Back to Journals » Cancer Management and Research » Volume 14
Profile of Glasdegib for the Treatment of Newly Diagnosed Acute Myeloid Leukemia (AML): Evidence to Date
Authors Iyer SG, Stanchina M, Bradley TJ, Watts J
Received 9 April 2022
Accepted for publication 18 June 2022
Published 1 August 2022 Volume 2022:14 Pages 2267—2272
DOI https://doi.org/10.2147/CMAR.S195723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Sunil Girish Iyer, Michele Stanchina, Terrence J Bradley, Justin Watts
Department of Medicine, Division of Hematology, University of Miami Miller School of Medicine, Miami, FL, USA
Correspondence: Terrence J Bradley, Department of Medicine, Division of Hematology, University of Miami Miller School of Medicine, 90 SW 3rd Street #2210, Miami, FL, 33130, USA, Tel +1 3052439290, Fax +1 305-243-9161, Email [email protected]
Abstract: Acute myeloid leukemia (AML) is an aggressive hematologic malignancy primarily affecting older adults. Historically, the highest rates of response have been achieved with intensive induction chemotherapy; however, a significant portion of older or unfit adults with AML are unable to tolerate intensive therapy or have chemotherapy-resistant disease, creating a large need for active and less intensive treatment strategies. Glasdegib, an oral inhibitor of the transmembrane protein Smoothened (SMO) involved in the Hedgehog (Hh) signaling pathway, was approved in 2018 for older or unfit adults with AML and attained a role in clinical practice after showing an overall survival (OS) advantage when combined with the established agent low-dose cytarabine (LDAC). Since that time, however, several other highly active lower intensity therapies such as venetoclax plus a hypomethylating agent (HMA) have garnered a dominant role in the treatment of this patient population. In this review, we summarize the role of glasdegib in the current treatment landscape of newly diagnosed AML and discuss ongoing investigations into its role in novel combination therapies.
Keywords: acute myeloid leukemia, glasdegib, lower intensity induction, Hedgehog signaling pathway
Introduction
Acute myeloid leukemia (AML) is an aggressive and biologically heterogeneous hematologic malignancy primarily affecting older adults. Standard of care intensive induction chemotherapy consists of a 7-day continuous infusion of cytarabine at 100–200 mg/m2 per day on days 1 to 7 and daunorubicin at 60–90 mg/m2 per day on days 1 to 3 (“7+3”), and can induce complete response (CR) rates as high as ≥80% with a 5-year overall survival (OS) of ~40–50% in younger patients without adverse cytogenetic or molecular risk factors.1–5 Despite encouraging response rates in young and fit patients, AML is typically diagnosed at a median age of 68 years in the United States, with 1/3 of newly diagnosed patients being ≥75 years of age.6,7 Although response rates in the elderly and unfit patient population have improved in the era of the hypomethylating agents (HMAs), 5-year survival remains <10% in patients over 65 years old and rates of cure remain low.1,8–11 The reason for poorer outcomes in older and unfit patients is multifactorial, owing to inability to tolerate intensive therapy, more deleterious genetic changes leading to reduced response rates and increased incidence of relapse, increased comorbidities, and ineligibility for curative allogeneic hematopoietic stem cell transplant (HSCT).1,5,8,12 Prior to the advent of the HMAs, the established non-intensive agent most widely used in clinical practice was low-dose cytarabine (LDAC). Although toxicity was low, response rates were a modest 7–18% with median OS of 5 months. In 2018 based on the preliminary results of the Phase II Bright AML 1003 trial, the Smoothened (SMO) inhibitor glasdegib was approved for the treatment of this patient population based on increased response rates and OS in combination with low-dose cytarabine (LDAC) vs LDAC alone.13,14 In recent years, the use of HMAs alone or in combination with the BCL2 inhibitor venetoclax has established a dominant role in the treatment of older/unfit adults with AML; however, ongoing studies may identify novel roles for glasdegib in the treatment of newly diagnosed AML, as part of both lower intensity and intensive approaches.
The Hedgehog Signaling Pathway
The canonical Hedgehog (Hh) signaling pathway, first discovered in Drosophila, is a highly conserved signaling pathway of key importance in embryological development, with roles including but not limited to primitive hematopoiesis and organogenesis. The majority of these roles appear to be epigenetically silenced in most human tissues after early development; roles in adults include extrathymic T cell development and pro-survival signaling in germinal center B cells, however the existence of an active role in adult hematopoiesis is unclear.15–17
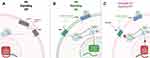
The human Hh signaling cascade, illustrated in Figure 1, begins with three ligands: Sonic Hedgehog (SHH), Indian Hedgehog (IHH), and Desert Hedgehog (DHH). These ligands bind to the transmembrane protein Patched (PTCH), releasing its inhibition of the 7-transmembrane G-like protein-couples receptor SMO. SMO, when uninhibited, can release transcription factors including GLI-1, GLI-2, and GLI-3 from their repressor complex Sufu, activating them and enabling transcription of target genes including cyclin-dependent kinases (eg, CCND1, CCND2) and pro-survival proteins (eg, BCL-2, BCL-XL). This leads to a myriad of downstream effects including pro-survival and anti-apoptotic signaling, as well as self-renewal and differentiation of hematopoietic stem cells.16–20
In 1987, the gli gene, which encodes for the transcription factors GLI-1/2/3, was found to be highly expressed in human glioma.21 Later, in 1996, aberrant Hh signaling was linked with the formation of basal cell carcinoma in the inherited basal cell nevus syndrome.22 Over time, deregulated Hh signaling and GLI transcription factor activation were implicated in a wide range of hematologic malignancies including AML, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), primary myelofibrosis, and multiple myeloma.17,23–26 Evidence began to mount that aberrant Hh signaling played a role in the survival, renewal, and expansion of the leukemic stem cell (LSC).25,27
Targeting Aberrant Hh Signaling and the Approval of Glasdegib
In an effort to modulate aberrant Hh signaling in hematologic malignancies, compounds with the ability to modulate or target Hh signaling were developed. In 2000, the plant-derived compound cyclopamine showed inhibition of the Hh pathway in mouse embryonic fibroblasts.28 Subsequently, the small molecule SMO inhibitor PF-04449913 (PF-913), now known as glasdegib, was developed. Preclinical studies, including in patient-derived xenografts (PDX), showed an ability to reduce tumor burden, sensitize quiescent malignant stem cells to chemotherapy such as cytarabine, and decrease chemoresistance mediated by the bone marrow microenvironment.29–31 In a Phase I study conducted in 2015, glasdegib showed efficacy in the treatment of multiple hematologic malignancies including AML: of 28 patients treated, some evidence of biological response (CR, complete or partial remission with incomplete hematological recovery, partial response, stable disease, and minor response) was noted in 16 patients (57%), including one patient achieving morphological CR with incomplete hematological recovery (CRi).32 Although clinical activity in AML with glasdegib monotherapy was modest, the landmark Phase 2 Bright AML 1003 trial demonstrated improved OS (8.8 vs 5.5 months) and rate of CR (19.2% vs 2.6%) in intensive chemotherapy-ineligible patients with AML or high-risk myelodysplastic syndrome (MDS) with the combination of Glasdegib/LDAC vs LDAC alone.13,14 Toxicity was similar in both arms without an increase in grade 3–4 adverse effects in the combination arm, with alopecia, dysgeusia, QTc prolongation, and muscle spasms thought to be linked to SMO inhibition.14 36-month post-hoc analysis confirmed these findings: Improved OS occurred across all cytogenetic risk groups, and a survival trend with glasdegib/LDAC was observed in patients with both de novo AML (hazard ratio 0.72) and even more pronounced in patients with secondary AML (hazard ratio 0.287).13 Additional post-hoc analysis revealed benefit with glasdegib/LDAC vs LDAC alone even in patients who did not attain CR, including improved rates of blood product transfusion independence (15% vs 2.9%) and durable recovery in the absolute neutrophil count (ANC) ≥1000/μL (45.6% vs 35.5%).33 Based on the preliminary results of the Bright AML 1003 trial, The Pfizer-developed glasdegib (brand name Daurismo) at the dose of 100 milligrams daily was approved by the United States Food and Drug Administration (FDA) in November 2018 in the USA for use in combination with low-dose cytarabine for the treatment of newly diagnosed AML in patients aged ≥75 years or those who have comorbidities that preclude use of intensive induction chemotherapy.34,35
The HMAs azacitidine and decitabine were approved by the FDA in 2004 and 2005, respectively, in the treatment of MDS. In 2008, azacitidine was approved in the use of AML with 20–30% blasts in patients ineligible for intensive therapy, and in clinical practice they became commonly utilized in this patient population regardless of blast count. Response rates including hematologic improvement were modest among trials (10–50%), a median of 3.5–4.3 months of therapy was needed to achieve best response, and median OS was under one year.36–40 After the FDA approval of glasdegib, certain comparative analyses found a possible survival advantage with glasdegib/LDAC vs HMA monotherapy.41,42 The treatment landscape changed in 2018 when the oral BCL2 inhibitor venetoclax was approved in combination with an HMA or LDAC in the treatment of newly diagnosed in AML in patients aged 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy. The Phase 1b trial leading to approval and the confirmatory Phase 3 VIALE-A trial, which compared azacitidine/venetoclax to azacitidine/placebo, showed previously unprecedented CR/Cri rates of 66–74% and median OS 16.9 months with nonintensive therapy.43–45 Although glasdegib/LDAC may have been preferred over HMA monotherapy by some providers, the novel combination of venetoclax/HMA soon became the leading lower intensity therapy for the older and unfit population. As a result of the timing of this approval, glasdegib saw limited uptake in clinical practice.
Novel Trials and Future Directions
Although the glasdegib/LDAC combination therapy has largely fallen out of favor due to the widespread adoption of venetoclax/HMA, numerous clinical trials (summarized in Table 1) are currently underway in an effort to harness glasdegib’s potential to eliminate LSCs and expose synergy with currently available therapies, both non-intensive and intensive. In 2015, an ex vivo study showed synergistic potential with the combined use of azacitidine and the SMO inhibitor erismodegib.18 Next, in 2017 a subsequent ex vivo study demonstrated that GLI3 signaling appeared to be abnormally methylated and silenced in most AML, independent of SMO activation, and that HMAs could restore this activity and sensitize AML cells to glasdegib.46 These preclinical discoveries have led to new clinical trials evaluating the use of glasdegib in novel combination regimens. The recently published phase 1b Bright AML 1012 (NCT02367456) studying glasdegib in combination with azacitidine has reported a median OS of 9.2 months and a CR and overall response rate of 20% and 30%, respectively, in patients with ND-AML, with a relatively low incidence of cytopenias and delayed marrow recovery. These results, although early with a median duration of follow-up of 8.5 months, appear to be at least comparable if not superior to those of glasdegib/LDAC. A signal of increased OS was noted for patients with FLT3 mutations with median OS not reached, which may expose a niche for this combination therapy should this trend be confirmed.47,48 The phase 2 GLAD-AML trial studying glasdegib in combination with decitabine enrolled one patient before being terminated due to failure to accrue patients in the setting of the COVID-19 pandemic.49
 |
Table 1 Recent and Ongoing Clinical Trials Examining Novel Glasdegib-Based Combinations in Newly Diagnosed AML |
The phase 2 trial NCT01546038 published in 2018 examined glasdegib in combination with intensive induction with 7+3 (intravenous cytarabine 100 mg/m2 on days 1–7 and daunorubicin 60 mg/m2 on days 1–3) in untreated AML or MDS with ≥10% blasts. CR rates were similar to historical controls; however, post-hoc review suggested a potential benefit in OS with a median OS of 14.7 months in AML patients ≥55 years old vs 8.7 months with historical control. The OS curve plateaued between 24 and 36 months with ~40% of patients alive at 36 months.50 An improvement in OS and possible cure, despite CR rates similar to historical control, can in theory be mechanistically explained by purported action of glasdegib on LSCs rather than tumor bulk cells. Sample size was limited; however, results were encouraging. The randomized, double-blinded phase 3 Bright AML 1019 trials evaluate the combination of glasdegib with intensive and lower intensity induction in newly diagnosed AML in two separate arms. The intensive arm combines glasdegib/placebo with 7+3 while the lower intensity trial combines glasdegib/placebo with azacitidine. The primary end point of both arms is OS, although long-term relapse-free survival will be another point of interest. The trail has been completed and is pending publication.51 The phase 2 NCT04231851 (CPX-351 and Glasdegib for Newly Diagnosed Acute Myelogenous Leukemia With MDS Related Changes or Therapy-related Acute Myeloid Leukemia) is currently enrolling.
Many exciting trials are currently underway; however, glasdegib continues to have a potential niche in the landscape of currently approved therapies. Although venetoclax/HMA produces considerably higher CR rates than glasdegib/LDAC, the toxicity is also significantly higher when venetoclax is added to an HMA: 98–100% of patients in the phase 1b study experienced an adverse event (AE) grade 3 or higher, with a 31–42% rate of febrile neutropenia and 4–7% incidence of sepsis.44,52 In the confirmatory Phase III VIALE-A trial, the incidence of grade 3–4 thrombocytopenia, neutropenia, and febrile neutropenia in the azacitidine/venetoclax vs azacitidine/placebo groups were 45% vs 38%, 42% vs 28%, and 42% vs 19%, respectively.45 30-day mortality with azacitidine/venetoclax and Glasdegib/LDAC were comparable at 7% vs 6%, respectively; however, the fatal adverse event rate was considerably higher with azacitidine/venetoclax at 23% vs 7%.14,44 The safety profile of glasdegib/LDAC in the Bright AML 1003 study compares favorably to venetoclax/azacitidine, with a 28.6% incidence of febrile neutropenia and a 31% incidence of grade 3–4 thrombocytopenia.14 The combination is better tolerated, less myelosuppressive, and results in fewer treatment-related hospitalizations. In elderly and unfit patients with less physiological reserve or value quality of life over treatment intensity, glasdegib/LDAC may be a preferable treatment option. Additionally, it can be utilized off-label in the second line or later after the failure of venetoclax/HMA and targeted therapies.53
Conclusions
Initially developed as a non-intensive and relatively nontoxic treatment of newly diagnosed AML in older and unfit adults, glasdegib has experienced limited utilization in the era of the HMAs and venetoclax combinations; however, in combination with LDAC, it remains an FDA approved and NCCN guideline-based option for first or subsequent line non-intensive therapy, including in the setting of HMA ± venetoclax failure. The promise of inhibition of the SMO protein and Hh pathway lies in the potential to suppress LSCs and prevent relapse, rather than a direct cytotoxic effect and an improvement in initial CR rate. Based on these preclinical findings with early clinical evidence, glasdegib is being tested in novel therapeutic combinations such as with HMAs and intensive induction chemotherapy. Success in ongoing clinical trials and clinical evidence of synergy in combination with currently approved treatments may renew and expand the role of glasdegib in the modern armamentarium of therapies to treat newly diagnosed AML.
Disclosure
Terrence J Bradley is part of the advisory board and a consultant for Novartis and is part of the adviosry board and speaker bureau for AbbVie, outside the submitted work. Dr Justin Watts reports grants and/or personal fees from Takeda, Reven Pharma, Rafael Pharma, BMS, and ISK, Ltd., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. doi:10.1182/blood-2015-08-604520
2. Fernandez H, Sun Z, Yao X, et al. Anthracycline dose intensification in Acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259. doi:10.1056/NEJMoa0904544
3. Luskin MR, Lee J-W, Fernandez HF, et al. High dose daunorubicin improves survival in AML up to age 60, across all cytogenetic risk groups including patients with unfavorable cytogenetic risk, and FLT3-ITD mutant AML: updated analyses from eastern cooperative oncology trial E1900. Blood. 2014;124(21):373. doi:10.1182/blood.V124.21.373.373
4. Lee J, Joo Y, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood. 2011;118(14):3832–3841. doi:10.1182/blood-2011-06-361410
5. Alibhai S, Leach M, Minden M, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115(13):2903–2911. doi:10.1002/cncr.24373
6. Key statistics for acute myeloid leukemia (AML). Atlanta: American Cancer Society; 2022. Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html.
7. Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975–2018. Bethesda; 2021. Available from: https://seer.cancer.gov/csr/1975_2018/.
8. Recher C, Rollig C, Berard E, et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: a large patient data set study from European registries. Leukemia. 2021;36(4):913–922. doi:10.1038/s41375-021-01425-9
9. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi:10.1016/j.blre.2019.04.005
10. Klepin H, Estey E, Kadia T. More versus less therapy for older adults with acute myeloid leukemia: new perspectives on an old debate. Am Soc Clin Oncol Edu Book. 2019;39:421–432. doi:10.1200/EDBK_239097
11. Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924. doi:10.3324/haematol.2012.066100
12. Podoltsev NA, Stahl M, Zeidan AM, Gore SD. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev. 2017;31:43–62. doi:10.1016/j.blre.2016.09.005
13. Heuser M, Smith BD, Fiedler W, et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: long-term analysis of a phase II randomized trial. Ann Hematol. 2021;100(5):1181–1194. doi:10.1007/s00277-021-04465-4
14. Cortes J, Heidel F, Hellman A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–389. doi:10.1038/s41375-018-0312-9
15. Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nat Med. 1980;287:795–801.
16. Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Pathol. 2012;180(1):2–11. doi:10.1016/j.ajpath.2011.09.009
17. Lainez-Gonzalez D, Serrano-Lopez J, Alonso-Dominguez JM. Understanding the hedgehog signaling pathway in acute myeloid leukemia stem cells: a necessary step toward a cure. Biology. 2021;10(4). doi:10.3390/biology10040255
18. Tibes R, Al-Kali A, Oliver GR, et al. The Hedgehog pathway as targetable vulnerability with 5-azacytidine in myelodysplastic syndrome and acute myeloid leukemia. J Hematol Oncol. 2015;8:114. doi:10.1186/s13045-015-0211-8
19. Tibes R, Mesa RA. Targeting hedgehog signaling in myelofibrosis and other hematologic malignancies. J Hematol Oncol. 2014;7(18). doi:10.1186/1756-8722-7-18
20. Terao T, Minami Y. Targeting Hedgehog (Hh) pathway for the Acute myeloid leukemia treatment. Cells. 2019;8(4):312. doi:10.3390/cells8040312
21. Kinzler KW, Bigner SH, Bigner DD, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236(4797):70–73. doi:10.1126/science.3563490
22. Hahn H, Wicking C, Zaphiropoulos PG, et al. Mutations of the human homolog of drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi:10.1016/S0092-8674(00)81268-4
23. Bai LY, Chiu CF, Lin CW, et al. Differential expression of sonic hedgehog and Gli1 in hematological malignancies. Leukemia. 2008;22:226–228. doi:10.1038/sj.leu.2404978
24. Kobune M, Iyama S, Kikuchi S, et al. Stromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasms. Blood Cancer J. 2012;2:e87.
25. Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood. 2012;119(10):2196–2204. doi:10.1182/blood-2011-10-383752
26. O’Brien C, Kreso A, Jamieson C. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16(12):3113–3120. doi:10.1158/1078-0432.CCR-09-2824
27. Beachy P, Karhadkar S, Berman D. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–331. doi:10.1038/nature03100
28. Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi:10.1038/35023008
29. Minami Y, Fukushima N, Sadarangani A, Hironobu M, Jamieson C, Naoe T. Abstract 1884: treatment with Hedgehog inhibitor PF-913 attenuates leukemia-initiation potential in acute myeloid leukemia cells. Cancer Res. 2014;74:1884.
30. Fukushima N, Minami Y, Kakiuchi S, et al. Small-molecule Hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci. 2016;107(10):1422–1429. doi:10.1111/cas.13019
31. Munchhof MJ, Li Q, Shavnya A, et al. Discovery of PF-04449913, a potent and orally bioavailable inhibitor of smoothened. ACS Med Chem Lett. 2012;3(2):106–111. doi:10.1021/ml2002423
32. Martinelli G, Oehler VG, Papayannidis C, et al. Treatment with PF-04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol. 2015;2(8):e339–e346. doi:10.1016/S2352-3026(15)00096-4
33. Cortes JE, Heidel FH, Fiedler W, et al. Survival outcomes and clinical benefit in patients with acute myeloid leukemia treated with glasdegib and low-dose cytarabine according to response to therapy. J Hematol Oncol. 2020;13(1):92. doi:10.1186/s13045-020-00929-8
34. Daurismo (glasdegib) [package insert]. New York, NY: Pfizer; 2018.
35. Hoy SM. Glasdegib: first global approval. Drugs. 2019;79(2):207–213. doi:10.1007/s40265-018-1047-7
36. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with.30% blasts. Blood. 2015;126(3):291–299. doi:10.1182/blood-2015-01-621664
37. Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–561. doi:10.1200/JCO.2009.23.9178
38. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi:10.1200/JCO.2011.38.9429
39. Al-Ali HK, Jaekel N, Junghanss C, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53(1):110–117. doi:10.3109/10428194.2011.606382
40. Zhang Y, Asghari HH, Chan O, et al. Hypomethylating agent and venetoclax combination therapy yields superior outcomes when compared to hypomethylating agent monotherapy in patients ≥70 years with acute myeloid leukemia. Blood. 2019;134:1368. doi:10.1182/blood-2019-127772
41. Tremblay G, Westley T, Cappalleri JC, et al. Overall survival of glasdegib in combination with low-dose cytarabine, azacitidine, and decitabine among adult patients with previously untreated AML: comparative effectiveness using simulated treatment comparisons. Clinicoecon Outcomes Res. 2019;11:551–565. doi:10.2147/CEOR.S203482
42. van Beekhuizen S, Hu Y, Gezin A, et al. The comparative effectiveness of glasdegib in combination with low-dose cytarabine versus azacitidine by bone marrow blasts counts among patients with newly-diagnosed acute myeloid leukemia who are ineligible for intensive chemotherapy. J Clin Oncol. 2020;38:e19512–e19512. doi:10.1200/JCO.2020.38.15_suppl.e19512
43. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. doi:10.1182/blood-2018-08-868752
44. Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208–217. doi:10.1002/ajh.26039
45. DiNardo CD, Jonas B, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629. doi:10.1056/NEJMoa2012971
46. Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129(26):3465–3475. doi:10.1182/blood-2016-05-718585
47. Zeidan A, Schuster M, Joris M, et al. Glasdegib in combination with azacitidine (AZA) in patients (pts) with untreated higher-risk myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML): effects on marrow recovery and transfusion Independence. J Clin Oncol. 2020;38:7526. doi:10.1200/JCO.2020.38.15_suppl.7526
48. Sekeres MA, Schuster M, Joris M, et al. A phase 1b study of glasdegib + azacitidine in patients with untreated acute myeloid leukemia and higher-risk myelodysplastic syndromes. Ann Hematol. 2022. doi:10.1007/s00277-022-04853-4
49. Shallis R, Podoltsev NA, Prebet T, et al. Trial in Progress: glad-AML - a randomized, Phase 2 trial of glasdegib with two standard decitabine regimens for older patients with newly-diagnosed, poor-risk acute myeloid leukemia. Blood. 2020;136:29. doi:10.1182/blood-2020-139428
50. Cortes JE, Douglas Smith BD, Wang ES, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. 2018;93(11):1301–1310. doi:10.1002/ajh.25238
51. Cortes J, Dombret H, Merchant A, et al. Glasdegib plus intensive/nonintensive chemotherapy in untreated acute myeloid leukemia: BRIGHT AML 1019 Phase III trials. Future Oncolo. 2019;15(31):3531–3545.
52. DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi:10.1016/S1470-2045(18)30010-X
53. Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, Version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721–749. doi:10.6004/jnccn.2019.0028
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.