Back to Journals » Cancer Management and Research » Volume 12
Prevention of Procedural Hypertension in the Irreversible Electroporation Ablation of Liver and Pancreatic Tumors Based on Distance from the Adrenal Gland
Received 18 October 2019
Accepted for publication 12 December 2019
Published 7 January 2020 Volume 2020:12 Pages 71—78
DOI https://doi.org/10.2147/CMAR.S235227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Gang Fang, Lizhi Niu, Jibing Chen
Fuda Cancer Hospital of Jinan University, Guangzhou 510665, People’s Republic of China
Correspondence: Jibing
Chen
Fuda Cancer Hospital of Jinan University, No. 2 Of Tangdexi Road, Tianhe District, Guangzhou 510665, People’s Republic of China
Email
[email protected]
Background and objective: When irreversible electroporation (IRE) ablation of abdominal tumors, procedural hypertension often
occurs, which often affects the progress of the ablation. Until now, there is no reasonable explanation for this phenomenon. The objective of this research was to explore the
cause and solution of procedural hypertension in percutaneous IRE.
Methods: In this study, the treatment data of 4 consecutive groups of patients were used
to confirm the cause of intraoperative hypertension and then verify the solution. A total of 155 patients with procedural hypertension were screened based on their medical
records of pancreatic or hepatic IRE treatment. Procedural hypertension was monitored in 21 new patients, the correlation between serum catecholamines and hypertension was
recorded and evaluated using regression analysis. Forty new patients were divided into two groups (distance from needle tip to adrenal gland, < 2 cm vs ≥ 2 cm), and the
blood pressure was recorded and compared with two-way ANOVA. Eleven patients with ablative distance < 2 cm were treated in advance with
phentolamine to observe for the occurrence of procedural hypertension.
Results: Of the 21 re-enrolled patients with ablation of the pancreas and liver tumors, 9 developed intraoperative hypertension with
significantly elevated serum catecholamines levels, epinephrine, norepinephrine and dopamine are all positively associated with hypertension, with P values were 0.0003, 0.0253,
and 0.0015, respectively. For the two groups with different needle-insertion distances, hypertension in the < 2 cm group was more significant than that in the other group
(for procedural hypertension, P< 0.01; for heart rate, P< 0.05), which was considered as a high-risk group. The occurrence of intraoperative hypertension could be
completely prevented by using phentolamine prior to treatment.
Conclusion: Hypertension occurs frequently during hepatic and pancreatic IRE because of the
damage of adrenal gland. The safe distance of ablation probe for the adrenal gland was 2 cm. For high-risk patients, early drug prevention works well.
Keywords: irreversible electroporation, abdominal tumor, procedural hypertension, adrenal gland, catecholamine, risky distance
Introduction
In the treatment of abdominal tumors, various ablation methods have been reported to cause intraoperative hypertension, such as radiofrequency ablation,1 cryoablation2 and irreversible electroporation (IRE).3 Hypertension caused by thermal or cryoablation was usually caused by direct ablation of adrenal lesions.4 As the adrenal gland appeared normal at the end of the IRE procedure and on follow-up computed tomography (CT) scans, the patient’s biochemistry profile showed no abnormality in adrenal function, and it is therefore generally overlooked as the cause of intraoperative hypertension.5
The use of IRE has developed over the past decade, and recent studies have focused on the efficacy of the technique to improve overall survival and disease-free survival.6,7 The use of general anesthesia, neuromuscular blockade, and an electrocardiogram synchronization device is necessary for IRE, because the current ablation device needs to be in diastole to discharge ablation.3 Standard use of the device requires the patient’s heart rate to be between 55 and 105 beats, as there will be discharge if the heart rate is too slow or too fast. Anesthesiologists often face the following difficulties in controlling intraoperative blood pressure and heart rate. First, after general anesthesia, the heart rate usually slows down.8 Second, during electroporation, an elevation of systolic and diastolic blood pressure is observed in most patients, with a median of 44 and 19 mm Hg compared with the baseline pressure; the heart rate is also known to show a moderate increase (median: 10/min).9 Third, procedural severe hypertension often occurs during the operation for reasons unknown—sometimes accompanied by arrhythmia or cardiovascular and cerebrovascular accidents.3 If antihypertensive drugs are used prophylactically, many patients will have bradycardia and cause machine shutdown; however, if not used in advance, patients with hypertension will be prone to a high risk of cardiovascular and cerebrovascular accidents. To confirm that injury to the adrenal gland is the cause of the severe intraprocedural hypertension, data of 366 patients who received IRE treatment in our hospital were reviewed from July 1, 2015, to June 30, 2018, determining the causes of and solutions for intraoperative hypertension.
Materials and Methods
Study Design
This prospective study of patients who received IRE for treatment of pancreatic or hepatic tumors was approved by the review board of Fuda cancer hospital (Jinan University) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patients provided written informed consent before their inclusion in our trials which have been registered in Clinical Trials.gov (NCT02352935 and NCT02343835). This study was carried out for four steps from July 2018 to May 2019 (see Figure 1 for details).
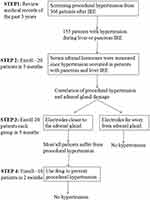 |
Figure 1 Study design for the causes and solutions of procedural hypertension. |
Inclusion and Exclusion Criteria
Patients who met the following criteria were included in this study: age, ≥20 years; Eastern Cooperative Oncology Group performance status, 0 or 1; unresectable pancreatic tumors; unresectable hepatic tumors being adjacent to the diaphragm, gallbladder, or hilar region; and blood pressure, <160/90 mmHg. Patients in whom the platelet count was <80×109/L, prothrombin time was >3 or prothrombin time international normalized ratio (INR) was >1.5, or those with primary or metastatic adrenal tumors were excluded.
IRE Procedure
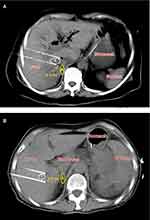
All patients underwent neuromuscular blockade, general anesthetic induction, and percutaneous IRE (NanoKnife system, AngioDynamics, Queensbury, NY, USA). Before treatment, the target heart rate was 70 beats per minute and blood pressure was approximately 105/60 mmHg. For patients with unsatisfactory heart rate and blood pressure, medication is needed to adjust. Computed tomography (CT) and ultrasound were used to guide the insertion of two electrodes, and IRE was synchronized to deliver electrical pulses in coordination with the cardiac rhythm. The distance between the electrodes was 1.5–2.0 cm. Energy was applied at 1200–1500 V/cm for 70–90 ms/pulse, for a total of 7–9 pulses. One or more pullbacks were performed if the target region measured >2 cm in diameter. Because pancreatic and liver tumors are usually deeply situated and obstructed by the ribs, the ablation is often conducted vertically through the intercostal space. In processes of ablation, the kidneys often make the percutaneous window very limited, and the ablation electrodes are usually very close to the adrenal gland. In STEP 3, treatments were divided into two groups: electrode tip close (< 2 cm, group I) or away (≥ 2 cm, group II) from the adrenal gland. Forty patients were enrolled consecutively over a five-month period, with 20 patients in each group. To ensure the objectivity of the enrollment, operators performed needle insertion according to the actual needs of complete tumor destroy, and the anesthesiologists and radiologists determined the enrollment according to the needle insertion location (Figure 2).
After ablation, the patient was transferred to the intensive care unit for overnight observation, and then transferred to the general ward if no acute complications resulted. Two interventionalists, two anesthetists, and two radiologists, all with 4–8 years of experience in image-guided tumor ablation performed all the procedures. The shortest distance between the electrode and the adrenal gland was measured under CT guidance by the radiologists.
Blood Sampling and Symptomatic Treatment According to Procedural Hypertension
Invasive blood pressure monitoring was performed before and after IRE was initiated. In STEP 2, blood sampling was done at the time when the radial artery systolic blood pressure rose to more than 170 mmHg during IRE; if hypertension did not present intraoperatively, blood was sampled at the end of treatment. Each time, 4 mL of blood was drawn, and at least 1.5 mL of plasma was separated by centrifugation. Epinephrine, norepinephrine, and dopamine were detected by radioimmunoassay, and the reference range of these three hormones were 0–100, 0–600, and 0–100 pg/mL, respectively.
Both a β-receptor blocker (esmolol hydrochloride injection, Qilu Pharmaceutical Co. Ltd., Jinan, China) and an α-receptor blocker (phentolamine mesilate injection, Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China) were prepared in the syringes before ablation. When radial artery systolic blood pressure rose to 170 mmHg, the treatment will be suspended, waiting for the blood pressure to recover on its own, and then reinserted the needle at a different angle. If the blood pressure continued to rise, phentolamine 2 mg will be injected. Meanwhile, if the heart rate rose to 100, esmolol 20–50 mg will be used to control it.
Statistical Analysis
In STEP 2, the correlation between serum catecholamine levels and procedural systolic blood pressure were evaluated using regression analysis. In STEP 3, patients were divided into two groups according to the shortest distance between the electrodes and adrenal gland, and two-way ANOVA was used for comparison of blood pressure between groups. Paired t-test was used for other inter- and intragroup comparisons. Statistical difference was indicated by P < 0.05; significant differences were indicated by P < 0.01 or P < 0.001. All analyses were conducted using GraphPad software (GraphPad, San Diego, CA, USA).
Results
Patients
In STEP 1, the medical records of 366 patients were reviewed and 155 patients (102 had pancreatic tumors and 53 had hepatic tumors) were found to have experienced intraoperative hypertension (> 170 mmHg), which needed to handle promptly during treatment. Some moderate clinical sequelae have been found after IRE ablation, such as headache (87 patients), nausea (85 patients), dizziness (93 patients), tinnitus (73 patients), insomnia (36 patients) and limb numbness (27 patients). According to the actual patient number that admitted to our hospital, enrollment time section of prospective trial and the inclusion and exclusion criteria, a total of 72 consecutive new patients with pancreatic or hepatic tumors were enrolled: 21 in STEP 2 (July 1, 2018–September 30, 2018), 40 in STEP 3 (October 1, 2018–Feb 28, 2019), and 11 in STEP 4 (March 1, 2019–May 15, 2019). Patient enrollment was carried out in strict accordance with the inclusion and exclusion criteria. All 72 patients did not take anti-tumor drugs before IRE ablation, nor received prior adrenal interventions.
Change in Blood Pressure and Catecholamine Levels (STEP 2)
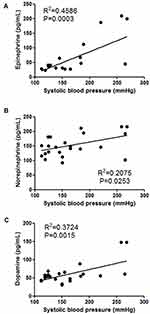
To test the association between procedural hypertension and adrenal injury, 21 patients were enrolled consecutively over a three-month period. There were 13 patients with pancreatic tumors and 8 patients with liver tumors, and 3 hypertensive patients returned to normal after strict drug control before ablation. Hypertension occurred intraoperatively in 9 patients – 5 of whom had pancreatic tumors and four, hepatic tumors. All 9 patients received symptomatic treatment during the operation, and the number of headache, nausea and dizziness after ablation was 5, 5, and 4, respectively. Serum catecholamines were quantified in all enrolled patients, which showed a positive correlation between the systolic blood pressure and serum catecholamine levels; notably, there was a significant correlation with epinephrine (Figure 3).
Differences in Blood Pressure and Ablation Distance (STEP 3)
In combination with the ablation range of IRE electric field, treatments were divided into two groups: electrode tip close (< 2 cm, group I) or away (≥ 2 cm, group II) from the adrenal gland. There were 31 patients with pancreatic tumors and 9 patients with liver tumors, and 6 hypertensive patients returned to normal after strict drug control before ablation. In group I, all patients suffered from procedural systolic hypertension, which was significantly higher than that before IRE (P< 0.001); at the same time, diastolic blood pressure increased significantly (P< 0.001) and the heart rate increased (P< 0.05). All 20 patients received symptomatic treatment during the operation, and the number of headache, nausea and dizziness after ablation was 12, 13, and 9, respectively. In group II, none of the patients had intraoperative hypertension, which was only slightly elevated at the end of treatment (P< 0.05 for both systolic and diastolic blood pressure). Intergroup comparison showed that patients in group I had higher blood pressure (P< 0.01) and heart rate (P< 0.05) than those in group II (Table 1).
 |
Table 1 Patient and Tumor Characteristics in STEP 3 |
Early Medication for High-Risk Patients (STEP 4)
“High risk” patients have tumors requiring probe position < 2 cm from the adrenal gland. To solve the problem of intraoperative hypertension, 11 new high-risk patients were enrolled within 2.5 months (STEP 4). There were 8 patients with pancreatic tumors and 3 patients with liver tumors, and 2 hypertensive patients returned to normal after strict drug control before ablation. After the interventionalist inserted the needle, the radiologist started to identify the distance between the electrode tip and adrenal gland; once a patient was confirmed as being high risk, an anesthesiologist injected 2 mg of phentolamine into the central vein before starting the ablation. None of the 11 high-risk patients developed elevated blood pressure, and only three patients had increased heart rate during the operation (~100 beats per minute); all were successfully treated with esmolol 20–50 mg. After ablation, all patients had no obvious symptoms of headache, nausea and dizziness.
Discussion
Our results have shown that the incidence of procedural hypertension was significantly higher when performing IRE near the adrenal gland, with the electrode tip at a distance of 2 cm from the adrenal gland qualifying as high risk. The procedural hypertension could be prevented by medication before ablation. Hypertension during IRE has been reported several times during the ablation of hepatic10 and pancreatic11,12 tumors, but only symptomatic treatment is recommended, and the cause of hypertension has not been explored. This phenomenon is consistent with the results of our screening on 366 patients, indicating that these two ablation sites are crucial for determining the cause of procedural hypertension. Based on the anatomical structure of the abdomen, tumors at the base of the pancreas and liver are close to the adrenal gland, which can produce transient severe hypertension once unintentionally electroporated. Different from direct ablation of adrenal tumors, this article is the first study of adrenal-related side effects when IRE ablation of other tumor types.
To verify whether IRE injury can cause massive release of catecholamines, a blood sampling test was performed as the intraoperative hypertension developed. Intraoperative hypertension was found to be associated with all three catecholamine levels, especially epinephrine (Figure 3). Similar results have been reported in radiofrequency ablation13 and cryoablation14 of adrenal tumors. Among the numerous patients with elevated blood pressure during IRE in our hospital, severe adrenal injury or Cushing’s syndrome was rarely reported postoperatively, which may be attributed to different principles of ablation equipment.
The purpose of the STEP 3 test was to quantify the intraoperative hypertension phenomenon and make it predictable. According to the ablation principle of IRE, the necrotic area formed by irreversible electroporation was 1 cm around the probe tip, and the apoptotic area formed by reversible electroporation was 1–2 cm.15 As even apoptotic areas can cause tissue damage, 2 cm was set from the electrode tip as the preferred quantitative indicator. As expected, all patients with tumors within the “risky distance” experienced intraoperative hypertension, while those with tumors outside the “risky distance” did not (Table 1). Therefore, the “2 cm rule” might be used to predict the occurrence of hypertension (Figure 2). Because interventionalists mainly consider surgical complications and adjust the puncture approach and angle to avoid blood vessels and important tissues to complete ablation of tumors, only the radiologists can assess the distance between the needle tip and the adrenal gland at different CT levels and caution the interventionalists.
The benefits of hypertension-associated premedication were obvious during IRE of the “risky distance”: first, treatment can proceed smoothly without frequent interruptions such as waiting for the blood pressure to return to normal or the need to undergo emergency treatment. Second, the risk of cardiovascular and cerebrovascular accidents might be greatly reduced. Third, it circumvents intraoperative hypotension caused by taking unnecessary medication in advance, which leads to the interruption of operation or emergency treatment.
The basis of procedural hypertension was investigated in the process of pancreatic or hepatic IRE ablation and a practical and active solution was found. The “2 cm rule” was thought to help prevent most or all these dangerous events. There are still a few points to note: first, in case of an emergency, blood-pressure lowering drugs still need to be prepared before the start of ablation; second, the success of this approach requires collaboration among interventionalists, anesthesiologists, and radiologists, to ensure sufficient time for position judgment and drug administration before the machine starts discharging. Third, when the operator has a choice of several needle-insertion options, he/she should try to stay clear of the adrenal gland and insert the needle outside the “risky distance.” There may be other factors involved in intraoperative blood pressure elevation, such as damage of sympathetic plexus, which will be further studied.
Data Sharing Statement
The authors intend to share individual deidentified participant data. All data in this article, including the reason and preventive method of IRE intraoperative hypertension, etc. No other study-related documents will be made available. All accessible data were in this paper, which are long-term available.
Acknowledgment
This work was supported by the Science and Technology Program of Tianhe District, Guangzhou, China [grant number: 2013kw050].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yamakado K, Takaki H, Yamada T, et al. Incidence and cause of hypertension during adrenal radiofrequency ablation. Cardiovasc Intervent Radiol. 2012;35(6):1422–1427. doi:10.1007/s00270-012-0348-6
2. Welch BT, Atwell TD, Nichols DA, et al. Percutaneous image-guided adrenal cryoablation: procedural considerations and technical success. Radiology. 2011;258(1):301–307. doi:10.1148/radiol.10100631
3. Kambakamba P, Bonvini JM, Glenck M, et al. Intraoperative adverse events during irreversible electroporation-a call for caution. Am J Surg. 2016;212(4):715–721. doi:10.1016/j.amjsurg.2016.07.001
4. Yamakado K. Image-guided ablation of adrenal lesions. Semin Intervent Radiol. 2014;31(2):149–156. doi:10.1055/s-00000068
5. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interventional Radiol. 2011;22(5):611–621. doi:10.1016/j.jvir.2010.12.014
6. Ansari D, Kristoffersson S, Andersson R, et al. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52(11):1165–1171. doi:10.1080/00365521.2017.1346705
7. Niessen C, Beyer LP, Pregler B, et al. Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interventional Radiol. 2016;27(4):480–486. doi:10.1016/j.jvir.2015.12.025
8. Martin RC, Schwartz E, Adams J, et al. Intra - operative anesthesia management in patients undergoing surgical irreversible electroporation of the pancreas, liver, kidney, and retroperitoneal tumors. Anesthesiol Pain Med. 2015;5(3):e22786. doi:10.5812/aapm.22786
9. Nielsen K, Scheffer HJ, Vieveen JM, et al. Anaesthetic management during open and percutaneous irreversible electroporation. Br J Anaesth. 2014;113(6):985–992. doi:10.1093/bja/aeu256
10. Coelen RJS, Vogel JA, Vroomen L, et al. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): a multicentre Phase I/II feasibility study protocol. BMJ Open. 2017;7(9):e015810. doi:10.1136/bmjopen-2016-015810
11. Martin RC
12. Yan L, Chen YL, Su M, et al. A single-institution experience with open irreversible electroporation for locally advanced pancreatic carcinoma. Chin Med J. 2016;129(24):2920–2925. doi:10.4103/0366-6999.195476
13. Lee SH, Seo DW, Oh D, et al. Cushing’s syndrome managed by endoscopic ultrasound-guided radiofrequency ablation of adrenal gland adenoma. Endoscopy. 2017;49(S 01):E1–E2. doi:10.1055/s-0042-118165
14. Atwell TD, Wass CT, Charboneau JW, et al. Malignant hypertension during cryoablation of an adrenal gland tumor. J Vasc Interventional Radiol. 2006;17(3):573–575. doi:10.1097/01.RVI.0000197370.83569.33
15. Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010;4(Suppl 1):S99–S104. doi:10.5009/gnl.2010.4.S1.S99
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.