–4596. doi:10.1200/JCO.2003.12.095
30. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74. doi:10.1245/s10434-011-2049-9
31. Zhang SS, Yang H, Xie X, et al. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus. 2014;27(6):574–584. doi:10.1111/dote.12073
32. Li S, Liu H, Diao C, et al. Prognosis of surgery combined with different adjuvant therapies in esophageal cancer treatment: a network meta-analysis. Oncotarget. 2017;8(22):36339–36353. doi:10.18632/oncotarget.16193
33. Rawat S, Kumar G, Kakria A, Sharma MK, Chauhan D. Chemoradiotherapy in the management of locally advanced squamous cell carcinama esophagus: is surgical resection required? J Gastrointest Cancer. 2013;44(3):277–284. doi:10.1007/s12029-013-9477-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.