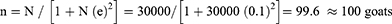Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Prevalence of Gastrointestinal Nematodes, Cestodes, and Protozoans of Goats in Nyagatare District, Rwanda
Authors Tumusiime M , Ndayisenga F, Ntampaka P
Received 24 September 2022
Accepted for publication 5 December 2022
Published 19 December 2022 Volume 2022:13 Pages 339—349
DOI https://doi.org/10.2147/VMRR.S389336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Young Lyoo
Margaret Tumusiime,* Festo Ndayisenga, Pie Ntampaka*
Department of Veterinary Medicine, University of Rwanda, Nyagatare, Eastern Province, Rwanda
*These authors contributed equally to this work
Correspondence: Pie Ntampaka, Email [email protected]
Introduction: Goat farming significantly contributes to the efficient use of land and socioeconomic development in developed and developing countries. During the fiscal year 2017– 2018, goats made up 13.5% of the total live livestock exported by Rwanda. Gastrointestinal parasites (GIPs) can negatively impact goat production, especially in developing countries like Rwanda. This study aimed to determine the impact of the goat’ age and location (administrative cell) on the prevalence of gastrointestinal nematodes, cestodes, and protozoans (GiNCPs) of goats in Nyagatare district, Rwanda.
Methods: In this cross-sectional study, 149 faecal samples were collected from apparently unwell goats and analyzed using the simple flotation technique. Strongyle-type nematodes (STNs) infections were graded using the McMaster method. Pearson chi-square tests of independence were calculated to assess the impact of the goat’ age and location on the prevalence of GiNCPs in the study area.
Results: All the goats (100%) were infected with GiNCPs. The identified types of parasites were STNs (96.0%), Coccidia (83.2%), Moniezia spp (14.8%), Strongyloides papillosus (12.8%), Nematodirus spp (0.7%) and Trichuris ovis (0.7%). Nearly 85.9% (128/149) of the goats were coinfected with 2 to 4 types of parasites and the coinfection of STNs and coccidia preponderated at 58.4%. The location (administrative cell) of the goats correlated with the prevalence of monieziasis (p< 0.05). The goat’s age category was also associated with the prevalence of strongyloidiasis (p< 0.05).
Conclusion: All the goats (100%) were infected with GiNCPs. The location (administrative cell) of the goat also correlated with the prevalence of monieziasis. In addition, the goat’s age category was associated with the prevalence of strongyloidiasis. These findings show that any control program for caprine gastrointestinal parasitoses in the study area should focus on STNs and Coccidia.
Keywords: caprine endoparasites, strongyle-type nematodes, Moniezia spp, Strongyloides papillosus, coccidia, Eastern province, Rwanda
Introduction
Goat farming significantly contributes to the efficient use of land and socioeconomic development both in developed and developing countries.3 Most households in Rwanda keep domestic goats because they are affordable for both small and big landholders and also for their economic, social, and cultural potential role.4 Goats require little funds for investment, attain maturity quickly, grow rapidly, and adapt to some environmental conditions more than cattle.5–7 Because of the significance of agriculture, and mainly livestock in improving the well-being of the underprivileged in rural areas, the government of Rwanda dedicated a development approach in which agriculture occupies a crucial function. During the fiscal year 2018–2019, livestock contributed to 4% of Rwanda’s GDP.8 Goats contribute to the self-reliance of farmers by providing them with food (animal protein and milk), skin, fertilizers from the droppings, urine, and direct cash income.2,7,9,10 Goats can also adapt to the harsh tropical environment and conveniently be reared on unproductive land where cattle farming is not economical.11,12
During the fiscal year 2016/2017, caprine population in Rwanda was estimated at 2.94 million and goats produced 13% of dressed meat in Rwanda. They were the fourth largest livestock that produced dressed meat after cattle, swine, and chicken.13 During the fiscal year 2017–2018, the goats represented 13.5% of total live livestock (n=941,046) exported by Rwanda and they were the third largest exported livestock after cattle and pigs.14
Despite the remarkable step made in increasing animal productivity in Rwanda, a lot of constraints and challenges exist in this sector. The economic benefit remains insignificant because of widespread diseases, lack of proper diets, poor husbandry practices, inappropriate reproductive skills, and lack of sufficient veterinary services. Even though farmers are faced with these challenges, the economic contribution of goats in Rwandan society is still very significant in alleviating poverty and in maintaining soil fertility.4,7,15
GIPs are common constraints on small ruminant farming and they impact the incidence and death rates, cost of treatment, and control measures for helminthiasis resulting in huge losses of livestock products.16–20 GIPs infections can result in loss of weight and poor growth in young animals, diarrhea, anemia, and protein loss.21,22 These parasites negatively impact the output and quality of milk and can also result in stunting, death as well as in condemnation of organs at slaughter.12,23,24 Goats are susceptible to different gastrointestinal helminths (nematodes, trematodes, and cestodes) and protozoa.25 STNs (Haemonchus contortus, Cooperia curticei, Trichostrongylus spp, Oesophagostomum columbianum, Teladorsagia circumcincta, Bunostomum trigonocephalum, Nematodirus spp) cause major caprine helminthiases.26 Other important caprine nematodes include Strongyloides papillosus and Trichuris ovis. Common cestodes and trematodes of goats include Moniezia spp, Fasciola spp, Paramphistomum spp, and Dicrocoelium spp. Eimeria spp are the major coccidia of goats. Goats infected with GIPs manifest various clinical signs depending on the causative parasite including anorexia, diarrhea, loss of weight, body weakness, rough hair coat, cough, oedema, and anaemia.22,27
These effects can predispose goats to secondary infections which result in overspending on veterinary care and increased risk for anthelmintic resistance due to treatment without proper diagnosis.24 Some small ruminant parasites are also zoonotic, and they are transmitted to humans. The latter include trematodes (Fasciola spp), nematodes (Trichostrongylus spp, H. contortus, Mashallagia marshalli, Nematodirus abnormalis, Ostertagia ostertagi, and T. circumcincta) and protozoa (Entamoeba spp, Balantidium spp, and Giardia spp).28–32
In Rwanda, there is little information documented about caprine GIPs. A study that investigated caprine and ovine H. contortus infections in Nyagatare District revealed a prevalence of 75.7%.2 Studies conducted elsewhere reported a prevalence of caprine GIPs infections that vary between 18% and 100%.33 This study, therefore, ascertained the impact of the goat’s age and location (administrative cell) on the prevalence of gastrointestinal nematodes, cestodes, and protozoans of goats in the study area. The findings can guide local animal health service providers to design the control and prevention program for caprine gastrointestinal parasitoses in the study district and Rwanda at large.
Materials and Methods
Study Area
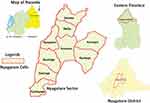
This study was carried out in Nyagatare administrative district (01°18′S 30°20′E) between February and March 2022. Administratively, Rwanda has five provinces and Nyagatare District is located in the Eastern province. Nyagatare is one of the 30 administrative districts of Rwanda and it is made up of 14 administrative sectors which are divided into 106 cells.14 Nyagatare District is bounded northerly by Uganda, easterly by Tanzania, southerly by Gatsibo District, and westerly by Gicumbi District.
Rwanda has a tropical climate and it is mainly divided into four climatic zones including the eastern plains, where Nyagatare District is located.34 In Rwanda, the mean temperature is around 20°C while the annual average temperature across the eastern plains varies between 20°C and 21°C while the annual rainfall is <900 mm.
Rwanda has two rain and dry seasons: the longest rain season occurs between March and May while the shortest one runs from October to December. The short dry season runs from January to February while the longest one occurs between June and September.35 The vegetation in Nyagatare District is mostly an afforested savanna and some forestry galleries.
In the first instance, Nyagatare District was selected on purpose: in an agricultural household survey conducted in Rwanda in 2017, the number of households owning livestock nationwide was estimated at 1.7 million. Goats were recorded as the second most kept livestock after cattle with 53.6% and goat-rearing was highest in the Eastern province with 70.2%.36 In addition, Nyagatare has the highest livestock population in Rwanda. For example, in 2013, Nyagatare District was home to 181,637 goats which was 6% (n= 2,970,780) of the national caprine population at the time.37 Lastly, Nyagatare sector was chosen because it has the highest caprine population in Nyagatare District. Six of nine administrative cells of Nyagatare sector were recruited in this study namely Barija, Bushoga, Nyagatare, Ryabega, Rutaraka, and Nsheke. The rest of the cells were home to a very limited caprine population and were not included in the present study. A map of the Nyagatare sector and the study cells is shown (Figure 1).38
Study Design and Sample Size
This was a cross-sectional study involving the collection of faecal samples from apparently unwell goats (i.e., with poor hair coat or diarrhea or body weakness or poor body condition). The goats were selected from small-scale farmers who reared their goats under a semi-intensive farming system. The goats older than 3 months were considered for this study and those aged 3 to 6 months were categorized as young ones while those aged at least 7 months were categorized as old goats. No formula was used to determine the goats’ age rather the researchers relied on the farmer’s information and their own judgment. The fact that the small-scale farmers preferred having more female goats than male ones resulted in that, the study did not use the goat’s sex as a factor. The animals were reared under an open grazing system on natural pastures and shrubs.
During the night, the goats were kept in a house at a farmer’s home while during the daytime, most of the goats were tethered though they sometimes moved freely. The study subjects were selected randomly from different goat farms in the selected administrative cells (locations). For each study farm, 2 goats were selected randomly that is a young one (3–6 months) and an old goat (aged at least 7 months). Registers of the Nyagatare sector level proved caprine population in the sector was approximated to 30,000 in 2021. With a margin error of 10% and a 90% level of confidence, we computed the minimum sample size using Yamane’s sample size formula for proportions39,40 as follows:
Where N is the population and e is the precision level. Given Nyagatare District is an area dedicated to farming and that the study was conducted when the residents were busy with agricultural activities and specifically the harvest, we had trouble meeting farmers at their homes. The computed sample size was increased by 50% to redress the probable non-response.41,42 This study thus aimed at sampling 150 goats of which 149 were successfully sampled.
Faecal Sample Collection and Parasitological Examination
Goats were restrained in a standing position and fresh faecal samples were collected directly from the rectum using a gloved index finger into a faecal pot. The collected samples were labeled, stored in a cool box, and transported to the parasitology laboratory of University of Rwanda, School of Veterinary Medicine for analysis. Faecal samples were stored in a refrigerator overnight and examined the next day. The faecal samples were processed using the flotation method and the McMaster method was only used to quantify the degree of infection for STNs. The identification of eggs and oocysts was done using the flotation method as described.43 Flotation solution was prepared using sodium chloride (specific gravity of 1.2g). Identification of GIPs was based on morphological characteristics of eggs and oocysts. To detect the degree of infection of caprine STNs, McMaster egg counting parasitological technique was used.43 To count STNs eggs, 4 grams of feces were suspended in 56 mL of the flotation fluid. The mixture was sieved and mixed and both chambers of the McMaster slide were loaded with the flotation fluid and left for 5 minutes before counting STNs eggs under a microscope at 10x magnification. The number of eggs per gram of feces was calculated by multiplying by 50.43
Statistical Analysis
The data generated was entered into Microsoft excel for Windows 2010 and transferred to the statistical package for social sciences (SPSS) version 23 for analysis. In order to calculate the prevalence, the totality of goats infected with GiNCPs was divided by that of study goats and multiplied by one hundred. To get the EPG (Eggs per gram of faeces) for STNs, the sum of counted eggs was multiplied by 50. The intensity of infection (EPG) was classified as mild (50–799), moderate (800–1200), and severe (>1200) as described previously.44 Pearson chi-square tests of independence45 were calculated to assess the impact of study goats’ age and location (administrative cell) on the prevalence of GiNCPs. Any p < 0.05 was considered statistically significant.
Results
Of all 149 goats sampled, 100% suffered from GiNCPs (Table 1). The Effect of the goat’s age category on the prevalence of caprine GI nematode, cestode, and protozoan infections in Nyagatare District is presented in Table 2. The prevalence of gastrointestinal nematode, cestode and protozoan mixed infections in goats in Nyagatare District is presented in Table 3. The intensity of Strongyle-type nematode infections in goats in Nyagatare District is presented in Table 4. The impact of the goat’ age on the intensity of STNs infections in Nyagatare District is presented in Table 5. The intensity of strongyle-type nematode infections in the goats vis-à-vis their locations (administrative cells) is presented in Table 6.
 |
Table 1 Overall Prevalence of Caprine Gastrointestinal Nematode, Cestode, and Protozoan Infections in Nyagatare District (n=149) |
 |
Table 2 Effect of the Goat’s Age Category on the Prevalence of Caprine Gastrointestinal Nematode, Cestode, and Protozoan Infections in Nyagatare District (N= 149) |
 |
Table 3 Prevalence of Gastrointestinal Nematode, Cestode and Protozoan Mixed Infections in Goats in Nyagatare District |
 |
Table 4 Intensity of Strongyle-Type Nematode Infections in Goats in Nyagatare District (N= 143) |
 |
Table 5 Impact of the Goat’s Age on the Intensity of STNs Infections in Nyagatare District (N= 143) |
 |
Table 6 Intensity of Strongyle-Type Nematode Infections in the Goats Vis-À-Vis Their Locations (Administrative Cells) |
Discussion
This study found that the overall prevalence of gastrointestinal nematodes, cestodes and protozoa of goats in Nyagatare District, Rwanda was 100%. The goat’s age category and location had a direct correlation with the prevalence of strongyloidiasis and Moniezia spp infections, respectively. This also shows that all the goats had equal chances of infection given that apart from Moniezia spp, there was no correlation between the prevalence of caprine parasites and locations. These findings can guide local animal health service providers to design a prevention and control program for caprine gastrointestinal parasitoses in the study district and Rwanda at large. This study’s overall prevalence of 100% agrees with that (100%) reported in Thailand and India42,46 but it is higher than 78.7%, 89.33%, 90.4%, and 93.2% reported in Ethiopia, Egypt, Cameroon, and Italy respectively.12,24,47,48 The high frequency of GiNCPs reported in the current study is comparable to previous findings reported in Ethiopia and Egypt.47,49 In addition, adults GIPs can produce a fair number of eggs that are shed in feces and hatched within 1–2 days, and this increases the parasite load in the environment.1
The prevalence of STNs infections (96%) recorded in this study was higher than 92%, 90%, 48.3%, 37.9%, 31%, and 12.88% reported in Slovakia, Kenya, Nigeria, Italy, Zimbabwe, and Egypt respectively.22,24,26,28,47,50 Coccidia was detected at the rate of 83.2% and this was higher than 78.4%, 76.89%, 43.87%, and 43% recorded in Italy, Egypt, Pakistan, and Zimbabwe respectively.24,26,27,47 The high frequency of STNs and Coccidia revealed by this study may be due to the confinement of goats in specific places or tethering on pegs to allow them to graze on the available surrounding pastures. This would increase the possibility of picking the infective larva or oocyst stages.51,52 This type of practice of keeping goats tethered on pegs has contributed significantly to economic losses in ruminant production in sub-Saharan countries.48,53 Some STNs are also zoonotic and thus transmitted from ruminants to humans.29–31 This indicates that some of the nematodes identified in this study might be zoonotic namely Nematodirus spp and other STNs.1 The present study prevalence of monieziasis (14.8%) was lower than 16%, 18.22%, and 19.4% reported in Kenya, Egypt, and Ethiopia respectively.12,28,47 The fact that the occurrence of monieziasis positively correlated with the location of the goats can indicate that some pastures across the study administrative cells (locations) harbored oribatid mites contaminated with Moniezia spp larvae.25,26 This study’s prevalence of Strongyloides papillosus (12.8%) was higher than 8.33% reported in Ethiopia54 but it was lower than 14.05% and 25.58% found in Slovakia and Somalia, respectively.22,55 This study’s prevalence of trichuriasis (0.7%) was lower than 35.45%, 7.84%, 5.33%, and 4.65% recorded in Pakistan, Slovakia, Egypt, and Somalia, respectively.22,27,47,55 The 0.7% prevalence of nematodirosis (Nematodirus spp) reported in this study was lower than 13%, 4.65%, and 3.98% reported in Pakistan, Somalia, and Slovakia respectively.22,27,55 The low prevalence of trichuriasis can be related to difficulty in detecting light infection of this nematode by the fecal flotation technique.25 Similar to other studies conducted on caprine parasitoses in Nigeria, Cameroon, and Iraq,19,47,48,56 85.9% of this study goats developed mixed infections. The latter could be attributed to the exposure of the goats to pastures contaminated by eggs, oocytes, and larvae of different types of parasites.
This study’s higher proportion of goats with mild STNs infections agrees with a study conducted on goats and sheep in Malaysia.25 Similar to a previous study, the prevalence of Moniezia spp infection was influenced by the location of the goats.29 Again, the goat’s age correlated with the prevalence of strongyloidiasis.
The present study had some limitations. The parasites were identified using the flotation method, a technique that can miss some infections. For instance, mild trichuriasis is difficult to detect with the fecal flotation technique.25 Again, with such a technique, we could not speciate the parasites. The sampling was also done once, and it was impossible to assess seasonal effects on the prevalence of GiNCPs in the study goats. In addition, the fact that the study recruited small-scale farmers who preferred keeping female goats than male ones resulted in not collecting the data on study goats’ sex. Further studies on caprine gastrointestinal parasites using other techniques, for instance, molecular tools would be most appropriate. Additionally, future studies should consider ascertaining the effect of season and goats’ sex on the prevalence of gastrointestinal parasites.
Conclusions
All the study goats (100%) were infected with nematodes, cestodes, and protozoa. The identified types of parasites were STNs (96.0%), Coccidia (83.2%), Moniezia spp (14.8%), Strongyloides papillosus (12.8%), Nematodirus spp (0.7%) and Trichuris ovis (0.7%). The location (administrative cell) of the goat also correlated with the prevalence of monieziasis. In addition, the goat’s age category was associated with the prevalence of strongyloidiasis. These findings show that any control program for caprine gastrointestinal parasitoses in the study area should focus on STNs and Coccidia. Although the identified parasites were not speciated due to the limitation of the method used, members of the identified STNs might have public health importance. For example, Haemonchus contortus and Nematodirus abnormalis are zoonotic.1 Indeed, a previous study that investigated ovine and caprine haemonchosis in Nyagatare District found that the prevalence of H. contortus infections was 75.7%.2
Abbreviations
EPG, Eggs per gram of faeces; GIPs, Gastrointestinal parasitoses; STNs, Strongyle-type nematodes; GiNCPs, Gastrointestinal nematodes, cestodes and protozoans.
Ethics Approval and Informed Consent
The study involved live goats rather than humans. It was approved by the academic council of the School of Veterinary Medicine at the University of Rwanda. Before collecting the data, the study was explained to the goat farmers, and only those who consented to admit their goats for faecal sampling were recruited. In addition, the animals were treated with the best practice of veterinary care.
Acknowledgments
The authors want to thank the University of Rwanda, Nyagatare Campus, for using their laboratory facilities. They are also grateful to the management of the Nyagatare sector for allowing them to collect data. Thanks to the goat farmers who allowed the collection of faecal samples from their goats.
Author Contributions
All authors significantly contributed to this work, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; participated in drafting, revising, or critically reviewing it. The authors also have approved the final version before submitting it; agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest regarding the publication of this paper.
References
1. Pestechian N, Kalani H, Faridnia R, Yousefi HA. Zoonotic gastrointestinal nematodes (Trichostrongylidae) from Sheep and Goats in Isfahan, Iran. Acta Scientiae Vet. 2014;42(1243):1–7.
2. Mushonga B, Habumugisha D, Kandiwa E, et al. Prevalence of Haemonchus contortus infections in sheep and goats in Nyagatare District, Rwanda. J Vet Med. 2018;2018:1–9. doi:10.1155/2018/3602081
3. Morand-Fehr P, Boutonnet J, Devendra C, et al. Strategy for goat farming in the 21st century. Small Rumin Res. 2004;51(2):175–183. doi:10.1016/j.smallrumres.2003.08.013
4. Rubangutsangabo J, Mulyungi DP. Rwanda goat production: a key element for economic growth and sustainable development. Int J Manag Commerce Innov. 2018;8(4):893–896.
5. Kenea T, Bekele J, Sheferaw D. Gastro-intestinal nematodes of sheep and goats in three districts of Kaffa and Bench Maji Zones, Southwest Ethiopia. Ethiop Vet J. 2015;19(2):67. doi:10.4314/evj.v19i2.6
6. Manzi M, Mutabazi J, Hirwa CD, Kugonza DR. Socio-economic assessment of indigenous goat production system in rural areas of Bugesera District in Rwanda. Livest Res Rural Dev. 2017;11:1–13).
7. Armson B, Ekiri AB, Alafiatayo R, Cook AJ. Small Ruminant Production in Tanzania, Uganda, and Ethiopia: a Systematic Review of Constraints and Potential Solutions. Vet Sci. 2020;8(1):5. doi:10.3390/vetsci8010005
8. Ministry of Agriculture and Animal Resources. Annual Report 2018-2019 by MINAGRI; 2019:76–77. Available from: https://www.minagri.gov.rw.
9. Mohanty M, Das BC, Nanda SM. Economic impact of goat farming on livelihood of goat farmers of Nabarangpur District of Odisha, India. IntJCurrMicrobiolAppSci. 2020;9(12):176–179. doi:10.20546/ijcmas.2020.901.020
10. Adams F, Ohene-Yankyera K, Aidoo R, Wongnaa CA. Economic benefits of livestock management in Ghana. Agric Econ. 2021;9(1):17. doi:10.1186/s40100-021-00191-7
11. Sutar AU, Kengar SB, Patil SS, Khan MR. Prevalence of gastrointestinal parasites in goats of Ahmednagar District of Maharashtra. Vet World. 2010;3(10):456–457.
12. Emiru B, Amede Y, Tigre W, Feyera T, Deressa B. Epidemiology of gastrointestinal parasites of small ruminants in Gechi District, Southwest Ethiopia. Adv Biol Res. 2013;7(5):167–174.
13. Shapiro BI, Gebru G, Desta S, Nigussie K. Rwanda livestock master plan; 2017.
14. National Institute of Statistics of Rwanda. Rwanda Statistical Yearbook 2018. Republic of Rwanda; 2018.
15. Hydock KL. Traditional methods of Rwandan goat production and management. IK. 2015;1:2. doi:10.18113/P8IK159751
16. Mukasa-Mugerwa E, Lahlou-Kassi A, Anindo D, et al. Between and within breed variation in lamb survival and the risk factors associated with major causes of mortality in indigenous Horro and Menz sheep in Ethiopia. Small Rumin Res. 2000;37(1–2):1–12. doi:10.1016/S0921-4488(99)00152-2
17. Mahusoon MM, Perera ANF, Perera ERK, Perera KA. Effect of Molybdenum Supplementation on Circulating Mineral Levels, Nematode Infection and Body Weight Gain in Goats as Related to Season. Postgraduate Institute of Agriculture, University of Peradeniya: Peradeniya; 2004:128–136.
18. Jittapalapong S, Saengow S, Pinyopanuwat N, Chimnoi W, Khachaeram W, Stich RW. Gastrointestinal Helminthic and. Protozoal infections of goats in Satun. Thailand. 2012;35(2):8.
19. Minnat TR. Detection of gastrointestinal parasite infection of sheep and goats in Diyala Province-Iraq. Al-Qadisiyah J Vet Med Sci. 2014;13(2):118–123.
20. Nabukenya I, Rubaire-Akiiki C, Olila D, Muhangi D, Höglund J. Anthelmintic resistance in gastrointestinal nematodes in goats and evaluation of FAMACHA diagnostic marker in Uganda. Vet Parasitol. 2014;205(3–4):666–675. doi:10.1016/j.vetpar.2014.07.019
21. Githigia SM, Thamsborg SM, Munyua WK, Maingi N. Impact of gastrointestinal helminths on production in goats in Kenya. Small Rumin Res. 2001;42(1):21–29. doi:10.1016/S0921-4488(01)00240-1
22. Babják M, Königová A, Urda-Dolinská M, Várady M. Gastrointestinal helminth infections of dairy goats in Slovakia. Helminthologia. 2017;54(3):211–217. doi:10.1515/helm-2017-0027
23. Stadalienė I, Petkevičius S, Šarkūnas M. The impact of grazing management on seasonal activity of gastrointestinal parasites in goats. Helminthologia. 2014;51(2):103–111. doi:10.2478/s11687-014-0217-8
24. Maurizio A, Stancampiano L, Tessarin C, et al. Survey on endoparasites of dairy goats in North-Eastern Italy using a farm-tailored monitoring approach. Vet Sci. 2021;8(5):69. doi:10.3390/vetsci8050069
25. Paul BT, Jesse FFA, Chung ELT, Che’Amat A, Mohd Lila MA, Factors R. Severity of gastrointestinal parasites in selected small ruminants from Malaysia. Vet Sci. 2020;7(4):208. doi:10.3390/vetsci7040208
26. Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, Dzama K. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin Res. 2016;143:75–83. doi:10.1016/j.smallrumres.2016.09.005
27. Gadahi JA, Arshed MJ, Ali Q, Javaid SB, Shah SI. Prevalence of gastrointestinal parasites of sheep and goat in and around Rawalpindi and Islamabad. Pakistan. 2009;2(2):51–53.
28. Kanyari PWN, Kagira JM, Mhoma RJ. Prevalence and intensity of endoparasites in small ruminants kept by farmers in Kisumu Municipality, Kenya; 2009:12.
29. Squire SA, Yang R, Robertson I, Ayi I, Squire DS, Ryan U. Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitol Res. 2018;117(10):3183–3194. doi:10.1007/s00436-018-6017-1
30. Lattes S. Trichostrongylus colubriformis Nematode Infections in Humans, France. Emerg Infect Dis. 2011;17(7):1301–1302. doi:10.3201/eid1707.101519
31. Ashrafi K, Sharifdini M, Heidari Z, Rahmati B, Kia EB. Zoonotic transmission of Teladorsagia circumcincta and Trichostrongylus species in Guilan province, northern Iran: molecular and morphological characterizations. BMC Infect Dis. 2020;20(1):28. doi:10.1186/s12879-020-4762-0
32. Mpofu TJ, Nephawe KA, Mtileni B. Prevalence and resistance to gastrointestinal parasites in goats: a review. Vet World. 2022. doi:10.14202/vetworld.2022.2442-2452
33. Atanásio-Nhacumbe A, Sitoe C. Prevalence and seasonal variations of eggs of gastrointestinal nematode parasites of goats from smallholder farms in Mozambique. Insights Vet Sci. 2019;3(1):023–029. doi:10.29328/journal.ivs.1001016
34. Rwanda Environment Management Authority. Rwanda Compendium of Environment Statistics. Rwanda Environment Management Authority; 2019.
35. Bonfils S. Trend analysis of the mean annual temperature in Rwanda during the last fifty-two years. J Environ Prot. 2012;2012:14.
36. National Institute of Statistics of Rwanda. Agricultural Household Survey, Republic of Rwanda. National Institute of Statistics of Rwanda; 2018.
37. Nyagatare District. District Potentialities Assessment for the Integrated and Self-Centered Local Economic Development. Nyagatare District; 2013.
38. Ntampaka P, Niragire F, Nkurunziza V, Uwizeyimana G, Shyaka A. Perceptions, attitudes and practices regarding canine zoonotic helminthiases among dog owners in Nyagatare District, Rwanda. Vet Med Sci. 2022;8(4):1378–1389. doi:10.1002/vms3.787
39. Yamane T. Statistics: an introductory analysis-3; 1973.
40. Singh AS, Masuku MB. Sampling techniques & determination of sample size in applied statistics research: an overview. Int J Econ Commer Manag. 2014;2(11):1–22.
41. Al-Subaihi AA. Sample size determination. Influencing factors and calculation strategies for survey research. Neuroscience. 2003;8(2):79–86.
42. Income N, Tongshoob J, Taksinoros S, et al. Helminth Infections in Cattle and Goats in Kanchanaburi, Thailand, with Focus on Strongyle Nematode Infections. Vet Sci. 2021;8(12):324. doi:10.3390/vetsci8120324
43. Hansen J, Perry BD. The epidemiology, diagnosis, and control of helminth parasites of ruminants. A handbook; 1994.
44. Dugassa J, Hussein A, Kebede A, Mohammed C. Multidisciplinary advances in veterinary science prevalence and associated risk factors of gastrointestinal nematodes of sheep and goats in Ziway Dugda District, Eastern Arsi Zone of Oromia Regional State, Ethiopia. Multidiscip Adv.Vet sci;2018;2(1):301–310.
45. McHugh ML. The chi-square test of Independence. Biochem Med. 2013;23(2):143–149. doi:10.11613/BM.2013.018
46. Ashraf A, Tramboo SR, Maqbool I, et al. Occurrence of GI parasites in ruminants of Kashmir and Ladakh. J Parasit Dis. 2022;46(1):196–201. doi:10.1007/s12639-021-01437-3
47. Hassan NMF, Farag TK, Abu El Ezz NMT, Abou-Zeina HAA. Prevalence assessment of gastrointestinal parasitic infections among goats in Giza Governorate, Egypt. Bull Natl Res Cent. 2019;43(1):127. doi:10.1186/s42269-019-0151-5
48. Ntonifor HN, Shei SJ, Ndaleh NW, Mbunkur GN. Epidemiological studies of gastrointestinal parasitic infections in ruminants in Jakiri, Bui Division, North West Region of Cameroon. J Vet Med. 2013;5(12):344–352.
49. Tefera M, Batu G, Prevalence BM. Of gastrointestinal parasites of sheep and goats in and around Bedelle, South-Western Ethiopia. Int J Vet Med. 2009;8(2):7.
50. Goria KP, David OFS, Abraham DG. Diversity of gastrointestinal parasites affecting some domestic animals in plateau state, north central Nigeria. Sci World J. 2018;13(1):82–86.
51. Magona JW, Musisi G. Influence of age, grazing system, season and agroclimatic zone on the prevalence and intensity of gastrointestinal strongylosis in Ugandan goats. Small Rumin Res. 2002;44:187–192. doi:10.1016/S0921-4488(02)00031-7
52. Nsereko G, Emudong P, Mulindwa H, Okwee-Acai J. Prevalence of common gastro-intestinal nematode infections in commercial goat farms in Central Uganda. Uganda J Agric Sci. 2016;16(1):99. doi:10.4314/ujas.v16i1.8
53. Ibrahim N, Tefera M, Bekele M, Alemu S. Prevalence of Gastrointestinal Parasites of Small Ruminants in and Around Jimma Town. Western Ethiopia. 2014;5(1):12–18.
54. Dugasa J, Hussein A, Kebede A, Mohammed C. Prevalence and associated risk factors of gastrointestinal nematodes of sheep and goats in ZiwayDugda District, Eastern Arsi Zone of Oromia Regional State, Ethiopia. Multidiscip Adv Vet Sci. 2018;2(1):301–310. doi:10.20431/2455-2518.0402002
55. Abdi-Soojeede MI. Common Gastro-intestinal parasites of goats (Capra aegagrus hircus) from Mogadishu, Somalia. OJVM. 2018;8(12):232–240. doi:10.4236/ojvm.2018.812020
56. Biu AA, Maimunatu A, Salamatu AF, Agbadu ET. A faecal survey of gastrointestinal parasites of ruminants on the University of Maiduguri Research Farm. Int J Biomed Sci. 2009;5(4):175–179.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.