Back to Journals » Clinical Ophthalmology » Volume 16
Prevalence and Factors Associated with Diabetic Retinopathy among Adult Diabetes Patients in Southeast Ethiopia: A Hospital-Based Cross-Sectional Study
Authors Sahiledengle B , Assefa T , Negash W , Tahir A, Regasa T, Tekalegn Y , Mamo A , Teferu Z , Solomon D , Gezahegn H , Bekele K, Zenbaba D, Tasew A , Desta F , Regassa Z , Feleke Z, Kene C, Tolcha F , Gomora D , Dibaba D , Atlaw D
Received 10 August 2022
Accepted for publication 11 October 2022
Published 20 October 2022 Volume 2022:16 Pages 3527—3545
DOI https://doi.org/10.2147/OPTH.S385806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Biniyam Sahiledengle,1 Tesfaye Assefa,2 Wogene Negash,2 Anwar Tahir,2 Tadele Regasa,3 Yohannes Tekalegn,1 Ayele Mamo,4 Zinash Teferu,1 Damtew Solomon,3 Habtamu Gezahegn,3 Kebebe Bekele,5 Demisu Zenbaba,1 Alelign Tasew,1 Fikreab Desta,1 Zegeye Regassa,2 Zegeye Feleke,2 Chala Kene,6 Fekata Tolcha,7 Degefa Gomora,6 Diriba Dibaba,1 Daniel Atlaw3
1Public Health Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 2Nursing Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 3Biomedical Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 4Pharmacy Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 5Surgery Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 6Midwifery of Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia; 7Pediatrics and Child Health Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia
Correspondence: Biniyam Sahiledengle, Public Health Department, Madda Walabu University, Goba Referral Hospital, Bale Goba, Ethiopia, Email [email protected]
Background: Diabetic retinopathy (DR) is the most prevalent microvascular consequence of diabetes mellitus, and it can result in blindness that is irreversible. Due to delayed diagnosis and limited access to diabetic care, the situation is even worse in developing countries. Scientific evidence on the prevalence of DR and its associated factors among diabetes patients in low-income countries, such as Ethiopia, is limited. This study aimed to determine the prevalence of DR and associated factors among adult diabetes patients in southeast Ethiopia.
Methods: A hospital-based cross-sectional study was conducted among diabetes patients who visited Madda Walabu University Goba Referral Hospital. Fundus and slit-lamp examination were performed for screening of DR. Multivariate binary logistic regression was computed to identify factors associated with DR.
Results: A total of 256 patients (144 men, 56.2%) aged 50.15± 15.71 years were included in the study. The prevalence of any DR was 19.9% (95% CI 15.4%– 25.3%), mild nonproliferative diabetic retinopathy (NPDR) 10.9% (95% CI 7.6%– 15.4%), moderate NPDR 5.9% (95% CI 3.5%– 9.5%), severe NPDR 0.9% (95% CI 0.2%– 3.9%), and proliferative DR 2.3% (95% CI 1.0%– 5.1%). Duration of diabetes ≥ 10 years (AOR 10.22, 95% CI 1.70– 61.44), central obesity (AOR 5.42, 95% CI 1.38– 21.19), overweight/obese (AOR 2.65, 95% CI 1.02– 6.92), lower high-density lipoprotein (HDL) cholesterol (AOR 5.82, 95% CI 1.86– 18.24), moderate triglyceride:HDL cholesterol ratio (AOR 4.13, 95% CI 1.13– 15.15), and urban dwelling (AOR 2.84, 95% CI 1.04– 7.78) were significantly associated with DR.
Conclusion: One in every five DM patients had DR. Sociodemographic, anthropometric, and blood lipids were independently associated with DR. To reduce the burden of diabetes, strategies that focus on lifestyle modifications targeted at identified modifiable risk factors are essential.
Keywords: diabetes mellitus, diabetic retinopathy, Ethiopia
Background
Diabetes mellitus (DM) describes a group of metabolic disorders characterized by high blood sugar levels.1 The global prevalence of DM has increased in recent decades, and this trend is expected to continue.2,3 According to the latest estimates from the International Diabetes Federation (IDF), 463 million people are living with DM worldwide, a figure that is set to reach 700 million by 2045, representing a 51% increase.2 The number of people with DM in the IDF Africa region is projected to have the highest increase of all regions by 2045.2
DM patients are at risk of developing a number of serious life-threatening health problems and microvascular complications, such as diabetic retinopathy (DR).1 This is one of the serious complications of DM.4,5 It is caused by long-term exposure to metabolic changes associated with DM, which cause damage to the retina’s microvasculature.4 The global burden of DR was estimated to be 103.12 million in 2020, and is expected to rise to 160.50 million by 2045, with low- and middle-income nations bearing a disproportionate share of the burden.5 DM is the leading cause of blindness, atraumatic lower-limb amputation, and chronic renal failure. According to the Global Burden of Disease 2019 study, DR was the fifth-leading cause of blindness and vision impairment worldwide.6
DR falls into two main classes: nonproliferative (NPDR) and proliferative (PDR).7 NPDR refers to the absence or presence of abnormal new blood vessels emanating from the retina. PDR is the more advanced form of the disease, where circulation problems deprive the retina of oxygen.4,7 Without treatment, almost 50% of diabetic patients with PDR will become blind within 5 years.8
Sub-Saharan Africa faces a rampant increase in DM prevalence.9 This will inevitably cause an increase in DM-associated complications. The predominant complications are blindness due to DR and diabetic cataracts.8,10 In DM clinic-based surveys, the reported prevalence of DR was 7.0%–62.4%.8 Population-based studies have also identified high DR prevalence of 35.9% in Kenya,11 20.5% in Nigeria,12 and 17.9% in Egypt.13 Despite yearly eye examinations reducing blindness by >95%, compliance with annual eye examinations remains 50% or less in African countries, including Ethiopia.14
Ethiopia is among the top four sub-Saharan Africa countries with the highest diabetic populations and with steadily increasing severe DM-related eye complications, such as DR.15–17 Clinic-based studies have reported prevalence of DR of 13%–42.2%,18–21 with pooled prevalence of 19.48%.15 Numerous risk factors have been associated with DR, including age,18,21 DM duration,18,21 hypertension,18,20,22 poor adherence to medication,22 poor glycemic control,22,23 and obesity.24
Despite the increasing prevalence of DR in Ethiopia, studies on the disease’s prevalence and risk factors remain limited in different parts of the country.15 A majority of those have been in northwest Ethiopia.19–21,25,26 In addition, a considerable number of diabetic patients in Ethiopia have either poor knowledge about DM-related eye complications26 or inadequate glycemic control,27–30 which increases the risk of developing DR. As DM and DR become more prevalent in Ethiopia, it is important that strategies are developed to enable the early detection and adequate management of this emerging epidemic. Additionally, a complete understanding of the scope of DR is required to prevent vision loss and control early-DM eye complications in the study setting. The purpose of the current study was thus to determine the prevalence of DR and associated factors among DM patients in southeast Ethiopia.
Methods
Study Design and Setting
A hospital-based cross-sectional study was conducted from June 1 to July 30, 2021 in the chronic Follow-up and ophthalmology units of Madda Walabu University Goba Referral Hospital in southeast Ethiopia. Goba Referral Hospital is the only referral hospital in Bale and East Bale zones, providing service for an estimated population of 1.5 million. On average, >115,442 patients receive inpatient and outpatient service annually. At the time of data collection, on average 1,422 diabetic patients received follow-up in the hospital. The hospital also serves as the headquarters for the Bale Zone Diabetic Association.
Population and Eligibility
The source population was all diabetic patients age ≥18 years who had been diagnosed with any type of DM and receiving regular follow-up at the chronic follow-up clinic. Patients who were critically ill and consequently unable to give informed consent for participation, had no perception of light in either eye, or had media opacity that obscured the view of their retina were excluded from the study.
Sample-Size Determination and Sampling Procedure
Sample size was determined using a single population–proportion formula considering parameters of prevalence of DR of 29.9%25 and taking into consideration a 5% margin of error, 95% CI, and a possible 10% nonresponse (p=29.9%, n=354). Since the source population was <10,000 we used a correction formula accordingly — 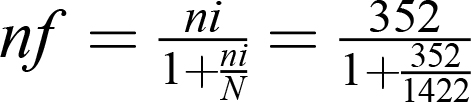 — and the overall sample size was 283. We used consecutive sampling to include study participants following the predefined eligibility criteria. To avoid double-counting of cases, identifiers for interview participants were documented each day, and any patient arriving at the DM clinic on a specific day was cross-checked with the document before the interview.
— and the overall sample size was 283. We used consecutive sampling to include study participants following the predefined eligibility criteria. To avoid double-counting of cases, identifiers for interview participants were documented each day, and any patient arriving at the DM clinic on a specific day was cross-checked with the document before the interview.
Study Variables
Dependent Variable
Prevalence of DR.
Independent Variables
Sociodemographic variables: age, sex, education, residence, family history of DM, member of DM association.
Behavioral, clinical, and DM care–related characteristics: type of DM, duration of DM, duration of DM medication, comorbidities, blood pressure (BP), fasting plasma blood glucose level, type of antidiabetic agents taken, knowledge about DR, glaucoma, regular exercise, and history of eye examinations.
Anthropometric measurement-related variables: central obesity, waist circumference, waist:hip ratio, and body-mass index (BMI).
Lipid profile-related variables: metabolic syndrome, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, TG:HDL cholesterol ratio, and total cholesterol (TC).
Measurements and Tools
An interviewer-administered structured questionnaire and eye-examination form were used to collect information pertaining to sociodemographic, behavioral, clinical, and DM care–related characteristics of respondents. The data-collection tools were developed by reviewing theoretical considerations and related literature. The questionnaire was first prepared in English and translated into Amharic and Afan Oromo, then translated back into English to make sure the data-collection tools were clear, understandable, and consistent.
Four senior data collectors (two optometrists and two nurses) were recruited from the ophthalmology unit. In addition, two senior health professionals were assigned to supervise the overall data-collection process along with the principal investigator. One day’s training was given to all data collectors on procedures. After collection of basic sociodemographic and clinical-related variables, ophthalmological evaluation was performed.
Eye Examination
The Snellen chart, slit-lamp microscopy, a Volk 90D, and direct ophthalmoscopy were used to ascertain visual acuity and DR status of patients. Retinal examination was carried out with a 90 D Volk lens with a slit-lamp biomicroscope by a trained senior optometrist after pupillary dilation had been done using 1% tropicamide eyedrops on both eyes. The anterior segment was assessed using the slit-lamp biomicroscope. Intraocular pressure was measured by Goldman tonometry. Presenting visual acuity was measured using projection charts placed at 6 m from the patient. Depending upon the smallest line that the patient could read, vision was recorded as 6/9, 6/12, 6/18, 6/24, 6/36, and 6/60. The presence of retinopathy was assessed using slit-lamp microscopy, the Volk 90D and direct ophthalmoscopy examination after dilating the pupils. DR was clinically graded according to disease severity. We used the Early Treatment Diabetic Retinopathy Study terminology scale: no apparent retinopathy, mild, moderate, or severe NPDR, and PDR. Cleanliness of hands and sterility of eye-examination instruments was ensured before each eye examination to reduce infection transmission.
Anthropometric Measurements
Weight was measured using electronic digital scale on a firm flat surface after the participant had removed their footwear and any heavy clothes and emptied their pockets. Height was measured with a portable height-measuring board by positioning the board on a firm surface against a wall. After the participant had removed their footwear, they stood with feet together facing data the collector with eyes level with ears. We measured height in centimeters to the nearest millimeter. Waist circumference was measured with a constant-tension tape at the end of a normal expiration with the arms relaxed at the sides at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest (hip bone). We read the measurement at the level of the tape to the nearest millimeter, making sure to keep the measuring tape snug but not tight enough to cause compression of the skin. Hip circumference was measured with the tape with arms relaxed at the sides at the maximum circumference over the buttocks to the nearest millimeter. Classifications followed WHO guidelines. Accordingly, BMI was calculated as weight in kilograms divided by height in meters squared: underweight <18.5, normal weight 18.5–24.9, overweight 25–29.99, and obese ≥30. Central obesity was classified as waist circumference >102 cm for men and >88 cm for women or waist:hip ratio of >0.9 for men and >0.85 for women.31
Blood Pressure
BP was measured from the right brachial artery with a standard mercury sphygmomanometer in the sitting position after 5 minutes of rest. BP measurements were performed to the nearest 2 mmHg. Three consecutive BP measurements were taken. The average of these three measurements was used in the analysis. Hypertension was defined as systolic BP (SBP) >140 mmHg, diastolic BP (DBP) >90 mmHg, or current use of antihypertensive medication.32,33
Blood Sugar Level and Blood Lipid Profiles
Blood samples for lipids and glucose were taken in the morning after fasting for at least 12 hours. Fasting antecubital venous blood was sampled to measure serum glucose and lipid profiles. Serum levels of fasting glucose, triglycerides (TGs), TC, HDL cholesterol, and LDL cholesterol were measured using a Hitachi 7600 automatic biochemical analyzer: raised TGs ≥150 mg/dL and reduced HDL cholesterol <40 mg/dL in men <50 mg/dL in women. A fasting blood sugar level <100 mg/dL is normal and >100 mg/dL is considered high.34
Metabolic Syndrome
Metabolic syndrome was determined according to the criteria of the IDF: central obesity defined as waist circumference ≥94 cm for men and ≥80 cm for women plus any two of the following four factors: raised TGs (≥150 mg/dL), reduced HDL cholesterol (for men <40 mg/dL and for women <50 mg/dL), raised BP (SBP ≥130 mmHg or DBP ≥85 mmHg, or treatment of previously diagnosed hypertension), raised fasting plasma glucose (≥100 mg/dL or previously diagnosed type 2 DM).35
Knowledge about DR
A composite score was constructed using six questionnaire items to compute overall knowledge score. Patients who answered all six questions correctly were considered to have good knowledge, otherwise poor.7,24
Member of DM Association
DM associations work to empower diabetic patients, their families, and the general public by providing current information on DM prevention, care, and treatment. They advocate for members to have access to free health care and anti-DM medications.
Operational Definitions
DR was classified into PDR and NPDR groups. NPDR was divided into mild, moderate, and severe.
No apparent retinopathy: no abnormalities.
Mild NPDR: microaneurysm only (one or more).
Moderate NPDR: more than just microaneurysms, but less than severe NPDR (microaneurysm, dot and blot hemorrhage, cotton-wool spot, venous beading, arteriolar narrowing, intraretinal microvascular abnormalities).
Severe NPDR: >20 intraretinal hemorrhages in four 4 quadrants, definite venous beading in two quadrants, prominent intraretinal microvascular abnormalities in one quadrant, or no signs of PDR.
PDR: one or both of neovascularization and vitreous/preretinal hemorrhage.
Data Processing and Analysis
After data collection, each questioner was checked manually for completeness and data were entered into EpiData Manager version 4.6.0.2 and exported to Stata version 14 for data analysis. Before data analysis, data exploration was carried out on the extent of outliers and missing values, and model fitness was checked. Primarily descriptive statistics (frequency, percentage, and mean) were computed to describe the characteristics of study participants. Both bivariate and multivariate binary logistic regression was performed. Variables in the bivariate logistic regression analysis with p<0.25 were included in the final model to identify factors showing significant associations with DR. Multicollinearity was checked using SE. Variables with a standard error ≥2 were dropped from the multivariate analysis. A receiver-operating characteristic curve was used to illustrate model performance. The Hosmer–Lemeshow goodness-of-fit test was used check model fitness (p=0.984). Finally, AORs with 95% CIs were calculated to indicate the direction and strength of associations.
Results
A total of 256 DM patients were included in our study, with a response rate of 90.4%. Of these, 112 (43.75%) were women. Mean age was 50.15±15.71 years, 16.41% were employed, 34.4% had attended primary education, and 68.7% were urban residents (Table 1). Table 2 indicates the behavioral, clinical, and DM care–related characteristics of respondents. Half (51.2%) of them had some form of comorbidity, and a majority (78.6%) had a history of hypertension. Half (50%) used insulin, while 43.3% took oral hypoglycemic agents for DM treatment. Close to two-thirds (57%) reported that they checked their glucose level monthly.
 |
Table 1 Sociodemographic characteristics of patients (n=256) |
 |
Table 2 Behavioral, clinical, and diabetes care–related characteristics (n=256) |
Blood Pressure and Anthropometric Measurement-Related Variables
Almost two-fifths (42.5%) of respondents’ BMI was normal and 39.1% were overweight/obese. Regarding waist circumference, 56.3% and 82.1% of male and female study participants had ≥94 cm and ≥80 cm, respectively. Overall, 67.6% of DM patients had high waist circumference and were at risk of metabolic complications (Table 3).
 |
Table 3 Blood pressure and anthropometric measurement-related variables (n=256) |
Lipid Profile
Mean fasting blood glucose was 194.08±72.95 mg/dL, and 216 (84.4%) had raised TGs. A total of 81 (31.6%) were at risk with respect to HDL. More than half (54.7%) had metabolic syndrome (Table 4).
 |
Table 4 Lipid profiles (n=256) |
Knowledge about Diabetic Retinopathy
A composite score was constructed using the six items to compute the overall knowledge score of study participants about DR. Overall, 179 (69.9%) of had poor knowledge about DR (Table 5).
 |
Table 5 Knowledge about diabetic retinopathy and history of DM-related eye examination (n=256) |
Presence of Diabetic Retinopathy
In all, 28 (10.94%), 14 (5.47%), three (1.17%), and two (0.78%) of them had mild NPDR, moderate NPDR, severe NPDR, and PDR, respectively (right eye). For the left eye, 23 (8.98%), 16 (6.25%), two (0.78%), and five (1.95%) had mild NPDR, moderate NPDR, severe NPDR, and PDR, respectively. The overall prevalence of DR among DM patients was 19.9% (95% CI 15.4%–25.3%). The prevalence of low vision among DM patients was 11.7% in right, left, or both eyes. The burden of severe visual impairment was 13.3% and 12.1% in the right and left eye, respectively (Table 6).
 |
Table 6 Presences of diabetic retinopathy and other eye diseases (n=256) |
Factors Associated with Diabetic Retinopathy
On bivariate logistic regression analysis, presence of DR had statistically significant associations with duration of DM and duration of DM medication (Table 7). Variables with p<0.25 on bivariate logistic analysis and clinically significant confounders were included in the multivariate logistic regression model. Before we analyzed the multivariate model, we checked for outliers and removed any values with standardized residuals >2.58. Further, we checked for any multicollinearity effect. Figure 1 illustrates the ability of the final model to predict diagnosed DR.
 |
Table 7 Bivariate binary logistic regression analysis of diabetic retinopathy and exposure |
 |
Figure 1 Receiver-operating characteristic (ROC) curve illustrating the ability of the final model to predict diagnosed DR. |
In the final model, the odds of developing DR among urban dwellers were 2.84 times those (95% CI 1.04–7.78) those of rural dwellers. The odds of developing DR among those who had longer DM duration (≥10 years) was tenfold that (AOR 10.22, 95% CI 1.70–61.44) of their counterparts. Participants with central obesity had a higher likelihood of developing DR than their counterparts (AOR 5.42, 95% CI 1.38–21.19). The likelihood of developing DR were higher in overweight/obese diabetic patients than those with normal BMI (AOR 2.65, 95% CI 1.02–6.92). The likelihood of developing DR among patients with lower HDL was almost six times (AOR 5.82, 95% CI 1.86–18.24) that of their counterparts. The likelihood of developing DR among patients who had moderate TG:HDL ratio were four times (AOR 4.13, 95% CI 1.13–15.15) that of those with optimal ratio (Table 8).
 |
Table 8 Multivariate binary logistic regression analysis of diabetic retinopathy and exposure |
Discussion
The purpose of thisy was to determine the prevalence of DR and associated factors among DM patients. The overall prevalence of DR among DM patients attending Madda Walabu University Goba Referral Hospital was found to be 19.9%. We found that place of residence, longer DM duration, abdominal obesity, overweight/obesity, and lower HDL were important factors associated with DR.
The prevalence of DR was in line with other studies conducted in Ethiopia, such as in Addis Ababa (18.57%),36 and Debre Markos (18.9%).20 This finding is also comparable with the national pooled estimate of 19.48%15 and a recent study from Egypt (17.9%).13 However, it is higher than that reported in studies conducted in Ethiopia and elsewhere: 13% in Arbaminch General Hospital, south Ethiopia,18 13.7% in Debre Tabor General Hospital, Northwest, Ethiopia,19 and 8.3% in Nepal.37 The discrepancy could be attributable to differences in study settings, methods, and duration of DM. For instance, the method of data collection used in Arbaminch General Hospital was retrospective record review. However, our study used primary data collection. In the case of Debre Tabor General Hospital, a majority of the participants (53.3% vs 25%) had type 1 DM.19 This can also be explained by the fact that type 1 is more common in younger individuals and type 2 more common in older ones, in whom microvascular complication is more common.
Additionally, our finding was lower than other related studies conducted in Ethiopia. For instance, a study conducted in Gondar Comprehensive Specialized Hospital reported a prevalence of DR was 29.9%,25 with 41.4% in Jimma University Hospital, southwest Ethiopia,38 42.2% in Gondar Tertiary Eye Care and Training Center, northwest Ethiopia,21 and 31.4% at Debre Tabor General Hospital, northwest Ethiopia.23 Studies conducted elsewhere have also reported higher prevalence of DR: China 27.9%,39 Cameroon 40.3%,40 Zimbabwe 28.4%,41 southern Iran 56.9%,42 and Khartoum 82.6%.43 Possible reasons for this inconsistency may be variations in population characteristics and design; the Khartoum study was population-based study,43 and the one from China was multihospital-based.39 It may also be due to differences in duration of DM, level of control of DM, age of subjects, differences in health-care facilities, and the quality of care provided to patients. The high prevalence of DR noted in our study was possibly due to longer DM duration, which is associated with DR.
In the current study, the odds of developing DR among urban-dwellers were higher than rural dwellers. This could be attributable to a lifestyle difference between the two populations, with rural residents working longer days with significantly more physical activity in the Ethiopian context. Our data also revealed that some risk factors, such as obesity, were significantly higher in urban dwellers than in rural dwellers (73.9% vs 26.01%, p=0.009). As a result, rural patients were less likely than urban patients to develop DR and other microvascular complications. In contrast, studies conducted in India44 and China45,46 reported that DM patients residing rurally were more prone to have DR than those in urban areas.
The odds of developing DR among patients who had had DM ≥10 years were higher than those of their counterparts. This finding is in line with studies conducted in Ethiopia,18,20,21 Kenya,11 Sudan,43 Tanzania,47 and Zimbabwe.41 Cross-sectional studies conducted in Asia reported similar findings.42,48 Long-term exposure to hyperglycemia is an established risk factor for developing DR, and the duration of DM strongly correlates with the severity of retinal damage.40
We also found out that the odds of developing DR among respondents with abdominal obesity were about five times those of patients with normal central obesity. In line with this finding, a study from China showed that abdominal obesity was associated with risk of DR (OR 1.07, 95% CI 1.03–1.10).49 In accordance with our findings, studies conducted in Ethiopia,19 China,48 Iran,42 and Australia24 reported a consistent association between obesity and risk of DR. In fact, obesity is a known risk factor for DM and may contribute to the pathogenesis of DR.50 Being obese causes increased blood viscosity, oxidative stress, vascular growth factors, leptin, and cytokines, which leads to DR among DM patients.51 In the current study, the likelihood of developing DR was 2.65 times in overweight/obese patients that of those with normal BMI. In line with our finding, a cohort study conducted in Europe indicated that high BMI was associated with the progression of DR,52 and a study from South Korea established that weight reduction was a key strategy in reducing the occurrence of DR.53 In contrast, a recent systematic review and meta-analysis of 27 studies revealed that neither being overweight nor obese was associated with an increased risk of DR.54 A study conducted in Singapore found that those with a high BMI were significantly less likely to have DR.55 The lack of consensus among these studies may be explained by methodological differences and differences in study participants. Further studies are needed.
We found that low HDL cholesterol were associated with DR. This indicated that those with reduced HDL had fourfold the likelihood of developing DR of DM patients who had desirable HDL levels. Nevertheless, there are conflicting reports regarding the association between blood lipid profiles, such as HDL, and the risk of developing retinopathy. For instance, Hove et al,56 Miljanovic et al,57 and Cui et al48 found no significant association between DR and HDL in diabetic populations. According to recent research, patients with HDL cholesterol <41 or >60 have a significantly increased chance of negative effects, exhibiting a “U-shaped” risk pattern.58 The explanation for this is still unclear and merits further investigation.
This study showed that the likelihood of developing DR was higher in patients with a moderate TG:HDL ratio than those with optimal ratio, but we did not found any significant association between DR and other blood lipids, such as TC (hyperlipidemia, TC >239 mg/dL), and very low-density lipoprotein cholesterol, as observed in Zhang et al’s study.59 Although some studies36,60 have indicated that potential risk factors, such as high TGs, were independent risk factors for DR, the results of our study did not reveal any association between TGs and DR. As a result, further studies are needed on the relationship between blood lipids and DR.
Although we did not assess antioxidant status, the role of oxidative stress in the development of DM complications cannot be overstated. Oxidative stress has been identified as a critical contributor to the pathogenesis of DR. Previous research has looked into the role of oxidative stress in the progression of DR. It is also stated that oxidative stress increases as a result of duration of DM, overweight/obesity and central obesity, and lower HDL cholesterol and TG:HDL ratio, and accordingly affects the development of DR.61–63
Limitations
The current study had some limitations. First, there is a possibility of a selection bias because the recruited individuals were visiting the hospital for a routine follow-up. Second, the lack of data on HbA1c data to measure glycemic control may affect the precision of the data. Third, behavioral factors were collected from the current data, which may not be the same as before the development of DR. Fourth, the use of self-report and review of the patient’s medical records for data collection may be subject to recall bias and missing data. Fifth, the study sample was institution-based, limiting the generalizability of the results to the overall Ethiopian population. Sixth, compared with multiview fundus examination, single-view fundus examination may underestimate the prevalence of DR. Seventh, because there are few trained ophthalmologists in the area, we relied on senior and well-trained optometrists to grade the DR, and the results should be considered cautiosly. Lastly, our study design was cross-sectional, and thus we could not take account of temporal relationships between potential risk factors and outcomes.
Conclusion
In our study population, we found that one in every five DM patients had DR. Urban residence, duration of DM (≥10 years), central obesity, overweight/obesity, lower HDL cholesterol and TG:HDL ratio were independently associated with DR. There is a need for coordinated DM eye-assessment services to detect DR in the early stages. To reduce the burden of DM, strategies that focus on lifestyle modifications targeted at the identified modifiable risk factors are required.
Abbreviations
BMI, body-mass index; DM, diabetes mellitus; DR, diabetic retinopathy; FBS, fasting blood sugar; NPDR, nonproliferative DR; PDR, proliferative DR; DBP, diastolic blood pressure; SBP, systolic blood pressure; WHO, World Health Organization.
Data Sharing
Data will be available upon request of the corresponding author.
Informed Consent
The protocol for the present study was reviewed and approved by the Institutional Review Board (IRB) committee of Madda Walabu University (RDD/0098/13, approval April 16, 2021). Our study was also performed in accordance with the Declaration of Helsinki guidelines for biomedical research involving human subjects. Written informed consent was obtained from all study participants. In addition, written permission was sought from the hospital medical director. All abnormal laboratory results and implications were explained to the patients and their providers immediately upon the next clinic visit to assist them in improving diabetic care and adjust therapy. Participants who were diagnosed with retinopathy were referred to the ophthalmology clinic for further investigation, counseling, and treatment. Study participants had the right to refuse to join, ask any question, or withdraw at any time. Privacy and confidentiality were assured.
Acknowledgments
We would like to acknowledge all Madda Walabu University Goba Referral Hospital Ophthalmology Department staffs, the hospital’s clinical director, and Madda Walabu University for the financial support. We also thank the study participants for their willingness to participate in the study and the Bale Zone Diabetic Association president.
Funding
Madda Walabu University funded this research.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
2. International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium; 2019.
3. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi:10.1016/j.diabres.2013.11.002
4. Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42(5):364–376. doi:10.4093/dmj.2018.0182
5. Teo ZL, Tham Y-C, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
6. Global Burden of Disease 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144ee160.
7. American Optometric Association. Evidence-based clinical practice guideline: eye care of the patient with diabetes mellitus. Available from: www.aoa.org/optometrists/tools-and-resources/evidence-based-optometry/evidence-based-clinical-practice-guidlines/cpg-3--eye-care-of-the-patient-with-diabetes-mellitus.
8. Burgess PI, MacCormick IJC, Harding SP, Bastawrous A, Beare NAV, Garner P. Epidemiology of diabetic retinopathy and maculopathy in Africa: a systematic review. Diabet Med. 2013;30(4):399–412. doi:10.1111/j.1464-5491.2012.03756.x
9. Mensah GA. Descriptive epidemiology of cardiovascular risk factors and diabetes in Sub-Saharan Africa. Prog Cardiovasc Dis. 2013;56(3):240–250. doi:10.1016/j.pcad.2013.10.014
10. Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-Saharan Africa: meeting the challenges of an emerging epidemic. BMC Med. 2013;11(1):157. doi:10.1186/1741-7015-11-157
11. Mathenge W, Bastawrous A, Peto T, et al. Prevalence and correlates of diabetic retinopathy in a population-based survey of older people in Nakuru, Kenya. Ophthalmic Epidemiol. 2014;21(3):169–177. doi:10.3109/09286586.2014.903982
12. Kyari F, Tafida A, Sivasubramaniam S, et al. Prevalence and risk factors for diabetes and diabetic retinopathy: results from the Nigeria national blindness and visual impairment survey. BMC Public Health. 2014;14:1299. doi:10.1186/1471-2458-14-1299
13. AlSawahli H, Mpyet CD, Ezzelarab G, et al. Population-based cross-sectional prevalence survey of diabetes and diabetic retinopathy in Sohag-Egypt, 2019. BMJ Open. 2021;11(6):e047757. doi:10.1136/bmjopen-2020-047757
14. Hartnett ME, Baehr W, Le YZ, et al. Diabetic retinopathy, an overview. Vision Res. 2017;139:1–6. doi:10.1016/j.visres.2017.07.006
15. Fite RO, Lake EA, Hanfore LK. Diabetic retinopathy in Ethiopia: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2019;13(3):1885–1891. doi:10.1016/j.dsx.2019.04.016
16. Abebe N, Kebede T, Addise D. Review article diabetes in Ethiopia 2000–2016–prevalence and related acute and chronic complications; a systematic review. African J Diabetes Med. 2017;25(2):7–12.
17. Lebeta KR, Argaw Z, Birhane BW. Prevalence of diabetic complications and its associated factors among diabetes mellitus patients attending diabetes mellitus clinics; institution based cross sectional study. Am J Health Res. 2017;5(2):38. doi:10.11648/j.ajhr.20170502.13
18. Chisha Y, Terefe W, Assefa H, Lakew S. Prevalence and factors associated with diabetic retinopathy among diabetic patients at Arbaminch General Hospital, Ethiopia: cross sectional study. PLoS One. 2017;12(3):e0171987. doi:10.1371/journal.pone.0171987
19. Alemu Mersha G, Tsegaw Woredekal A, Tilahun Tesfaw M. Sight-threatening diabetic retinopathy and associated risk factors among adult diabetes patients at Debre Tabor General Hospital, Northwest Ethiopia. Clin Ophthalmol. 2020;14:4561–4569. doi:10.2147/OPTH.S285606
20. Tilahun M, Gobena T, Dereje D, Welde M, Yideg G. Prevalence of diabetic retinopathy and its associated factors among diabetic patients at Debre Markos Referral Hospital, Northwest Ethiopia, 2019: hospital-based cross-sectional study. Diabetes Metab Syndr Obes Targets Ther. 2020;13:2179–2187. doi:10.2147/DMSO.S260694
21. Ejigu T, Tsegaw A. Prevalence of diabetic retinopathy and risk factors among diabetic patients at university of Gondar tertiary eye care and training center, North-West Ethiopia. Middle East Afr J Ophthalmol. 2021;28(2):71. doi:10.4103/meajo.meajo_24_21
22. Garoma D, Merga H, Hiko D. Determinants of diabetic retinopathy in Southwest Ethiopia: a facility-based case-control study. BMC Public Health. 2020;20(1):503. doi:10.1186/s12889-020-08652-2
23. Mersha GA. Prevalence of diabetic retinopathy and associated risk factors among adult diabetes attending at Debre Tabor General Hospital, Northwest Ethiopia. J Diabetes Metab. 2021;12(872):7.
24. Dirani M, Xie J, Fenwick E, et al. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci. 2011;52(7):4416–4421. doi:10.1167/iovs.11-7208
25. Asemu MT, Ahunie MA. The impact of diabetes on visual acuity in Ethiopia, 2021. PLoS One. 2021;16(8):e0256145. doi:10.1371/journal.pone.0256145
26. Assem AS, Tegegne MM, Alemu DS, Woredekal AT, Tefera TK. Knowledge about diabetic retinopathy, eye check-up practice and associated factors among adult patients with diabetes mellitus attending at debark hospital, Northwest Ethiopia. BMC Ophthalmol. 2020;20(1):453. doi:10.1186/s12886-020-01730-4
27. Gebrie A, Tesfaye B, Sisay M. Evaluation of glycemic control status and its associated factors among diabetes patients on follow-up at referral hospitals of Northwest Ethiopia: a cross-sectional study, 2020. Heliyon. 2020;6(12):e05655. doi:10.1016/j.heliyon.2020.e05655
28. Nigussie S, Birhan N, Amare F, et al. Rate of glycemic control and associated factors among type two diabetes mellitus patients in Ethiopia: a cross sectional study. PLoS One. 2021;16(5):e0251506. doi:10.1371/journal.pone.0251506
29. Feleke BE, Feleke TE, Kassahun MB, et al. Glycemic control of diabetes mellitus patients in referral hospitals of Amhara Region, Ethiopia: a cross-sectional study. Biomed Res Int. 2021;2021:1–6. doi:10.1155/2021/6691819
30. Tekalegn Y, Addissie A, Kebede T, Ayele W. Magnitude of glycemic control and its associated factors among patients with type 2 diabetes at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS One. 2018;13(3):e0193442. doi:10.1371/journal.pone.0193442
31. World Health Organization. Waist circumference and waist-Hip ratio: report of a WHO expert consultation [Internet]; [cited December 2, 2021]. Available from: https://www.who.int/publications-detail-redirect/9789241501491.
32. Stewart A, Marfell-Jones M, Olds T, de Ridder H International standards for anthropometric assessment. International Society for the Advancement of Kinantropometry. Lower Hutt, Churchil Livingstone. 2011; 3.
33. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
34. Pasternak RC. Report of the adult treatment panel III: the 2001 National Cholesterol Education Program guidelines on the detection, evaluation and treatment of elevated cholesterol in adults. Cardiol Clin. 2003;21(3):393–398. doi:10.1016/S0733-8651(03)00080-8
35. Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
36. Azeze TK, Sisay MM, Zeleke EG. Incidence of diabetes retinopathy and determinants of time to diabetes retinopathy among diabetes patients at Tikur Anbessa Hospital, Ethiopia: a retrospective follow up study. BMC Res Notes. 2018;11(1):542. doi:10.1186/s13104-018-3660-7
37. Thapa R, Joshi DM, Rizyal A, Maharjan N, Joshi RD. Prevalence, risk factors and awareness of diabetic retinopathy among admitted diabetic patients at a tertiary level hospital in Kathmandu. Nepal J Ophthalmol. 2014;6(1):24–30. doi:10.3126/nepjoph.v6i1.10760
38. Sharew G, Ilako DR, Kimani K, Gelaw Y. Prevalence of diabetic retinopathy in Jimma University Hospital, Southwest Ethiopia. Ethiop Med J. 2013;51(2):105–113.
39. Zhang G, Chen H, Chen W, Zhang M. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol. 2017;101(12):1591–1595. doi:10.1136/bjophthalmol-2017-310316
40. Jingi AM, Noubiap JJN, Ellong A, Bigna JJR, Mvogo CE. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC Ophthalmol. 2014;14(1):1–5. doi:10.1186/1471-2415-14-19
41. Machingura PI, Macheka B, Mukona M, Mateveke K, Okwanga PN, Gomo E. Prevalence and risk factors associated with retinopathy in diabetic patients at Parirenyatwa Hospital outpatients’ clinic in Harare, Zimbabwe. Arch Med Biomed Res. 2017;3(2):104. doi:10.4314/ambr.v3i2.6
42. Ghaem H, Daneshi N, Riahi S, Dianatinasab M. The prevalence and risk factors for diabetic retinopathy in Shiraz, Southern Iran. Diabetes Metab J. 2018;42(6):538. doi:10.4093/dmj.2018.0047
43. Elwali ES, Almobarak AO, Hassan MA, Mahmooud AA, Awadalla H, Ahmed MH. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: population based study. Int J Ophthalmol. 2017;10(6):948–954. doi:10.18240/ijo.2017.06.18
44. Rani PK, Raman R, Sharma V, et al. Analysis of a comprehensive diabetic retinopathy screening model for rural and urban diabetics in developing countries. Br J Ophthalmol. 2007;91(11):1425–1429. doi:10.1136/bjo.2007.120659
45. Yao X, Pei X, Yang Y, et al. Distribution of diabetic retinopathy in diabetes mellitus patients and its association rules with other eye diseases. Sci Rep. 2021;11(1):16993. doi:10.1038/s41598-021-96438-w
46. Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010803. doi:10.7189/jogh.08.010803
47. Cleland CR, Burton MJ, Hall C, et al. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop Med Int Health. 2016;21(3):417–426. doi:10.1111/tmi.12652
48. Cui J, Ren J-P, Chen D-N, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ Open. 2017;7(8):e015473. doi:10.1136/bmjopen-2016-015473
49. Zhou J-B, Yuan J, Cai Y-H, Shu L-P, Yang J-K. 590-P: Is abdominal obesity associated with diabetic retinopathy in Chinese individuals: an exploratory study. Diabetes. 2019;68(Supplement 1). doi: 10.2337/db19-590-P
50. Vazquez G, Duval S, Jacobs DR, Silventoinen K. Comparison of body mass index, waist circumference, and waist/Hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29(1):115–128. doi:10.1093/epirev/mxm008
51. Dorchy H, Claes C, Verougstraete C. Risk factors of developing proliferative retinopathy in type 1 diabetic patients. Diabetes Care. 2002;25(4):798–799.
52. Hammes HP, Welp R, Kempe HP, et al. Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV database. PLoS One. 2015;10:e0132492. doi:10.1371/journal.pone.0132492
53. Lim S, Kim KM, Kim MJ, et al. The association of maximum body weight on the development of type 2 diabetes and microvascular complications: MAXWEL study. PLoS One. 2013;8(12):e80525. doi:10.1371/journal.pone.0080525
54. Zhou Y, Zhang Y, Shi K, Wang C. Body mass index and risk of diabetic retinopathy: a meta-analysis and systematic review. Medicine. 2017;96(22):e6754. doi:10.1097/MD.0000000000006754
55. Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(9):4458–4463.
56. Hove MN, Kristensen JK, Lauritzen T, Bek T. The prevalence of retinopathy in an unselected population of type 2 diabetes patients from Arhus County, Denmark. Acta Ophthalmol. 2004;82(4):443–448. doi:10.1111/j.1600-0420.2004.00270.x
57. Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53(11):2883–2892. doi:10.2337/diabetes.53.11.2883
58. Could Too Much “Good” HDL Cholesterol Be Bad for You? [Internet]. WebMD; [cited December 5, 2021]. Available from: https://www.webmd.com/cholesterol-management/news/20180827/could-too-much-good-hdl-cholesterol-be-bad-for-you.
59. Zhang HY, Wang JY, Ying GS, et al. Serum lipids and other risk factors for diabetic retinopathy in Chinese type 2 diabetic patients. J Zhejiang Univ Sci B. 2013;14(5):392–399. doi:10.1631/jzus.B1200237
60. Wright AD, Dodson PM. Medical management of diabetic retinopathy: fenofibrate and ACCORD eye studies. Eye. 2011;25(7):843–849. doi:10.1038/eye.2011.62
61. Bokhary K, Aljaser F, Abudawood M, et al. Role of oxidative stress and severity of diabetic retinopathy in type 1 and type 2 diabetes. Ophthalmic Res. 2021;64(4):613–621. doi:10.1159/000514722
62. Sabaner MC, Akdogan M, Doğan M, et al. Inflammatory cytokines, oxidative and antioxidative stress levels in patients with diabetic macular edema and hyperreflective spots. Eur J Ophthalmol. 2021;31(5):2535–2545. doi:10.1177/1120672120962054
63. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi:10.1016/j.redox.2020.101799
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
