Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
PRO Hair Safe Study: The Patient’s Perspective on the Effects of Scalp Cooling on Hair Preservation
Authors Brunner C, Egle D , Ritter M, Kofler R, Giesinger JM, Schneitter L, Sztankay M, Emmelheinz M , Abdel Azim S, Wieser V, Oberguggenberger A
Received 13 March 2023
Accepted for publication 11 June 2023
Published 17 July 2023 Volume 2023:15 Pages 485—494
DOI https://doi.org/10.2147/BCTT.S412338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Christine Brunner,1 Daniel Egle,1 Magdalena Ritter,1 Ricarda Kofler,1 Johannes M Giesinger,2 Lisa Schneitter,1 Monika Sztankay,2 Miriam Emmelheinz,1 Samira Abdel Azim,1 Verena Wieser,1 Anne Oberguggenberger2
1Department of Gynecology and Obstetrics, Medical University of Innsbruck, Innsbruck, Austria; 2Department of Psychiatry, Psychotherapy, Psychosomatics and Medical Psychology, Psychiatry II, Medical University of Innsbruck, Innsbruck, Austria
Correspondence: Christine Brunner, Department of Gynecology and Obstetrics, Medical University of Innsbruck, Anichstraße 35, Innsbruck, 6020, Austria, Tel +43 512 504 81194, Email [email protected]
Purpose: Alopecia has been reported a distressing side-effect of chemotherapy for breast cancer patients (BCP) that is highly relevant for quality of life during treatment. For the prevention of chemotherapy-induced alopecia, scalp cooling (SC) has been reported to be an effective and safe intervention. However, data on the patient’s perspective on effectiveness and applicability of SC in a clinical routine setting are scarce. In this comparative study, we aimed at a longitudinal assessment of patient-reported outcome (PRO) data on the effect of SC on alopecia and its effect on symptoms and functional health when applied in clinical routine in BCP receiving taxane or anthracycline-based chemotherapy.
Patients and Methods: Study participants were allocated either to the intervention group receiving SC or to the control group based on patient preference (non-randomized study). All patients completed PRO-measures on hair preservation (EORTC Item Library items on hair loss), symptom and functional health measures (EORTC QLQ-C30 and -BR23) and the Body Image Scale (BIS). Outcomes were assessed at chemotherapy start (baseline), mid-chemotherapy, last chemotherapy cycle, 3 months follow-up and 6– 9 months follow-up.
Results: Overall, we included 113 patients: 75 patients underwent SC (mean age = 51.3 years, 52.7% premenopausal); 38 patients standard care (mean age = 55.6 years, 39.5% premenopausal). A total of 53 patients (70.7%) discontinued SC, with 39 patients (73.5%) stating alopecia as the primary reason. On average, BCP stayed on treatment with the cooling cap for 40.2% of the duration of their chemotherapy (SD 25.3%). In an intention-to-treat analysis, we found no difference between the SC group and the control group with regard to their patient-reported hair loss (p=0.831) across the observation period, overall QOL (p=0.627), emotional functioning (p=0.737), social functioning (p=0.635) and body image (p=0.463) did not differ between groups.
Conclusion: We found a high rate of SC-decliners and no beneficial effects of SC for patient-reported hair loss, symptoms and functional health. The efficacy and tolerability of SC applied in a clinical routine setting hence appeared to be limited. The further determination and up-front definition of criteria prognostic for effectiveness of SC may be helpful to identify patient subgroups that may experience a treatment benefit.
Keywords: cooling cap, self-report, alopecia, chemotherapy
Introduction
Breast cancer is the predominant cancer disease in women worldwide.1 For women, this life-threatening disease constitutes an interruption of their life in multiple ways. Disease and treatment side-effects do not only cause severe physical impairments but also changes of the women’s emotional stability and social relationships. The patient’s perspective of life, self-perception and quality of life (QOL) is changed.2,3
A well-known side-effect of chemotherapy is chemotherapy induced alopecia (CIA). Though not life-threatening, CIA impacts on the patients’ social appearance as it contributes to the visibility of the cancer disease not only for the patient herself but also for others. Patients report the experience of hair loss as a limitation of their feeling of femininity, attractiveness, body image and self-esteem. Numerous studies have proved alopecia to be associated with patients’ psychological and social well-being.4–7 Some patients even decline chemotherapy treatment because of fear for alopecia.8 In order to support patients in keeping life as “stable” as possible, targeted supportive treatment strategies for CIA can contribute to reducing symptoms and sustaining or improving the patient’s functional health. Within the last few years, several studies have demonstrated scalp cooling efficacious and successful for the management of CIA.9–12 Evidence seems to prove the cooling of the scalp to reduce the delivery of chemotherapy to the scalp thereby reducing cellular damage. A reduction of more than 50% of CIA by scalp cooling (SC) has been reported in clinical studies.13 However, most studies have not lived up to requirements of a clinical routine setting, eg SC was mostly applied to single-agent chemotherapy regimens rather than the much more frequently applied combined chemotherapy regimens. Moreover, established evidence has predominantly highlighted SC efficacy in terms of the quantification of effective hair loss. In the evaluation of clinical efficacy and applicability of SC, research should focus on the subjective patient experience with the intervention in a clinical routine setting. This also includes a comprehensive assessment of patient-reported symptom burden over the treatment course, ie implications of SC on body image, emotional and social well-being and overall QOL outcome.14
The authors hence conducted a multi-method study on the assessment of SC applicability and effectiveness in a routine clinical treatment setting – The Hair Safe Study. This included the evaluation of the grade of hair preservation by clinicians using the Common Terminology Criteria for Adverse Events (CTCAE) grading system and patients using the Patient-Reported Outcomes (PRO) version CTCAE (PRO-CTCAE) grading for hair loss (primary endpoint). According to clinician ratings, an effectiveness of 72% was observed. Details of the clinical results of this study have been published elsewhere.15 In addition, we aimed at evaluating the patient perspective on SC efficacy. For this purpose, a longitudinal patient-reported outcome assessment on SC efficacy for the prevention of alopecia and patient-reported symptoms and functional health was conducted. Herein, we present PRO results of the Hair Safe study.
Materials and Methods
Study Design
The study is an observational, prospective, single-center study with two study arms conducted at the Department of Gynecology and Obstetrics. Medical University of Innsbruck from May 2018 to February 2021 (clinical registration number NCT04117815). In this study, based on patient preference, participants were allocated to either an intervention group receiving scalp cooling or a control group with standard care without scalp cooling (allocation ratio 2:1). We were not able to use a study design with group randomization due to the following ethical considerations: the treatment with SC has been proved effective in admission trials/Phase 4 studies and is an approved medical product. Denying patients an approved available treatment (due to randomization) when offered as part of routine treatment would have caused ethical problems, ie treatment-disadvantage for the control group. The study was approved by the ethics committee of the Medical University of Innsbruck (EK Nr. 1049/2018) and complies with the Declaration of Helsinki.
Participants
Breast cancer patients treated at the Department of Gynecology and Obstetrics, Medical University of Innsbruck, were eligible for the study according to the following inclusion criteria:
- A treatment indication for chemotherapy (neoadjuvant, adjuvant or palliative)
- Treatment with a maximum of two lines of chemotherapy (adjuvant chemotherapy is considered as one line)
- Chemotherapy regime associated with alopecia, ie taxane or anthracycline-based chemotherapy regimens
- Chemotherapy duration planned for at least 4 cycles
- Written informed consent
- Age 18 and older
- No overt cognitive impairment
- Fluency in German
- No contra-indication for scalp cooling (ie migraine, scalp metastasis, alopecia of any reasons, hematological malignancies, cold allergy/agglutinins).
Treatment Allocation
Patients fulfilling the inclusion criteria were offered scalp cooling. BCP who decided to undergo scalp cooling were allocated in the intervention group. Details on the SC procedure have been published elsewhere.15 In short, patients underwent SC using the Orbis Paxman Hair Loss Prevention Scalp cooling system (16–20 °C) at every CT cycle.
Patients having a contraindication for scalp cooling or refused scalp cooling were allocated to the control group (reference sample).
Assessment of Self-Reported CIA and Patient-Reported Symptoms and Functional Health
All patients completed PRO measures on CIA (European Organization for Research and Treatment of Cancer [EORTC] Item Library items on hair loss), symptom and functioning health measures (EORTC QLQ-C30 and -BR23) and the Body Image Scale (BIS). Please find a detailed description of questionnaires below. PRO outcomes were assessed at the following five time points: chemotherapy start (baseline), mid-chemotherapy, last chemotherapy cycle, 3 months follow-up and 6–9 months follow-up. At each assessment time point, patients completed the questionnaires (paper–pencil version) themselves during the chemotherapy application. A study nurse provided support when required.
Assessment Instruments
EORTC QLQ-C30, QLQ-BR23 and Additional Items from the EORTC Item Library
The EORTC QLQ-C30 is a patient self-report measure assessing 15 dimensions of health-related quality of life in cancer patients. It comprises 30 items with a 4-point Likert scale response format (1=not at all, 2=a little, 3=quite a bit, 4=very much). The measure targets on functioning (physical, role emotional, cognitive and social functioning and global health/QoL scale) and symptoms (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties). High scores indicate high level of functioning/QoL (functioning scales) and high levels of symptom (for the symptom scales).16
The core questionnaire can be supplemented by disease specific modules. For the purpose of this study, we used the breast cancer specific module – The EORTC QLQ-BR23 – as a supplement. It consists of 23 items comprising 2 functioning scales (body image and sexuality) and 3 symptom scales (arm symptoms, breast symptoms, systemic therapy symptoms).17 In addition, we included the following four items specific for hair loss from the EORTC Item Library (https://qol.eortc.org/item-library/, accessed 05/2018):
- Have you lost any hair?
- Were you upset by the loss of your hair?
- Have you been upset by how the treatment has affected your hair?
- Have you had thin or lifeless hair as a result of your disease or treatment?
Corresponding to the response format of EORTC QLG questionnaires, hair-loss questions are answered on the same 4-point Likert scale with higher values indicating hair symptoms.
Body Image Scale (BIS)
The BIS is a well-validated and widely used PRO measure for the assessment of body image in cancer patients.18 It is a 10-item instrument with a response format of a 4-point Likert scale. A total score is calculated from all items. High values indicate good body image.
Statistical Analysis
Descriptive statistics for sociodemographic and clinical variables are given as means, medians, standards deviations, and absolute and relative frequencies. Baseline comparisons for these variables between the two study arms relied on a t-test for independent sample and 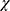 ²-tests. For the longitudinal analysis of the PRO parameters, we used linear mixed models with a first-order autoregressive covariance matrix. These models included the PRO parameter as a dependent variable and the following fixed effects: study group, time point, and the group-by-time interaction. In this model a treatment effect is reflected by the interaction term that indicates a group difference in change over time. Based on the linear mixed models we calculated the estimated marginal means and their 95% confidence intervals for each time point and study group. All analyses were conducted in the software SPSS 27.0.
²-tests. For the longitudinal analysis of the PRO parameters, we used linear mixed models with a first-order autoregressive covariance matrix. These models included the PRO parameter as a dependent variable and the following fixed effects: study group, time point, and the group-by-time interaction. In this model a treatment effect is reflected by the interaction term that indicates a group difference in change over time. Based on the linear mixed models we calculated the estimated marginal means and their 95% confidence intervals for each time point and study group. All analyses were conducted in the software SPSS 27.0.
Results
Patient Characteristics
Between May 2018 and February 2020, 113 breast cancer patients were recruited at the start of (neo-)adjuvant chemotherapy. Out of the 113 patients in the analysis, 75 patients were in the scalp cooling group and 38 patients were in the control group. Reasons for patients’ decision for not choosing the cooling group were pre-existing migraine (10 patients, 26.3%), no specific reason stated (14 patients, 36.8%), hematological, neurological or vascular diseases (4 patients, 10.5%), or participation in a study with cooling gloves (5 patients, 13.1%) (Figure 1).
 |
Figure 1 Study flow chart. |
Patients received different CT protocols that comprised either a taxane monotherapy or a taxane- and anthracycline-based CT (either taxane first, anthracycline first or both CT regimens simultaneously). More than 80% of patients in both groups received taxane- and anthracycline-based CT. Details on the impact of CT regimens on the effectiveness of SC have been published previously (Hair Safe Study).15
Mean age was 51.3 years (SD 13.5) in the cooling group compared to 55.6 years (SD 12.4) in the control group (p=0.102). Menopausal status did not differ statistically significantly between two groups (p=0.232), with 52.7% of premenopausal patients in the scalp cooling group and 39.5% in the control group. Tumor grade was statistically significantly higher in the cooling group than in the control group (grade 3: 44.0% vs 21.1%; p=0.015). One patient discontinued chemotherapy after 3 of 4 cycles but was included in the ITT analysis. For further details please see Table 1.
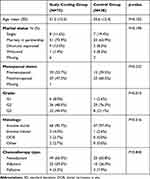 |
Table 1 Sample Characteristics of the Intention-to-Treat Population (N=113) |
Self-Reported CIA
In the cooling group 53 patients (70.7%) reported treatment discontinuation with the cooling cap, with 39 patients (73.5%) stating alopecia as the primary reason (Figure 1). Mean time to treatment discontinuation was 6.2 weeks (median 4.7; SD 4.5). On average patients stayed on treatment with the cooling cap for 40.2% of the duration of their chemotherapy (SD 25.3%). We found no difference regarding self-reported CIA and burden by CIA between groups over the course of treatment to follow-up. Please find details in Table 2. In addition, patient examples on the effect of SC are provided in Figure 2 (“patient examples of SC results over the course of CT treatment”), presenting examples of a patient with a successful SC with sufficient hair preservation, a patient who discontinued SC due to alopecia and a patient from the control group.
 |
Table 2 Self-Reported CIA by Means of EORTC QLQ – Items Hair Loss |
 |
Figure 2 Patient examples of scalp cooling results over the course of CT treatment. |
Patient-Reported Symptoms Across the Course of Chemotherapy to Follow-Up
The intention-to-treat analysis revealed no difference between the SC and no SC groups regarding patient-reported symptom burden. SC had no beneficial effect on body image, emotional or social functioning. Please find details in Table 3. Results for all EORTC QLQ-C30/ BR23 are presented as Supplemental Material.
Discussion
Self-Reported CIA
In our study, the SC group did not experience significantly better hair preservation compared to the group without SC across the course of treatment. We observed a high rate of patients discontinuing SC very early in the treatment course because of CIA. The majority of patients (ie 70.7%) declined from SC after a median time of 4.7 weeks due to increasing CIA. This drop-out rate seems to exceed rates reported in the literature. Chan et al19 observed about 40–45% decliners from SC depending on the chemotherapy regimen received. In an Italian study, 32% declined early in the course of SC treatment.10 Previous evidence illustrates better effectiveness of SC for CIA.20
However, the comparability of results seems to be highly limited as treatment regimens (in terms of agents used, dosage and cycle numbers), study sample size or design and population characteristics differ distinctly across studies. In a systematic review of SC studies, the authors identified effectivity rates ranging from 27 to 90% hair preservation, with a high impact of the chemotherapy regimens applied. Also, others highlighted differing study designs to explain the lack of consistency of results on SC efficacy.11,21 Our “real life”, heterogeneous study sample in terms of various CT regimens seems to reflect these previous observations and might contribute to the explanation of our SC drop-out rate. To the best of our knowledge, our study is among the first presenting data from a “clinical real life” setting. In contrast to a Phase 3 (admission) trial, eligibility criteria hence allowed for a more heterogeneous sample, ie comprising all patients receiving any type of chemotherapy (different regimes neo-adjuvant, adjuvant or palliative) at different disease stages. Patients were offered SC as part of a routine care strategy rather than a new treatment investigated for its effectiveness. These factors seem to be relevant for patient expectations, motivation for participation and adherence behavior which in turn impacts on study outcome. It is well-known that the generalizability of clinical trial data into clinical routine, to “real-world” patients, underlies limitations.22–24 Observational studies such as our Hair Safe study provide complementary data to the essential, “gold-standard” data derived from clinical trials.24
In addition, the patient perspective was a central focus of this study. The patient’s appraisal of treatment effectiveness is highly driven by patient expectations and perception of “adequate” hair preservation. Most studies rate hair preservation of >50% as successful. For patients, this “cut-off” seems to be inadequate, ie even small hair loss might change the patient’s appearance and makes patients experience CIA as burdensome. With increasing symptom burden patients’ willingness to undergo additional treatments might decrease resulting in early treatment discontinuation. The subjective perception of hair loss hence seems to be central in the evaluation of efficacy. These considerations are also supported by findings of the Hair Safe study published elsewhere:15 a significant difference between patient ratings and proxy ratings by health care experts was found in terms of grading of hair loss. Patients graded hair loss significantly higher than the health care experts. Further research should focus on the determination of cut-offs for hair loss that patients are satisfied with in routine care. We need to define what constitutes “adequate” hair loss that can contribute to a preservation of functional health.
Patient-Reported Symptom Burden
We could not show SC to be superior to no SC with regard to patient-reported hair loss and functional health including body image, emotional and social functioning. This finding supplements evidence reported over the last years. Marks et al14 presented mixed evidence on the effect of SC for QOL outcome in their systematic review. However, the majority of studies (62%) reported no QOL improvement by SC. For instance, Chan et al found no advantageous effect of SC on body image, depression or anxiety.19 Concurrently, Smetanay et al reported comparable results for SC.12 However, our results can be attributed to the high number of patients discontinuing SC at an early stage within the course of chemotherapy. Only 30% of the SC group finished SC, so that group comparability for patient-reported symptom burden is highly limited. However, we can assume that our observational data derived in clinical routine might present a practical and realistic reflection of SC applicability compared to referenced clinical trials.
In order to develop a more detailed understanding of the patient experience of SC until their treatment decline, a PRO assessment with smaller intervals between assessments would have been advantageous. Most patients declined at some point in between the baseline and second assessment (mid chemotherapy), which was unexpectedly early in the treatment course. PRO data at each CT cycle would have given better insight into the development of hair loss and its implication for functional health.
Conclusion
SC has been demonstrated an efficacious supportive treatment in previous clinical studies. Findings in our observational study seem to reflect the well-known limitation of data generalizability from trial outcome data to clinical routine. SC implementation in a routine clinical treatment setting seems to require some further considerations and preparations: further research should focus on the determination and up-front definition of criteria prognostic for effectiveness of SC so that patient subgroups with highest treatment benefit can be identified. Another valuable method for this purpose might be the use of biomarkers for predicting alopecic severity. As Chae, Ng and Chan pointed out, biomarkers might contribute to a better understanding on how to improve scalp cooling delivery.25 The advancement of supportive care strategies such as SC should be a major focus in research and patient care.
Data Sharing Statement
The authors intend to share deindividualized data upon reasonable request for academic use only. Data sharing includes the data published in this manuscript. No other documents will be made available. Data can be accessible via the corresponding author (request via email). Data will be available for at least one year after publication.
Acknowledgments
We gratefully acknowledge the work of the team of the breast outpatient clinic of the Department of Gynecology and Obstetrics, Medical University of Innsbruck, substantially supporting this study, and thank all study participants for sharing their knowledge. This paper was presented at the 13th European Breast Cancer Conference as a poster presentation with interim findings. The poster’s abstract was published in “Abstract Book” in European Journal of Cancer.
Disclosure
Christine Brunner has received grant funding from Paxman UK. Daniel Egle reports personal fees from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Novartis, Pfizer, Sirius; grants from Sirius; non-financial support from Roche, outside the submitted work. All other authors declare no conflicts of interest in this work.
References
1. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128(1):47–58.
2. Kaptein AA, Schoones JW, Fischer MJ, Thong MS, Kroep JR, van der Hoeven KJ. Illness perceptions in women with breast cancer-a systematic literature review. Curr Breast Cancer Rep. 2015;7(3):117–126.
3. Fanakidou I, Zyga S, Alikari V, Tsironi M, Stathoulis J, Theofilou P. Mental health, loneliness, and illness perception outcomes in quality of life among young breast cancer patients after mastectomy: the role of breast reconstruction. Qual Life Res. 2018;27(2):539–543.
4. Zdenkowski N, Tesson S, Lombard J, et al. Supportive care of women with breast cancer: key concerns and practical solutions. Med J Aust. 2016;205(10):471–475.
5. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psycho-Oncology. 2008;17(4):317–328.
6. Abu-Helalah M, Al-Hanaqta M, Alshraideh H, Abdulbaqi N, Hijazeen J. Quality of life and psychological well-being of breast cancer survivors in Jordan. Asian Pac J Cancer Prev. 2014;15(14):5927–5936.
7. Helms RL, O’Hea EL, Corso M. Body image issues in women with breast cancer. Psychol Health Med. 2008;13(3):313–325.
8. Batchelor D. Hair and cancer chemotherapy: consequences and nursing care--a literature study. Eur J Cancer Care (Engl). 2001;10(3):147–163.
9. Bajpai J, Kagwade S, Chandrasekharan A, et al. ”Randomised controlled trial of scalp cooling for the prevention of chemotherapy induced alopecia”. Breast. 2020;49:187–193.
10. Munzone E, Bagnardi V, Campennì G, et al. Preventing chemotherapy-induced alopecia: a prospective clinical trial on the efficacy and safety of a scalp-cooling system in early breast cancer patients treated with anthracyclines. Br J Cancer. 2019;121(4):325–331.
11. Rugo HS, Klein P, Melin SA, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. 2017;317(6):606–614.
12. Smetanay K, Junio P, Feißt M, et al. COOLHAIR: a prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing (neo)adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat. 2019;173(1):135–143.
13. de Barros Silva G, Donati A, van den Hurk CJ. Comments regarding “Hair regrowth during chemotherapy after scalp cooling technique”. Int J Dermatol. 2017;56(3):e57–e59.
14. Marks DH, Okhovat JP, Hagigeorges D, et al. The effect of scalp cooling on CIA-related quality of life in breast cancer patients: a systematic review. Breast Cancer Res Treat. 2019;175(2):267–276.
15. Brunner C, Emmelheinz M, Kofler R, et al. Hair safe study: effects of scalp cooling on hair preservation and hair regrowth in breast cancer patients receiving chemotherapy - A prospective interventional study. Breast. 2022;64:50–55.
16. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
17. Michels FA, Latorre Mdo R, Maciel Mdo S. Validity, reliability and understanding of the EORTC-C30 and EORTC-BR23, quality of life questionnaires specific for breast cancer. Br j epidemiol. 2013;16(2):352–363.
18. Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189–197.
19. Chan A, Bauwens A, Pontre S, et al. Efficacy of scalp cooling in reducing alopecia in early breast cancer patients receiving contemporary chemotherapy regimens. Breast. 2018;41:127–132.
20. Wang S, Yang T, Shen A, Qiang W, Zhao Z, Zhang F. The scalp cooling therapy for hair loss in breast cancer patients undergoing chemotherapy: a systematic review and meta-analysis. Support Care Cancer. 2021;29(11):6943–6956.
21. Komen MM, Smorenburg CH, van den Hurk CJ, Nortier JW. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist. 2013;18(7):885–891.
22. Fraser J, Steele N, Al Zaman A, Yule A. Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with FE100C-D chemotherapy in a non-trial population of node positive breast cancer patients compared with PACS-01 trial group. Eur J Cancer. 2011;47(2):215–220.
23. Blanco C, Olfson M, Goodwin RD, et al. Generalizability of clinical trial results for major depression to community samples: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(8):1276–1280.
24. He Z, Tang X, Yang X, et al. Clinical trial generalizability assessment in the big data era: a review. Clin Transl Sci. 2020;13(4):675–684.
25. Jung-Woo Chae RN. Alexandre Chan Chemotherapy drug concentrations in hair follicles: a potential biomarker to monitor the effectiveness of scalp cooling for chemotherapy-induced alopecia. Support Care Cancer. 2018;26(11):3669–3670.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

