Back to Journals » Journal of Inflammation Research » Volume 16
Primary Angiitis of the Central Nervous System in Adults: A Comprehensive Review of 76 Biopsy-Proven Case Reports
Authors Lu P , Cui L , Zhang X
Received 3 September 2023
Accepted for publication 31 October 2023
Published 7 November 2023 Volume 2023:16 Pages 5083—5094
DOI https://doi.org/10.2147/JIR.S434126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Ping Lu,1 Lingyun Cui,1 Xinghu Zhang2
1Center for Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 2Department of Neuroinfection and Neuroimmunology, Center for Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China
Correspondence: Xinghu Zhang, Department of Neuroinfection and Neuroimmunology, Center for Neurology, Beijing Tiantan Hospital, Capital Medical University, No. 119, South Fourth Ring Road West, Beijing, 100070, People’s Republic of China, Tel + 86 10 5997 6585, Email [email protected]
Purpose: Primary angiitis of the adult central nervous system (PACNS) is an increasingly recognized but limited disease. Using previous case reports, we sought to summarize the clinical symptoms, imaging manifestations, treatment, and prognosis of patients with biopsy-confirmed PACNS to guide clinical diagnosis and management.
Methods: We searched the Web of Science database for studies published from January 2000 to April 2023, with the language set to English and the document type limited to [Article or Review or Letter or Editorial Material]. A systematic review of all case reports met the inclusion and exclusion criteria was performed. These patients’ clinical, pathological, and imaging characteristics were analyzed, and treatment and prognostic data were summarized.
Results: We analyzed 69 articles, including 76 patients with biopsy-confirmed PACNS. And 57.9% of the patients were male, the median age at presentation was 47.0 years, and focal neurological deficits were the most common symptom in patients (78.9%), followed by headache (52.6%). The median duration of biopsy was 1.1 months, of which 49 (64.5%) patients were lymphocytic, 13 (17.1%) were granulomatous, 11 (14.5%) were amyloidotic, and 3 (3.9%) were necrotizing PACNS. Relapse events occurred in 41 (53.9%) patients, including 34 (44.2%) relapses and 8 (10.5%) deaths. Univariate logistic regression analysis revealed that symptomatic epilepsy, prolonged biopsy time window, and CD20 expression in pathological tissues might be independent risk factors for recurrent events in patients (HR=4.69, 95% CI: 1.51– 14.54, p=0.007; HR=1.11, 95% CI: 1.00– 1.22, p=0.043; HR=5.33, 95% CI: 1.07– 26.61, p=0.041).
Conclusion: Adult PACNS is associated with frequent relapses and high mortality. Symptomatic epilepsy, prolonged biopsy time window, and CD20 expression in pathological tissue may be associated with recurrent events.
Keywords: PACNS, biopsy, recurrence, CD20, comprehensive review
Introduction
Primary angiitis of the central nervous system (PACNS) in adults is a rare idiopathic inflammatory disease affecting only intracranial and spinal cord vessels, predominantly small and medium-sized vessels. Harbitz first reported this vasculitis of unknown origin in 1922.1 Subsequently, Calabrese and Mallek systematically reported eight cases of this type of disease in 1988, unified them under the name PACNS, and proposed clinical diagnostic criteria.2 In 2005, Scolding further named granulomatous vasculitis due to β-amyloid as Aβ-related angiitis (ABRA).3 Clinical symptoms include focal neurological deficits, headache, symptomatic epilepsy, and cognitive impairment, and the diagnosis is mainly confirmed by biopsy or angiography. Easily confused with intracranial tumors in terms of imaging.4 The pathological types are lymphocytic, granulomatous, necrotizing, and ABRA.5 The low incidence and diagnostic difficulties have led to a lack of prospective randomized studies on PACNS in adults.
The 191 cases of PACNS reported by Salvarani et al at the Mayo Clinic, USA, in 2020 is the most extensive sample report on the disease. These cases were collected over 35 years (1983–2017), with 120 patients diagnosed by angiographic findings and 71 diagnosed by biopsy findings.6 The following most significant sample was the French multicenter registry project (COVAC), initiated in 2010, which reported 110 patients in 2018, 78 of whom were diagnosed by angiographic findings and 32 by biopsy findings.7 Beuker et al reviewed PubMed articles related to PACNS from 1988 to 2020, resulting in a systematic review and meta-analysis of 46 cohort studies including a total of 911 patients, of which 41% were biopsy confirmed, 43% angiographically guaranteed, and 16% not assigned to a diagnostic procedure.8 Although knowledge of PACNS is becoming more advanced, it still needs to be improved, and therefore a systematic review of previous case information is warranted.
In this study, we summarized the clinical, pathological, and imaging characteristics of adult patients with PACNS diagnosed by previous biopsy through a systematic review of case reports of these patients. We reported the treatment outcomes, recurrence events, and mortality rates of different drugs to facilitate understanding and research on this disease.
Method
Data Sources and Search Strategies
We searched the Web of Science Core Collection (WOSCC) database for articles related to PACNS reported between January 1, 2000, and April 30, 2023. Our search strategy was based on the following combination of medical subject terms and keywords: subject set to [TS=“primary angiitis of the central nervous system” OR “primary angiitis of the central nervous system” OR “primary central nervous system vasculitis”], document type set to [Article or Review or Letter or Editorial Material], and the language was set to English. In addition, we performed a manual search for the list of references in the retrieved articles.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) article type as case report (2) diagnosis confirmed by pathological biopsy (3) several reported cases ≤3; Exclusion criteria: (1) missing pathological information (2) missing follow-up information.
Study Selection and Data Extraction
We screened all retrieved literature for titles and abstracts, and for those that met the criteria, we conducted a full-text review and finalized the literature for inclusion. Two authors (LP and CL) screened the literature, and consensus resolved disagreements. Predefined data were extracted using standardized forms. Extracted study and patient characteristics included article information (year of publication, number of reports), baseline characteristics (sex, age), clinical symptoms (focal neurological deficits, headache, symptomatic epilepsy, cognitive impairment), cerebrospinal fluid characteristics (white blood corpuscle and protein content), biopsy information (time window, number, pathological classification), imaging characteristics (site of involvement, vascular status, occupancy effects, enhancement), treatment modalities (Glucocorticoid, cyclophosphamide, azathioprine, rituximab, mycophenolate mofetil, methotrexate, gamma globulin, and etanercept), and prognostic information (follow-up time, recurrent events, and mortality outcome). If not specified in the article, the default time window from hospitalization to biopsy is 0.25 months.
Outcome
Relapse was defined as a recurrence or worsening of neurological symptoms or new evidence of neuroimaging findings. Death was defined as all-cause mortality. A relapse event was defined as the occurrence of a recurrence and death.
Statistical Analysis
Continuous variables are represented by median and Quartile spacing, and Categorical variable are represented by frequency (percentage). Analysis of variance or Wilcoxon rank sum test was used to compare continuous variables between groups, while Pearson Chi-squared test was used to compare Categorical variable. Calculate the hazard ratio (HR) of the 95% confidence interval using univariate logistic regression. A two-sided test of P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 23 software, and graphs were created in Excel (Microsoft® Excel 2019).
Results
The literature search included 383 articles, including 246 papers in [Article], 68 articles in [Review], 32 articles in [Letter], and 37 articles in [Editorial Material]. After title and abstract screening, 259 articles were excluded. The remaining 125 articles were reviewed in full text, of which 22 were diagnosed with PACNS confirmed by imaging, 11 had incomplete biopsy information, and 22 were pediatric-related case reports. Finally, 69 studies met the inclusion criteria,9–77 including 76 adult PACNS patients (Figure 1).
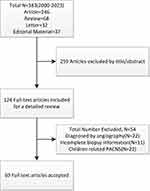 |
Figure 1 Study population flowchart. |
Publications Analysis
Sixty-nine studies reported 76 cases of PACNS in adults between 2001 and 2022. All analyses were cited 517 times, with a mean of 7.49 citations and a single highest source of 44. All studies were primarily from 49 journals, 22 countries, and 383 authors, of which Appendix Table 1 lists the top 10 journals, governments, and authors in terms of the number of articles published.
Baseline Characteristics
Of the 76 adult patients with biopsy-confirmed PACNS, 57.9% (44/76) were male, and the median age of onset was 47 years. Focal neurological deficits were the most common symptom in patients (60/76, 78.9%), followed by headache (41/76, 52.6%), cognitive deficits (29/76, 38.2%), and seizures (23/76, 30.3%). And 77.6% (59/76) of the patients reported the results of cerebrospinal fluid analysis, with missing information in the remaining patients. Of 59 patients, 47 (79.7%) had abnormal cerebrospinal fluid, of which 55.9% (33/59) had an elevated white blood cell count (> four cells/μL) and 76.3% (45/59) had a high protein level (>0.45 g/L). Most patients had lesions involving the brain (65/76, 85.5%), a few involved the spinal cord (5/76, 6.6%), and only six patients (7.9%) had both brain and spinal cord involvement. Angiography suggested stenosis in 28.8% (15/52) of the patients, a dominant effect was present in 61.8% (47/76) of the lesions, and enhancement was present in 92.3% (60/65) of the lesions (Table 1).
 |
Table 1 Baseline Characteristics of 76 PACNS Patients Confirmed by Histopathology |
Biopsy Characteristics
The median time window from onset to biopsy was 1.1 (0.3–6.8) months in 76 patients, of which 10.5% (8/76) were diagnosed by second biopsy. A total of 63 (82.9%) patients reported biopsy sites, including 5 (7.9%) patients whose diagnosis was confirmed by autopsy, with the most common being the temporal lobe (20/63, 31.7%), followed by the frontal lobe (15/63, 23.8%), parietal (11/63, 17.5%), spinal cord (6/63, 9.5%), cerebellum (4/63, 6.3%), occipital (1.6%) and thalamus (1/63, 1.6%). The final pathological findings were lymphocytic in 64.5% (49/76) of the patients, granulomatous in 17.1% (13/76), ABRA in 14.5% (11/76), and necrotizing PACNS in 3.9% (3/76). Histopathological staining was reported in 32 (42.1%) patients, of whom 68.8% (22/32) were positive for CD3/4/8 antibodies, 34.4% (11/32) for CD20 antibodies, and 50.0% (16/32) for CD68 antibodies β-amyloid staining was reported in 24 (31.6%) patients, of which 45.8% (11/24) were positive (Table 1).
In a dichotomous analysis, other pathological types were compared, and we found that ABRA patients were older (72.0 vs 44.0 years, p < 0.001) and more likely to have cognitive decline (100.0% vs 27.3%, p < 0.001), with no significant differences seen in the rest (Appendix Table 2).
Treatment and Follow-Up
Eight immunotherapeutic agents were used in combination, including glucocorticoid in 90.8% (69/76) of the patients, cyclophosphamide in 63.2% (48/76), azathioprine in 11.8% (9/76), rituximab in 10.5% (8/76), mycophenolate mofetil in 10.5% (8/76), methotrexate in 5.3% (4/76) patients were treated with methotrexate, 3.9% (3/76) patients with gamma globulin, and 1.4% (1/76) patients with etanercept (Table 2). The median time from onset to last follow-up was 17.3 (6.3–32.4) months, with 53.9% (41/76) of patients experiencing a relapse event, including 34 (44.2%) relapses and 8 (10.5%) deaths (Table 1, Figures 2 and 3).
 |
Table 2 Baseline Characteristics of 7 Therapeutic Drugs |
In terms of recurrent events, patients with recurrent events had a higher incidence of symptomatic epilepsy (43.9% vs 14.3%, p=0.005), a longer time window for biopsy (4.0 vs 0.5, p=0.003), more CD20 expression in pathological tissue (53.3% vs 17.6%, p=0.034), and a higher percentage of intracranial enhancement compared to patients without recurrent events higher (100% vs 83.9%, p=0.049). In univariate logistic regression, symptomatic epilepsy, biopsy time window, and CD20 expression in pathological tissues may be independent risk factors for patients presenting with recurrent events (HR=4.69, 95% CI: 1.51–14.54, p=0.007; HR=1.11, 95% CI: 1.003–1.22, p= 0.043; HR=5.33, 95% CI: 1.07–26.61, p=0.041, Table 3).
 |
Table 3 Characteristics of Patients with Recurrent Events and Death Outcomes |
In terms of mortality outcomes, patients who died had a higher incidence of secondary biopsies (37.5% vs 7.4%, p=0.043), lower rates of immunosuppression (25.0% vs 76.5%, p=0.009), and shorter follow-up (3.3 vs 19.0 months, p=0.012, Table 3) compared to those who survived.
Discussion
We systematically reviewed 69 PACNS-related case reports, including 76 adult patients. Adult PACNS was associated with frequent relapses and high mortality. Symptomatic epilepsy, prolonged biopsy time window, and CD20 expression in pathological tissue may be associated with recurrent events.
To our knowledge, this is the first study to pool case reports of adult PACNS. All of our patients were confirmed by biopsy, and some reported histopathological staining results, while the Mayo Clinic cohort and the French COVAC cohort had approximately 30% of biopsy-confirmed cases.6,7 The sex ratio was comparable to the two large cohorts. The median age was consistent with the COVAC cohort and younger than the Mayo Clinic cohort (47.0 vs 58.0 years), possibly due to the lower proportion of patients with ABRA. There was heterogeneity in the four pathological patterns of adult PACNS, with different histopathological ways leading to variability between cohorts. The pathological typing in our cohort was similar to that of the French COVAC cohort, which was predominantly lymphocytic with more than half (64.5%). In contrast, the Mayo Clinic cohort was predominantly ABRA (33.8%), followed by granulomatous (28.2%). Necrotizing vasculitis may be a less common pattern, with the lowest percentage in the Mayo Clinic cohort (14.1%) and present in only 3.9% of the patients in our cohort. Salvarani et al summarized ABRA in the Mayo Clinic cohort and found that ABRA patients were older and more likely to have cognitive changes and seizures. Significant inflammatory vessel wall disruption was seen in ABRA that was not found in inflammatory cerebral amyloid angiopathy, suggesting that ABRA represents a distinct subset of PACNS.78 So, like previous PACNS studies, we included patients with ABRA. The median age of ABRA patients in our study was 72 years, consistent with previous studies (65.2 years), significantly higher than sarcoidosis (35 years) and lymphocytic PACNS (46 years), and had significant cognitive impairment but no difference in the occurrence of symptomatic epilepsy.
Since no drug-related randomized clinical trials exist for adult PACNS, its treatment strategies are primarily derived from other vasculitis cohorts. The eight different immunologic agents in this study were combined, reflecting variability in disease perception and management. In two large cohorts, almost all patients were initially treated with steroid hormones (96% vs 98%). In our study, 69 (90.8%) patients were initially treated with steroid hormones, including 34 in combination with cyclophosphamide; 3 (3.9%) patients were initially treated with immunosuppressants only; and 4 (5.3%) patients were not treated, of whom 2 had a fatal outcome. A recent prospective observational study found that MMF significantly reduced the incidence of new neurological events in adults with PACNS.79 In our cohort, the use of MMF was present only in relapsed patients (20% vs 0%, P=0.012), mainly as second-line treatment. In the future, MMF may act as an effective immunosuppressive agent for the induction and maintenance of PACNS in adults, reducing the incidence of relapse events.
More than half of the patients in our study had recurrent events, higher than the two large cohorts (53.9% vs 30% vs 34%), which may be due to the different cohort structure, with previous studies showing that patients with angiographically negative PACNS had more frequent recurrences.80,81 Unfortunately, the data on stenosis in this study were incomplete and did not allow for a valid comparison. The French COVAC cohort showed that symptomatic epilepsy and focal intensification were associated with frequent recurrences, partially consistent with our study. However, no correlation between focal intensification and recurrent events was found in our research, despite the trend.81,82 Also, our study found that a prolonged biopsy time window was associated with recurrence events, which may be because a lengthy biopsy time window increases the time to diagnosis and initial treatment of PACNS. Interestingly, we found that CD20 expression in pathological tissues may be associated with relapse events, and to our knowledge, this is the first study to summarize the expression of PACNS pathological tissues, and this finding provides a basis for precision medicine, and CD20 testing is recommended routinely for brain tissue staining in patients with suspected PACNS. In this study, the use of rituximab was higher in the CD20-expressing group compared to the CD20 non-expressing group. However, the difference was not statistically significant due to the small sample size (0% vs 27.3%, p=0.061), and the existence of effectiveness of target-specific therapy needs to be verified in a larger cohort. Our cohort had a mortality outcome in 10.5% of the patients between the two large cohorts (15.0% vs 6.0%), including five rapidly progressive deaths and three long-term follow-up deaths. Although the Mayo Clinic cohort showed deaths only with sarcoidosis and necrotizing PACNS, 8.2% of the lymphocytic PACNS in our cohort died, and the higher proportion of lymphocytic PACNS and case report bias may be potential causes.
Our study has some limitations. First, although our cohort is the largest pathological biopsy cohort available, however, due to the rarity of PACNS and the diversity of pathological types, classification into four pathological types does not allow statistical comparison due to the small sample size. Secondly, all data were extracted from case reports and the completeness of the data relied on the article itself, for most of the data we could only count qualitatively and could not distinguish the severity and know the exact time of biopsy, follow-up and medication administration. Considering the difficulty of large-scale prospective studies of PACNS, a global large-scale retrospective study of case reports may be useful.
Conclusion
PACNS, associated with frequent relapses and high mortality, is a collective term for various pathological types of neurovascular inflammatory diseases with heterogeneity in clinical features and drug response. A large global retrospective case report study may help us to further explore the treatment and prognostic factors of different PACNS subtypes.
Data Sharing Statement
Data are available upon reasonable request. All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Acknowledgments
We are grateful to the authors and patients of all the articles.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (No. 82271374) and Beijing Natural Science Foundation (Nos. 7212030, 7212029).
Disclosure
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Harbitz F. Unknown forms of arteritis, with special reference to their relation to syphilitic arteritis and periarteritis nodosa. Am J Med Sci. 1922;163(2):250–271. doi:10.1097/00000441-192202000-00010
2. Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine. 1988;67(1):20–39. doi:10.1097/00005792-198801000-00002
3. Scolding NJ, Joseph F, Kirby PA, et al. Abeta-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain. 2005;128(Pt 3):500–515. doi:10.1093/brain/awh379
4. Gandham EJ, Patel B, Mathew V, Raju KP. Primary central nervous system angiitis mimicking a space-occupying lesion. Indian J Neurosurg. 2022. doi:10.1055/s-0042-1743263
5. Miller DV, Salvarani C, Hunder GG, et al. Biopsy findings in primary angiitis of the central nervous system. Am J Surg Pathol. 2009;33(1):35–43. doi:10.1097/PAS.0b013e318181e097
6. Salvarani C, Brown RD, Christianson TJH, Huston J, Giannini C, Hunder GG. Long-term remission, relapses and maintenance therapy in adult primary central nervous system vasculitis: a single-center 35-year experience. Autoimmun Rev. 2020;19(4):102497. doi:10.1016/j.autrev.2020.102497
7. de Boysson H, Parienti JJ, Mawet J, et al. Primary angiitis of the CNS and reversible cerebral vasoconstriction syndrome: a comparative study. Neurology. 2018;91(16):e1468–e1478. doi:10.1212/WNL.0000000000006367
8. Beuker C, Strunk D, Rawal R, et al. Primary angiitis of the CNS: a systematic review and meta-analysis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1093. doi:10.1212/NXI.0000000000001093
9. Campi A, Benndorf G, Martinelli V, Terreni MR, Scotti G. Spinal cord involvement in primary angiitis of the central nervous system: a report of two cases. Am J Neuroradiol. 2001;22(3):577–582.
10. Iwase T, Ojika K, Mitake S, et al. Involvement of CD45RO+T lymphocyte infiltration in a patient with primary angiitis of the central nervous system restricted to small vessels. Eur Neurol. 2001;45(3):184–185. doi:10.1159/000052120
11. Ay H, Sahin G, Saatci I, Soylemezoglu F, Saribas O. Primary angiitis of the central nervous system and silent cortical hemorrhages. Am J Neuroradiol. 2002;23(9):1561–1563.
12. Hassan AS, Trobe JD, McKeever PE, Gebarski SS. Linear magnetic resonance enhancement and optic neuropathy in primary angiitis of the central nervous system. J Neuro Ophthalmol. 2003;23(2):127–131. doi:10.1097/00041327-200306000-00004
13. Schwab P, Lidov HGW, Schwartz RB, Anderson RJ. Cerebral amyloid angiopathy associated with primary angiitis of the central nervous system: report of 2 cases and review of the literature. Arthritis Rheum. 2003;49(3):421–427. doi:10.1002/art.11049
14. Paisansinsup T, Manno EM, Moder KG. Cauda equina syndrome as a clinical presentation of primary angiitis of the central nervous system (PACNS). JCR. 2004;10(5):265–268. doi:10.1097/01.rhu.0000141508.17269.8e
15. Lukas C, Keyvani K, Bornke C. Primary angiitis of the central nervous system presenting with subacute and fatal course of disease: a case report. BMC Neurol. 2005;5(1):4. doi:10.1186/1471-2377-5-16
16. Bhibhatbhan A, Katz NR, Hudon M, Clark AW, Hurlbert RJ, Zochodne DW. Primary angiitis of the spinal cord presenting as a conus mass: long-term remission. Surg Neurol. 2006;66(6):622–626. doi:10.1016/j.surneu.2006.01.023
17. Arias M, Osorio XR, Dapena D, Arias-Rivas S, Vazquez F. Recurrent leukoencephalopathy with microhemorrhages: gradient-echo MRI study diagnostic value in CNS primary angiitis. Mult Scler J. 2008;14(8):1139–1141. doi:10.1177/1352458508094642
18. Duhon B, Renner D, Jensen R. Primary CNS angiitis presenting as short-term memory loss: a case report and literature review. Int J Rheum Dis. 2008;11(3):303–307. doi:10.1111/j.1756-185X.2008.00370.x
19. Nabika S, Kiya K, Satoh H, et al. Primary angiitis of the central nervous system mimicking dissemination from brainstem neoplasm: a case report. Surg Neurol. 2008;70(2):182–185. doi:10.1016/j.surneu.2007.05.008
20. Salvarani C, Brown RD, Calamia KT, et al. Efficacy of tumor necrosis factor a blockade in primary central nervous system vasculitis resistant to immunosuppressive treatment. Arthritis Rheum. 2008;59(2):291–296. doi:10.1002/art.23337
21. Chenevier F, Renoux C, Marignier R, et al. Primary angiitis of the central nervous system: response to mycophenolate mofetil. J Neurol Neurosurg Psychiatry. 2009;80(10):1159–1161. doi:10.1136/jnnp.2008.154567
22. Qu SB, Khan S, Liu H. Primary central nervous system vasculitis mimicking brain tumour: case report and literature review. Rheumatol Int. 2009;30(1):127–134. doi:10.1007/s00296-009-0914-7
23. Kinsella JA, O’Brien W, Mullins GM, Brewer J, Whyte S. Primary angiitis of the central nervous system with diffuse cerebral mass effect and giant cells. J Clin Neurosci. 2010;17(5):674–676. doi:10.1016/j.jocn.2009.09.031
24. Morishige M, Abe T, Kamida T, et al. Cerebral vasculitis associated with amyloid angiopathy-case report. Neurol Med Chir. 2010;50(4):336–338. doi:10.2176/nmc.50.336
25. Rigby H, Easton A, Bhan V. Amyloid beta-related angiitis of the central nervous system: report of 3 cases. Can J Neurol Sci. 2011;38(4):626–630. doi:10.1017/S0317167100012178
26. Tanei T, Nakahara N, Takebayashi S, Ito M, Hashizume Y, Wakabayashi T. Primary angiitis of the central nervous system mimicking tumor-like lesion -case report. Neurol Med Chir. 2011;51(1):56–59. doi:10.2176/nmc.51.56
27. Coronel-Restrepo N, Bonilla-Abadia F, Cortes OA, et al. Primary angiitis of the central nervous system: a report of three cases from a single Colombian center. Case Rep Neurol Med. 2013;2013:4. doi:10.1155/2013/940438
28. Hirano K, Fukae J, Hieda S, et al. Eosinophilic Meningitis Caused by Primary Angiitis of the Central Nervous System. Intern Med. 2013;52(12):1393–1396. doi:10.2169/internalmedicine.52.9578
29. Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Polish J Radiol. 2013;78(3):66–69. doi:10.12659/PJR.884011
30. Rao NM, Prasad PS, Flippen CC, et al. Primary angiitis of the central nervous system presenting as unilateral optic neuritis. J Neuro Ophthalmol. 2014;34(4):380–385. doi:10.1097/WNO.0000000000000147
31. Shiner EA, Zagami AS. An illustrative case of primary angiitis of the central nervous system. SAGE Open Med Case Rep. 2014;2:4. doi:10.1177/2050313X14559638
32. Bajaj BK, Pandey S, Ramanujam B, Wadhwa A. Primary angiitis of central nervous system: the story of a great masquerader. J Neurosci Rural Pract. 2015;6(3):399–+. doi:10.4103/0976-3147.158781
33. Fang CW, Chen YC, Liao IC, Lin CCK. Primary granulomatous angiitis of the central nervous system with amyloid angiopathy a case report and literature review. Neurologist. 2015;19(3):73–78. doi:10.1097/NRL.0000000000000014
34. Killeen T, Jucker D, Went P, et al. Solitary tumour-like mass lesions of the central nervous system: primary angiitis of the CNS and inflammatory pseudotumour. Clin Neurol Neurosurg. 2015;135:34–37. doi:10.1016/j.clineuro.2015.05.008
35. Kim S, Kim DK. Psychosis in primary angiitis of the central nervous system involving bilateral thalami: a case report. Gen Hosp Psychiatry. 2015;37(3):3. doi:10.1016/j.genhosppsych.2015.03.006
36. Kim SI, Kim SH, Cho HJ, et al. Mass-forming primary angiitis of central nervous system with Rosai-Dorfmann disease-like massive histiocytosis with emperipolesis. Pathol Int. 2015;65(8):420–425. doi:10.1111/pin.12317
37. Benson CE, Knezevic A, Lynch SC. Primary central nervous system vasculitis with optic nerve involvement. J Neuro Ophthalmol. 2016;36(2):174–177. doi:10.1097/WNO.0000000000000328
38. Bonstrup M, Ott K, Glatzel M, Magnus T. Frontal lobe dementia syndrome as a first manifestation of primary angiitis of the central nervous system (PACNS). Clin Neurol Neurosurg. 2016;141:92–94. doi:10.1016/j.clineuro.2015.12.016
39. Sun L, Zhu LJ, Zhao T, et al. A rare case of tumor-mimicking primary angiitis of the central nervous system. Mol Clin Oncol. 2016;4(5):827–829. doi:10.3892/mco.2016.784
40. Al Share B, Zakaria A, Hiner E, Iskenderian Z, Warra N. Primary angiitis of the center nervous system: a clinical challenge diagnosed postmortem. Case Rep Neurol Med. 2017;2017:4. doi:10.1155/2017/3870753
41. Fisher A, Rahman H, Farrell M, Hennessy M. Progressive fatal myelopathy secondary to isolated spinal cord vasculitis. Front Neurol. 2017;8:4. doi:10.3389/fneur.2017.00705
42. Matar RK, Alshamsan B, Alsaleh S, et al. New onset refractory status epilepticus due to primary angiitis of the central nervous system. Epilepsy Behav Case Rep. 2017;8:100–104. doi:10.1016/j.ebcr.2017.07.005
43. Zhu DS, Yang XL, Lv HH, et al. Seizure syndrome as a first manifestation of solitary tumor-like mass lesion of PACNS Two case reports. Medicine. 2017;96(9):5.
44. Al-Sharydah AM, Al-Abdulwahhab AH, Al-Suhibani SS, Al-Issawi WM, Al-Safran FS. Primary central nervous system vasculitis disguised as tumor-like granulomatous angiitis and multifocal subdural hematomas: a case report and literature review. Interdiscip Neurosurg. 2018;13:119–123. doi:10.1016/j.inat.2018.05.002
45. He DA, Cai G, Li Y, et al. Primary angiitis of the central nervous system mimicking sporadic Creutzfeldt-Jakob disease: a case study. Alzheimer Dis Assoc Disord. 2018;32(3):258–261. doi:10.1097/WAD.0000000000000242
46. Caputi L, Erbetta A, Marucci G, et al. Biopsy-proven primary angiitis of the central nervous system mimicking leukodystrophy: a case report and review of the literature. J Clin Neurosci. 2019;64:42–44. doi:10.1016/j.jocn.2019.03.021
47. Jin H, Qu Y, Guo ZN, Cui GZ, Zhang FL, Yang Y. Primary Angiitis of the Central Nervous System Mimicking Glioblastoma: a Case Report and Literature Review. Front Neurol. 2019;10:8. doi:10.3389/fneur.2019.01208
48. Kon T, Funamizu Y, Suzuki C, et al. A long interval from a spinal cord lesion to a subsequent brain lesion in primary central nervous system vasculitis. Intern Med. 2019;58(10):1485–1489. doi:10.2169/internalmedicine.1667-18
49. Leonardi J, Saleh C, Jaszczuk P, et al. Primary angiitis of the central nervous system: from psychiatry to neurology. Case Rep Neurol Med. 2019;2019:4. doi:10.1155/2019/8074258
50. Spence S, Ng D, Casault C. Atypical presentation of fulminant primary central nervous system angiitis. J Neuroimmunol. 2019;330:1–4. doi:10.1016/j.jneuroim.2019.01.019
51. Arif S, Arif S, Liaqat J, Muhammad WW, Palwa AR. Need for swift diagnosis of primary angiitis of central nervous system: a case with focal motor seizures of hand progressing to aphasia. Cureus. 2020;12(10):13.
52. Bernstein JE, Podkovik S, Kashyap S, Ghanchi H, Ananda AK. Primary angiitis of the central nervous system presenting as a cerebral mass lesion: a case report and literature review. Cureus. 2020;12(6):8.
53. Borcheni M, Abdelazeem B, Malik B, Gurugubelli S, Kunadi A. Primary central nervous system vasculitis as an unusual cause of intracerebral hemorrhage: a case report. Cureus. 2021;13(3):8.
54. Su X, Han L, Li MX, et al. Novel method using DW-MRI and ADC images to guide stereotactic biopsy for the diagnosis small primary angiitis of the central nervous system: a case report. Eur J Med Res. 2021;26(1):10. doi:10.1186/s40001-021-00529-3
55. Zhang GD, Yang CX, Chang JJ, et al. Primary angiitis of the central nervous system mimicking a cerebellar tumor. Br J Neurosurg. 2021;35(3):367–369. doi:10.1080/02688697.2018.1464122
56. Sarhan FM, Al-Jasim A, Alaraj RS, Abedalkhader SF, Ghanim Z. Right arm weakness and mouth deviation as a presentation of Primary Angiitis of the Central Nervous System treated with rituximab: a case-report. Ann Med Surg. 2022;79:5. doi:10.1016/j.amsu.2022.104040
57. Tanner JA, Richie MB, Cadwell CR, et al. Amyloid-beta related angiitis presenting as eosinophilic meningitis: a case report. BMC Neurol. 2022;22(1):7. doi:10.1186/s12883-022-02638-w
58. Tamargo RJ, Connolly ES, McKhann GM, et al. Clinicopathological review: primary angiitis of the central nervous system in association with cerebral amyloid angiopathy. Neurosurgery. 2003;53(1):136–143. doi:10.1227/01.NEU.0000068864.20655.31
59. Kumar RS, Singh A, Rathore C, Kesavadas C. Primary angiitis of central nervous system: tumor-like lesion. Neurol India. 2010;58(1):147–149. doi:10.4103/0028-3886.60417
60. Ho MG, Chai WX, Vinters HV, et al. Unilateral hemispheric primary angiitis of the central nervous system. J Neurol. 2011;258(9):1714–1716. doi:10.1007/s00415-011-5993-1
61. De Boysson H, Arquizan C, Guillevin L, Pagnoux C. Rituximab for primary angiitis of the central nervous system: report of 2 patients from the French COVAC cohort and review of the literature. J Rheumatol. 2013;40(12):2102–2104. doi:10.3899/jrheum.130529
62. Lyra TG, Martin MDM, Carvalho RD, et al. Pseudotumoral presentation of primary central nervous system vasculitis. Arq Neuropsiquiatr. 2013;71(5):333–335. doi:10.1590/0004-282X20130032
63. Pizzanelli C, Tavoni A, Pelliccia V, et al. Multiple life-threatening relapses in a woman with primary angiitis of the central nervous system mimicking brain tumour: a case report. Clin Exp Rheumatol. 2014;32(2):S143–S144.
64. Safouris A, Stricker J, Michotte A, Voumvourakis K, Gazagnes MD, Tsivgoulis G. Biopsy-proven fulminant primary angiitis of the central nervous system with normal arteriography: a challenging diagnosis of recurrent ischemic strokes. Neurol Sci. 2014;35(1):135–137. doi:10.1007/s10072-013-1528-0
65. Salvarani C, Brown RD, Morris JM, Huston J, Hunder GG. Unilateral chronic relapsing primary central nervous system vasculitis. Clin Exp Rheumatol. 2014;32(2):S139–S140.
66. Moussaddy A, Levy A, Strbian D, Sundararajan S, Berthelet F, Lanthier S. Inflammatory cerebral amyloid angiopathy, amyloid-beta-related angiitis, and primary angiitis of the central nervous system similarities and differences. Stroke. 2015;46(9):E210–E213. doi:10.1161/STROKEAHA.115.010024
67. Burghaus L, Kabbasch C, Deckert M, et al. FET PET in primary central nervous system vasculitis. Clin Nucl Med. 2018;43(9):E322–E323. doi:10.1097/RLU.0000000000002197
68. Chu L, Eustace M, Pittman N. Primary angiitis of the central nervous system presenting with headache and ataxia. Can J Neurol Sci. 2018;45(5):583–584. doi:10.1017/cjn.2018.264
69. Van Rooij JLM, Rutgers DR, Spliet WGM, Frijns CJM. Vessel wall enhancement on MRI in the diagnosis of primary central nervous system vasculitis. Int J Stroke. 2018;13(9):NP24–NP27. doi:10.1177/1747493018789276
70. Wang CR, Peng SL, Huang HW. Isolated cervical cord involvement in primary central nervous system vasculitis. J Rheumatol. 2018;45(7):962–963. doi:10.3899/jrheum.171212
71. Moseley BD, Smith JH, Dhamija RR, Jones LK. Primary angiitis of the central nervous system presenting with microhemorrhages on gradient echo imaging. Neurol India. 2019;67(5):1374–1375. doi:10.4103/0028-3886.271267
72. Agarwal A, Reddy PS, Vishnu VY, Garg A. Primary central nervous system vasculitis presenting with isolated headache. J Neurosci Rural Pract. 2020;11(04):678–679. doi:10.1055/s-0040-1715999
73. Messmer B, Butts M. Relapsing primary central nervous system vasculitis treated with rituximab. J Clin Rheumatol. 2020;26(6):E206–E207. doi:10.1097/RHU.0000000000001075
74. Muccio CF, Tedeschi E, Elefante A, Caranci F, Cerase A. Primary central nervous system vasculitis mimicking a brain tumor on conventional magnetic resonance imaging: the usefulness of perfusion-weighted imaging. A case report. Acta Neurol Belg. 2020;120(2):475–477. doi:10.1007/s13760-018-0907-y
75. Paramasivan NK, Sundaram S, Sharma DP, Sreedharan SE, Sylaja PN. Rituximab for refractory primary angiitis of the central nervous system: experience in two patients. Mult Scler Relat Disord. 2021;51:3. doi:10.1016/j.msard.2021.102907
76. Santyr B, Pejhan S, Zhang Q, Budhram A. Unilateral primary angiitis of the central nervous system. Ann Neurol. 2021;90(6):999–1000. doi:10.1002/ana.26234
77. Han X, Pang Z, Wang Z, Xu S, Lin Y. A case of primary angiitis of the central nervous system presenting with diffuse cerebral microbleeds and recurrent intracranial hemorrhage. Neurol Sci. 2019;40(2):417–419. doi:10.1007/s10072-018-3595-8
78. Salvarani C, Hunder GG, Morris JM, Brown RD, Christianson T, Giannini C. Aβ-related angiitis: comparison with CAA without inflammation and primary CNS vasculitis. Neurology. 2013;81(18):1596–1603. doi:10.1212/WNL.0b013e3182a9f545
79. Das S, Goswami RP, Sinha D, Shobhana A, Purkayastha S, Datta A. Mycophenolate mofetil as induction and maintenance immunosuppressive therapy in adult primary central nervous system vasculitis: a prospective observational study. Clin Rheumatol. 2023;42(8):2155–2162. doi:10.1007/s10067-023-06602-y
80. de Boysson H, Boulouis G, Aouba A, et al. Adult primary angiitis of the central nervous system: isolated small-vessel vasculitis represents distinct disease pattern. Rheumatology. 2017;56(3):439–444. doi:10.1093/rheumatology/kew434
81. de Boysson H, Parienti JJ, Arquizan C, et al. Maintenance therapy is associated with better long-term outcomes in adult patients with primary angiitis of the central nervous system. Rheumatology. 2017;56(10):1684–1693. doi:10.1093/rheumatology/kex047
82. de Boysson H, Arquizan C, Touze E, et al. Treatment and long-term outcomes of primary central nervous system vasculitis: updated results from the French Registry. Stroke. 2018;49(8):1946–1952. doi:10.1161/STROKEAHA.118.021878
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


