Back to Journals » Cancer Management and Research » Volume 15
Profile of Capmatinib for the Treatment of Metastatic Non-Small Cell Lung Cancer (NSCLC): Patient Selection and Perspectives
Authors Fraser M, Seetharamu N , Diamond M, Lee CS
Received 5 May 2023
Accepted for publication 19 September 2023
Published 3 November 2023 Volume 2023:15 Pages 1233—1243
DOI https://doi.org/10.2147/CMAR.S386799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Madison Fraser,1 Nagashree Seetharamu,2 Matthew Diamond,2 Chung-Shien Lee2,3
1Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hofstra University, Hempstead, NY, USA; 2Division of Medical Oncology and Hematology, Northwell Health Cancer Institute, Lake Success, NY, USA; 3Department of Clinical Health Professions, St. John’s University, Queens, NY, USA
Correspondence: Chung-Shien Lee, Email [email protected]
Abstract: Aberrant c-MET (Mesenchymal–Epithelial Transition) signaling contributes to cancer cell development, proliferation, and metastases of non-small cell lung cancer (NSCLC). MET exon 14 (METex14) skipping mutation is noted in approximately 4% of NSCLC cases and is targetable with the recently approved tyrosine kinase inhibitors capmatinib and tepotinib. Capmatinib, the focus of this review article, is a highly selective MET inhibitor approved for use in patients with METex14 mutated NSCLC. In this review, we discuss cMET as a target, the pharmacology of capmatinib, key trials of capmatinib in MET-altered lung cancer, and toxicity profile. We highlight some ongoing capmatinib clinical trials that expand their role to other subsets of patients, especially those with EGFR mutations, who develop MET alterations as a resistance pathway. We further provide our perspective on the management of METex14 NSCLC, strategies for sequencing agents, and toxicity management.
Keywords: non-small cell lung cancer, Mesenchymal–Epithelial Transition gene, MET exon 14 skipping mutation, tyrosine kinase inhibitor, capmatinib
Introduction
Among both men and women, lung cancer remains the leading cause of cancer-related deaths, with death rates of 44.5% and 30.7%, respectively.1–3 Fortunately, lung cancer mortality has also been decreasing steadily. From 2015 to 2019, lung cancer, on average showed one of the steepest declines (>4% per year) in annual death rate.1 This is especially true for non-small cell lung cancers (NSCLC), which make up over 82% of diagnosed lung cancers.2 These improvements are likely due to a combination of advances in lung cancer diagnostics and therapeutics. Survival has improved across all stages of lung cancer, including stage IV, which still accounts for the majority of patients diagnosed with lung cancer today. Major advances in the treatment of advanced lung cancer have been made in the domains of targeted and immune-directed therapies. Targeted drugs have expanded exponentially since their introduction in the late 1990s and the early 2000s. These include angiogenesis inhibitors and therapies that target molecular alterations in specific genes, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma (KRAS), c-ros oncogene 1 (ROS1), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), rearranged during transfection (RET), Mesenchymal–Epithelial Transition (MET), neurotrophic tyrosine receptor kinase (NTRK) and human epidermal growth factor receptor 2 (HER2). With the advancement of sequencing technologies, the list of targetable genomic alterations and approved targeted therapies is increasing expeditiously over time. This review focuses on the more promising recent developments of targeted therapies for MET gene alterations in NSCLC.
Met Gene
The MET gene is located on the human chromosome 7q21-31 and encodes the tyrosine kinase hepatocyte growth factor receptor (HGFR). c-MET is a transmembrane glycoprotein consisting of an extracellular alpha chain and an intracellular beta chain connected by a disulfide bridge. Three domains form the extracellular portion: the Sema domain, PSI domain, and four immunoglobulin-plexin transcriptional (IPT) repeats (Figure 1). The intracellular region consists of the tyrosine kinase domain, which is bordered by the juxta membrane (JM) domain and the carboxyl-terminal sequences/docking site.4,5 In NSCLC, mutations have been found in the Sema, JM, and tyrosine kinase domains. The well-known exon 14 skipping mutation (METex14) is located within the JM domain.4
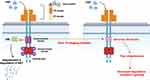 |
Figure 1 MET Exon 14 Skipping Activity. |
When hepatocyte growth factor (HGF) or its splicing isoforms (NK1 and NK2), which are the only known ligands of the MET receptor, bind to c-MET/HGFR, it results in receptor homodimerization and two tyrosine residues become phosphorylated.5 This subsequently leads to the activation of many signaling pathways, including but not limited to P13K-AKT, Ras-RAF ERK/MAPK, and STAT-JNK.4
c-MET, also called HGFR is found pervasively throughout the body, starting at embryogenesis, and lasting through adulthood, with the highest levels found in epithelial cells and placental cells.6 The actions of c-MET signaling leads to cell proliferation, differentiation, cytoskeletal restructuring, and cellular movement.4 As a result, c-MET is highly expressed in epithelial and placental cells, and c-MET is a major player in the processes of embryogenesis and wound and organ repair.4,7 However, when MET is mutated or overexpressed, it can serve as an oncogene and is associated with poor prognosis, where it triggers tumor growth, angiogenesis, and metastasis. MET is dysregulated in a multitude of malignancies including lung cancer.
Met Oncogenesis
As a protooncogene, MET was initially identified in 1984 in human osteosarcoma, but has been connected to numerous solid tumors since then, including NSCLC.8 Aberrant c-MET signaling is thought to contribute both to cancer cell development and proliferation in early stages, and later to metastasis and invasion via changes in cytoskeletal functioning.4 In fact, reduced HGF-MET signaling has resulted in inhibition of lung cancer cell growth, invasion, and metastasis via blockage of epithelial–mesenchymal transformation (EMT).9 Additionally, c-MET signaling is thought to support the development and sustenance of the tumor microenvironment, leading to tumor cells harboring an immune escape from T-cell killing.10 Genetic alterations in MET have therefore shown to produce unchecked cell signaling that can enhance cancer cell growth and potentiate spread through multiple pathways.
Met in NSCLC
In NSCLC, three main types of oncogenic MET alterations have been identified: c-METex14 skipping mutations, gene amplification of c-MET, and c-MET protein overexpression.10 In this review, we will focus on all of these aberrations since amplification, overexpression and exon 14 skipping mutations have been associated with a poorer prognosis in NSCLC patients and have been the focus of recent drug development.11,12
Exon 14 Skipping Mutations
METex14 skipping mutations are the most commonly reported MET alterations in NSCLC, with up to 4% of NSCLC displaying such mutations.13,14 Exon 14 skipping can be caused by point mutations, deletions, or insertions.10 These mutations result in impaired transcriptional splicing of the MET gene and subsequent loss of exon 14, which encodes the juxtamembrane (JM) domain of the MET receptor.15 The impact on the JM domain is typically deletion of its tyrosine-1003 residue. This leads to poor ubiquitination of the c-MET receptor, thereby impairing receptor internationalization and degradation.16 Continued expression of receptors that would have otherwise been degraded results in overactive c-MET signaling, including ligand-independent activation, and therefore in the case of tumor cells, continued cell growth.15
Patients with METex14 mutations tend to be older on average (median 70–73 years old) with more extensive smoking history.15 Interestingly, METex14 skipping mutations are theorized to contribute to early-onset NSCLC development, with subsequent additional mutations, such as amplification and/or overexpression producing a more aggressive phenotype.15 Similarly, numerous studies have shown that there to be co-existence of METex14 with other oncogenic mutations, such as MDM2, CDK4, and TP53.17,18 The clinical phenotype that results from such combinations, however is yet to be fully elucidated.
Met Amplification
An increase in the number of MET genes may occur either from amplification of the gene itself or via polysomy, which is an increase in the number of chromosome 7.19 However, polysomy (aneuploidy) is less likely to progress as an oncogenic driver than true amplification. True MET amplification (METamp) typically occurs as a local gain of one arm of chromosome 7q31.15 METamp may present alone or in association with METex14, with a varying co-occurrence rate of 0–40%.14 MET gene copy numbers >5 have been correlated with poorer prognosis in NSCLC patients.20 Overexpression at the protein level has also been implicated on oncogenesis (Figure 2).21 In general, MET amplification occurs in about 4% of patients with NSCLC that have not been previously exposed to systemic therapy. When exposed to EGFR TKIs, this rate can increase to 20% of patients due to an acquired amplification.22
 |
Figure 2 MET Amplification Activity. Notes: Data from Drusbosky et al.23 |
Importantly, METamp has been recognized as a form of treatment resistance to tyrosine kinase inhibitors (TKIs) in NSCLC, specifically in EGFR mutation-positive NSCLC.14,24 METamp was found in approximately 3% of untreated NSCLC, and this rate increased to 5–22% in patients who acquired resistance to treatment with first- or second generations EGFR-TKIs.19 The aforementioned poor prognosis seen with METamp may correlate with this observed treatment resistance in select patients.
Role of TKIs in NSCLC
Numerous TKIs have been approved for use in NSCLC and have been shown to be efficacious against a wide range of alterations including EFGR, ALK, and MET.25 Their appeal comes from the fact that they are orally bioavailable and provide treatment options to patients with advanced NSCLC harboring specific genetic alterations. Following the footsteps of successful targeting of EGFR, ALK, ROS and RET, cMET targeting has emerged as one of the major advancement in NSCLC therapeutics over the last few years. Capmatinib was the first targeted agent for alteration of the MET gene, specifically exon 14 mutations.26 Results from the GEOMETRYmono-1 trial reported in May 2020 resulted in accelerated approval by the United States (US) Food and Drug Administration (FDA) for the treatment of metastatic NSCLC with exon 14 skipping mutations. Capmatinib went on to receive regular FDA-approval in August 2022.27
Capmatinib
Administration and Mechanism of Action
Capmatinib is an oral agent, which is a highly selective small-molecule inhibitor of cMET. It has been shown to have anti-tumor properties against MET-driven in vitro and in-vivo solid tumor models.28 The agent is 30 times more potent than crizotinib with IC50 values of 0.13 nmol/L and 4 nmol/L, respectively, and slightly more potent than tepotinib which has an IC50 of ~1.7 nmol/L.29,30
Pharmacokinetics and Pharmacodynamics
After oral administration of capmatinib, Cmax was attained within 1–2 hours (Tmax). Absorption of oral medications was noted to be >70%. The postprandial or fasting state did not significantly affect Cmax or capmatinib exposure (AUC 0–12 hours). The drug is mostly protein-bound (96%) in circulation, and its half-life is estimated to be 6.5 hours. Capmatinib is primarily metabolized by the liver via enzymes CYP3A4 and aldehyde oxidase. In healthy subjects, 78% of the total radioactivity was recovered in feces and 22% in urine after a single oral dose of radiolabelled capmatinib.31
Historical Perspectives Leading Up to FDA Approval and Beyond
Most of the initial studies were performed on MET amplified lung cancer as assessed by immunohistochemistry and were found to have inconsistent results. Subsequent studies used FISH to assess gene copy number (CGN) and showed promising activity. Further studies, especially in lung cancer patients with oncogenic METex14 skipping mutations, have demonstrated that this drug has the highest activity in this subset of patients. MET overexpression is a common resistance pathway in patients treated with EGFR TKI for EGFR mutated lung cancer, and capmatinib has been shown to be effective preclinically and clinically in this setting.32–35
The first in-human, Phase I, dose-escalation/dose-expansion study in solid tumors that harbored MET alterations, which included 55 patients (NCT01324479), established 400 mg tablets twice daily or 600 mg capsules twice daily as the Phase II dose. The agents were well-tolerated, with gastrointestinal (GI) events, edema, fatigue, and anorexia being the most common adverse events. Adverse events (AEs) that were grade ≥3 were rare. The disease control rate (DCR) was 51%, with a 20% partial response (PR). The responses were most robust in patients with MET GCN ≥6.36
The Geometry Mono-1 (NCT02414139), a Phase II, open-label, multicenter study of capmatinib in NSCLC enrolled patients with EGFR and ALK wild-type NSCLC belonging to several cohorts, was initiated in 2015.37 The various cohorts of patients included in this study were as follows: cohort 1, MET amplified (GCN ≥6) previously treated NSCLC; cohort 2-previously treated NSCLC with GCN ≥4 but <6; cohort 3-previously treated NSCLC with MET CGN <4; cohort 4-previously treated NSCLC with METex14 skipping mutation; and cohort 5-treatment naïve MET amplified (CGN≥10) or METex14 skipping mutation-positive NSCLC. There were two additional expansion cohorts: cohort 6 for pre-treated patients with MET GCN ≥10 without MET exon 14 mutation or METex14 skipping mutation, regardless of MET GCN, and cohort 7 for treatment-naïve patients with METex14 skipping mutation only. Recently, the activity of capmatinib in some of the aforementioned cohorts has been reported. The study enrolled a total of 364 patients, 96 of whom had a METex14 skipping mutation. This group experienced an overall response rate (ORR) of 41% (95% CI, 29–53) among those who had received prior lines of therapy and 68% (95% CI, 48–84) among those who were treatment-naïve. The median duration of response was 9.7 months (95% CI 5.6 to 13) for pre-treated patients and 12.6 months (95% CI 5.6 to NE) for treatment-naïve group. There was very little activity in MET amplified NSCLC, in which GCN was less than 10% and 29% (95% CI 19 to 41) in pre-treated NSCLC with GCN ≥ 10% and 40% (95% CI 16 to 68) in treatment-naïve high MET expressors. Edema and nausea were the most common adverse events occurring in 51% and 45%, respectively.37
On May 6, 2020, the US FDA granted accelerated approval for the use of capmatinib in NSCLC with METex14 skipping mutations.26 The updated results of Geometry Mono-1 were presented at the American Society of Clinical Oncology in 2021. Efficacy analysis was performed on treatment-naïve patients with METex14 NSCLC (cohorts 5b and 7) as well as those who had received other lines of treatment (cohorts 4 and 6). This updated analysis involving 160 patients who were treated with capmatinib 400 mg twice daily showed an ORR of 65.6% (95% CI 46.8 to 81.4) and a median PFS of 10.8 months (95% CI 8.6 to 22.2) for treatment-naïve patients in the expansion cohort 7. Mature overall survival (OS) survival data for cohort 5b (treatment-naïve) and cohort 4 (pre-treated) populations were also provided during this updated analysis at 20.8 months (95% CI 12.4 to NE) and 13.6 months (95% CI 8.6 to 22.2), respectively. No new safety signals were observed. After this update, involving 63 additional patients and a longer follow-up of 22 months, the FDA granted regular approval for the drug in METex14 NSCLC.27,38
Real-world experience with capmatinib has been reported in a retrospective, multicenter study. Patients in this study were adults with locally advanced or metastatic NSCLC with confirmed METex14 skipping mutation and were part of the early access program. Of note, 30% of patients in this study had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 2 or 3. ORR was 50% (95% CI, 35–65) in patients who had received prior lines of therapy and 68% (95% CI, 50–82) in patients were treatment-naïve. PFS was 9.1 months (95% CI, 4.7–14.3) and 10.6 months (95% CI, 5.5–15.7), respectively. Overall efficacy results were similar to that seen in the Geometry Mono-1 study.39
Central Nervous System (CNS) Activity
Capmatinib was found to have CNS activity in an ad hoc analysis of Geometry Mono-1, which included 13 patients with brain metastases. The CNS response rate was 54% and included complete response (CR). In a subsequent real-world analysis of 68 patients with METex14 NSCLC and brain metastases, the ORR in the first-line setting (n=55) was 90.9% systemically and 87.3% intracranially. In patients who did not receive cranial radiotherapy (n=20), the ORR was 85% extra- and intracranially.40 In a separate real-world study, CNS response rate was 46% in 22 patients with brain lesions.39
Adverse Events (AE)
Toxicity data is available from GEOMETRY mono-1 Cohorts 1–6 (n=373) and real-world populations, showed that the drug is well-tolerated.39,41 The most frequent AEs occurring in ≥10% of patients included edema, which was the most common AE and occurred in 48–59% of patients, muscle aches, fatigue, fever, weight loss, nausea, vomiting, constipation, diarrhea, dyspnea, cough, anorexia, rash, and dizziness. Most AEs were grade 1 or 2, but grade ≥3 AEs occurred in some patients, with edema as the top AE on the list at 13%.
Dose Reductions, Interruptions and Discontinuations
The recommended starting dose of capmatinib is 400 mg twice daily, to be taken whole with or without food. Dose reduction due to an AE occurred in 26–40% of patients with edema, and biochemical abnormalities in the liver and renal function were the most common AEs requiring dose modification. The recommended first and second dose reduction doses were 300 mg and 200 mg twice daily, respectively.29 Dose interruptions occurred in 26–57% of patients with edema, and abnormal liver, pancreatic, and renal function tests were the most common reasons. GI side effects, fatigue, cough, dyspnea, pneumonia, and musculoskeletal pain are among the other reasons for treatment interruption. AEs leading to permanent discontinuation, occurred in 10–12% of patients, included edema, pneumonitis, fatigue, and pneumonia.
Impact on Health-Related Quality of Life (HRQoL)
Importantly, HRQoL was assessed by patient-reported outcomes (PRO) and global health scale (GHS) in Geometry Mono-1, and results were published recently.42 At the time of data cut-off for this study in January 2020, 92 patients had completed PROs at baseline and >70% remained engaged throughout the study by completing PRO at different time points. Most patients noted improvement in lung cancer-related symptoms, particularly cough, which improved early (by seven weeks) and persisted over time (43 weeks). HRQoL, as assessed by the GHS, also improved by 7 weeks in both the first-line and subsequent line cohorts. Median time to definitive deterioration (TTDD) in GHS/QoL was 16.6 months (95% CI: 9.7 to NE) in 1st line and 12.4 months (95% CI: 12.4 to NE) in subsequent line cohort.42
Other Agents Targeting Met in Lung Cancer
Similar to capmatinib, tepotinib is also a small-molecule tyrosine kinase inhibitor, which has received accelerated approval by the US FDA for the treatment of metastatic NSCLC with METex14 skipping mutation.43 Paik et al found that tepotinib administered once daily at a dose of 500 mg resulted in a partial response in approximately half of the evaluated patients.30 Given that capmatinib and tepotinib are orally bioavailable, they are frequently the first choice for treating lung cancer with a METex14 skipping mutation. The toxicity profile of tepotinib is very similar to that of capmatinib with lower rates of nausea seen (26% vs 45%), but higher rates of edema (63% vs 48–51%).30,39,41 However, other agents, such as the bispecific antibody amivantamab, and investigational compounds, such as the antibody–drug conjugate telisotuzumab vedotin, may also be options for patients. Amivantamab is a bispecific antibody that engages both EGFR and MET was granted accelerated FDA approval in May 2021.44 This bispecific antibody demonstrates activity in patients with METex14 skipping mutations as well as EGFR exon 20 insertions. The CHRYSALIS study has found that amivantamab exerts anti-tumor activity in patients with alterations in MET.45 On the experimental front, the antibody–drug conjugate telisotuzumab vedotin has demonstrated promising activity in patients with metastatic NSCLC with c-myc overexpression who have progressed on platinum-based therapy. This comes from the phase II LUMINOSITY trial, which was designed to determine the ORR of telisotuzumab vedotin in a selected population of patients with NSCLC whose tumors overexpress c-myc. The trial is ongoing and has been granted breakthrough status by the FDA.
Perspectives
METex14-driven NSCLC represents a unique subset of NSCLC for which there are two approved therapeutic options. Current NCCN guidelines recommend using TKIs as the first-line for METex14 positive NSCLC.46 We agree with this recommendation. Given the fact that capmatinib was approved earlier and has a higher IC50 than other TKIs, our preference has been capmatinib in the first-line setting. We typically start patients at the recommended FDA starting dose of 400 mg twice daily; however, in patients with higher/borderline performance status and multiple comorbidities, we start with a lower dose and titrate accordingly. It is very common for dose reductions and dose interruptions due to toxicity, as observed in the Geometry Mono-1 study and other real-world experiences.37,39 Use of MET inhibitors in patients with MET amplification tumors is undefined at this point and use of MET inhibitors in these patients should not be done outside of clinical trials. Due to a very unique, potentially serious AE profile, we believe that it is imperative to have clinical pharmacists on the team managing these patients.
We provide an in-depth education regarding the administration of capmatinib and its potential AEs to all patients. We specifically educate them about timely communication regarding any signs or symptoms of pulmonary toxicity, including dyspnea, cough, and fever, in which case we ask them to stop capmatinib immediately while elucidating alternate causes. We permanently discontinued capmatinib if pulmonary toxicity is determined to be a direct result of the capmatinib treatment.
Patients are also monitored closely with blood tests, specifically liver function tests, lipase, amylase, and creatinine levels. Biochemical abnormalities are not infrequent and dose adjustments and interruptions are frequently necessary as a result. In most cases, biochemical abnormalities subside with drug interruption.
The most common difficult-to-treat side effect is peripheral edema, which can range from mild to severe. The actual mechanism of AE is unknown, but it is thought that the HGF/MET inhibition may disrupt the permeability of the vascular endothelium and result in edema. We educate patients at the onset of this possibility and advise them to use compression stockings, maintain an active lifestyle, and elevate their lower extremities. We also measure limb girth before initiating treatment so it can serve as a reference when edema occurs. If peripheral edema occurs, we advise elevation of the extremities, compression stockings, and low-dose diuretics for grade 2 peripheral edema. For grade 3 or higher, we recommend dose interruption until it resolution to grade ≤1 and then restart with a lower dose.
Progression on targeted therapies, including capmatinib occurs often. Although there is no data to provide guidance on second-line treatment for these patients after progression on capmatinib, there are a multitude of options. Clinical trials are the best options for these patients, but these are not available or applicable to many patients. There are anecdotal reports of switching to tepotinib successfully and this is concordant with our experience.47,48 Alternatively, platinum-based chemotherapy or chemoimmunotherapy may be an option.46 Unlike other genomic drivers, METex14 does not seem to render tumors resistant to immunotherapy, although there are some conflicting reports regarding this. Newer agents, including ADC and bispecifics, can also be used in these patients.
Conclusions and Future Directions
Targeting specific genetic alterations is an illustration of the advances in oncology and medicine as a whole. We have entered an era in the field of precision medicine that allows us to analyze and target a patient’s particular genetic aberrations to achieve therapeutic outcomes. Among these aberrations identified, METex14 skipping and MET amplification mutations have emerged as important targets for NSCLC treatment. The development and approval of capmatinib has been a milestone that offer promise for the treatment of cancers driven by MET alterations.
Looking to the future, capmatinib may possibly serve a role in other subsets of lung cancer driven by other aberrations in MET such as amplifications and overexpressions. Studies have also focused on targeting MET aberrations that occur as “escape” mechanisms in EGFR and ALK-positive cancers. Further ongoing and future studies with capmatinib are summarized in Table 1. In addition, capmatinib is also being studied in a variety of solid tumors by investigators globally.
 |
Table 1 Ongoing and Future Studies with Capmatinib |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. Cancer. 2022;128(24):4251–4284. doi:10.1002/cncr.34479
2. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–436. doi:10.3322/caac.21731
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
4. Yang X, Liao HY, Zhang HH. Roles of MET in human cancer. Clin Chim Acta. 2022;525:69–83. doi:10.1016/j.cca.2021.12.017
5. Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. doi:10.1177/1758834011422556
6. Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327(6119):239–242. doi:10.1038/327239a0
7. Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–705. doi:10.1038/373702a0
8. Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311(5981):29–33. doi:10.1038/311029a0
9. Shen Y, Chen Q, Li L. Endostar regulates EMT, migration and invasion of lung cancer cells through the HGF-Met pathway. Mol Cell Probes. 2019;45:57–64. doi:10.1016/j.mcp.2019.05.003
10. Dempke WCM, Reuther S, Hamid Z, Thoennissen NH. Oncogene alterations in non-small cell lung cancer-have we MET a new target? Transl Lung Cancer Res. 2022;11(10):1977–1981. doi:10.21037/tlcr-22-648
11. Okuda K, Sasaki H, Yukiue H, Yano M, Fujii Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008;99(11):2280–2285. doi:10.1111/j.1349-7006.2008.00916.x
12. Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–3056. doi:10.1158/1078-0432.CCR-15-2061
13. Cortot A, Le X, Smit E, et al. Safety of MET tyrosine kinase inhibitors in patients with MET exon 14 skipping non-small cell lung cancer: a clinical review. Clin Lung Cancer. 2022;23(3):195–207. doi:10.1016/j.cllc.2022.01.003
14. Socinski MA, Pennell NA, Davies KD. MET exon 14 skipping mutations in non-small-cell lung cancer: an overview of biology, clinical outcomes, and testing considerations. JCO Precis Oncol. 2021;5. doi:10.1200/PO.20.00516
15. Remon J, Hendriks LEL, Mountzios G, et al. MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–435. doi:10.1016/j.jtho.2022.10.015
16. Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859. doi:10.1158/2159-8290.CD-15-0285
17. Lee JK, Madison R, Classon A, et al. Characterization of non-small-cell lung cancers with MET exon 14 skipping alterations detected in tissue or liquid: clinicogenomics and real-world treatment patterns. JCO Precis Oncol. 2021;5. doi:10.1200/PO.21.00122
18. Le X, Hong L, Hensel C, et al. Landscape and clonal dominance of co-occurring genomic alterations in non-small-cell lung cancer harboring MET exon 14 skipping. JCO Precis Oncol. 2022;6:e2200175. doi:10.1200/PO.21.00135
19. Fan Y, Sun R, Wang Z, et al. Detection of MET amplification by droplet digital PCR in peripheral blood samples of non-small cell lung cancer. J Cancer Res Clin Oncol. 2023;149(5):1667–1677. doi:10.1007/s00432-022-04048-4
20. Zhang Y, Yang Q, Zeng X, et al. MET amplification attenuates lung tumor response to immunotherapy by inhibiting STING. Cancer Discov. 2021;11(11):2726–2737. doi:10.1158/2159-8290.CD-20-1500
21. Sterlacci W, Fiegl M, Gugger M, Bubendorf L, Savic S, Tzankov A. MET overexpression and gene amplification: prevalence, clinico-pathological characteristics and prognostic significance in a large cohort of patients with surgically resected NSCLC. Virchows Arch. 2017;471(1):49–55. doi:10.1007/s00428-017-2131-1
22. Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937. doi:10.1073/pnas.0710370104
23. Drusbosky LM, Dawar R, Rodriguez E and Ikpeazu CV. Therapeutic strategies in METex14 skipping mutated non-small cell lung cancer. J Hematol Oncol. 2021;14(1);129. doi:10.1186/s13045-021-01138-7
24. Landi L, Minuti G, D’Incecco A, Salvini J, Cappuzzo F. MET overexpression and gene amplification in NSCLC: a clinical perspective. Lung Cancer. 2013;4:15–25. doi:10.2147/LCTT.S35168
25. Griffin R, Ramirez RA. Molecular targets in non-small cell lung cancer. Ochsner J. 2017;17(4):388–392. doi:10.3322/caac.21387
26. U.S. Food and Drug Administration. Center for drug evaluation and research. Resources for information on approved drugs. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-capmatinib-metastatic-non-small-cell-lung-cancer.
27. U.S. Food and Drug Administration. Center for drug evaluation and research. Resources for information on approved drugs. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capmatinib-metastatic-non-small-cell-lung-cancer.
28. Vansteenkiste JF, Van De Kerkhove C, Wauters E, Van Mol P. Capmatinib for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther. 2019;19(8):659–671. doi:10.1080/14737140.2019.1643239
29. Liu X, Wang Q, Yang G, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17(22):7127–7138. doi:10.1158/1078-0432.CCR-11-1157
30. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943. doi:10.1056/NEJMoa2004407
31. Tabrecta [Package Insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2023.
32. Wilgucki M, Yeung V, Ho G, et al. Osimertinib and capmatinib combination therapy to overcome MET Y1003N-mediated resistance in EGFR-mutant NSCLC: a case report. JTO Clin Res Rep. 2022;3(10):100396. doi:10.1016/j.jtocrr.2022.100396
33. Zhu K, Lv Z, Xiong J, et al. MET inhibitor, capmatinib overcomes osimertinib resistance via suppression of MET/Akt/snail signaling in non-small cell lung cancer and decreased generation of cancer-associated fibroblasts. Aging. 2021;13(5):6890–6903. doi:10.18632/aging.202547
34. McCoach CE, Yu A, Gandara DR, et al. Phase I/II study of capmatinib plus erlotinib in patients with MET-positive non-small-cell lung cancer. JCO Precis Oncol. 2021;1. doi:10.1200/PO.20.00279
35. Wu YL, Han JY, Kato T, et al. Capmatinib plus osimertinib versus platinum-pemetrexed doublet chemotherapy as second-line therapy in patients with stage IIIb/IIIc or IV EGFR-mutant, T790M-negative NSCLC harboring MET amplification. J Clin Onc. 2022;40(16_suppl):TPS9153–TPS9153. doi:10.1200/JCO.2022.40.16_suppl.TPS9153
36. Esaki T, Hirai F, Makiyama A, et al. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci. 2019;110(4):1340–1351. doi:10.1111/cas.13956
37. Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957. doi:10.1056/NEJMoa2002787
38. Wolf J, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated, advanced NSCLC: updated results from the GEOMETRY mono-1 study. J Clin Onc. 2021;39(15_suppl):9020. doi:10.1200/JCO.2021.39.15_suppl.9020
39. Illini O, Fabikan H, Swalduz A, et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): a retrospective analysis from an early access program. Ther Adv Med Oncol. 2022;14:17588359221103206. doi:10.1177/17588359221103206
40. Paik PK, Goyal RK, Cai B, et al. Real-world outcomes in non-small-cell lung cancer patients with MET Exon 14 skipping mutation and brain metastases treated with capmatinib. Future Oncol. 2023;19(3):217–228. doi:10.2217/fon-2022-1133
41. Heist RS, Garon EB, Green HJM, et al. Capmatinib safety update in MET dysregulated NSCLC from the GEOMETRY mono-1 trial. Ann Oncol. 2021;32:S986. doi:10.1016/j.annonc.2021.08.1859
42. Wolf J, Garon EB, Groen HJM, et al. Patient-reported outcomes in capmatinib-treated patients with METex14-mutated advanced NSCLC: results from the GEOMETRY mono-1 study. Eur J Cancer. 2023;183:98–108. doi:10.1016/j.ejca.2022.10.030
43. U.S. Food and Drug Administration. Center for drug evaluation and research. Resources for information on approved drugs. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tepotinib-metastatic-non-small-cell-lung-cancer.
44. U.S. Food and Drug Administration. Center for drug evaluation and research. Resources for information on approved drugs. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-amivantamab-vmjw-metastatic-non-small-cell-lung-cancer.
45. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391–3402. doi:10.1200/JCO.21.00662
46. National Comprehensive Cancer Network, Inc. The NCCN non-small cell lung cancer clinical practice guidelines in oncology (version 32023). 2023; Available from: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
47. Hashiguchi MH, Sato T, Yamamoto H, et al. Successful tepotinib challenge after capmatinib-induced interstitial lung disease in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation: case report. JTO Clin Res Rep. 2021;3(2):100271. doi:10.1016/j.jtocrr.2021.100271
48. Tseng L-W, Chang JW-C, Wu C-E. Safety of tepotinib challenge after capmatinib-induced pneumonitis in a patient with non-small cell lung cancer harboring MET exon 14 skipping mutation: a case report. Int J Mol Sci. 2022;23(19):11809. doi:10.3390/ijms231911809
49. Asan Medical Center. An open-label, multicenter, phase II Study of capmatinib in patients with non-small cell lung cancer harboring METex14 14 skipping mutation. Available from: http://www.clinicaltrials.gov/ct2/show/NCT03693339.
50. Timothy Burns. A Phase 2 open-label study to determine the central nervous system efficacy of capmatinib in NSCLC patients with brain metastases with cfDNA positive MET alterations. Available from: http://www.clinicaltrials.gov/ct2/show/NCT05567055.
51. Collin Blakely. A multicenter phase I/Ib Study of capmatinib plus trametinib in patients with metastatic MET exon 14 skipping mutation positive NSCLC. Available from: http://www.clinicaltrials.gov/ct2/show/NCT05435846.
52. Novartis Pharmaceuticals. A prospective, multicenter, open-label, Phase IV, interventional Study to assess the safety and efficacy of capmatinib in Indian patients with mesenchymal epithelial transition (MET) exon 14 skipping mutation positive advanced Nonsmall Cell Lung Cancer (NSCLC). Available from: http://www.clinicaltrials.gov/ct2/show/NCT05110196.
53. Novartis Pharmaceuticals. A phase ii, multicenter, two-cohort study of oral MET inhibitor capmatinib in Chinese adult patients with EGFR wild-type (wt), ALK rearrangement negative, MET exon 14 skipping mutations, Advanced Non-small Cell Lung Cancer (NSCLC) who are treatment naive or failed one or two prior lines of systemic therapy. Available from: http://www.clinicaltrials.gov/ct2/show/NCT04677595.
54. Novartis Pharmaceuticals. A randomized, open label, multicenter phase ii study evaluating the efficacy and safety of capmatinib (INC280) plus pembrolizumab versus pembrolizumab alone as first line treatment for locally advanced or metastatic non-small cell lung cancer with PD-L1≥ 50%. Available from: http://www.clinicaltrials.gov/ct2/show/NCT04139317.
55. Novartis Pharmaceuticals. A Phase III, randomized, controlled, open-label, multicenter, global study of capmatinib versus soc docetaxel chemotherapy in previously treated patients with EGFR wt, ALK negative, locally advanced or metastatic (stage IIIB/IIIC or IV) NSCLC harboring met exon 14 skipping mutation (METΔex14). Available from: http://www.clinicaltrials.gov/ct2/show/NCT04427072.
56. Novartis Pharmaceuticals. Phase II trial of neoadjuvant and adjuvant capmatinib in participants with stages IB-IIIA, N2 and Selected IIIB (T3N2 or T4N2) NSCLC with MET exon 14 skipping mutation or high MET amplification (geometry-N). Available from: http://www.clinicaltrials.gov/ct2/show/NCT04926831.
57. Novartis Pharmaceuticals. A double-blind, placebo controlled, randomized, phase ii study evaluating the efficacy and safety of capmatinib and spartalizumab vs capmatinib and placebo as 1st line treatment for advanced NSCLC patients with MET exon 14 skipping mutations. Available from: http://www.clinicaltrials.gov/ct2/show/NCT04323436.
58. Janssen Research & Development, LLC. A Phase 1/2 study evaluating the safety and efficacy of amivantamab and capmatinib combination therapy in unresectable metastatic non-small cell lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT05488314.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
