Back to Journals » International Journal of Women's Health » Volume 15
Preterm Birth: Screening and Prediction
Authors Creswell L, Rolnik DL , Lindow SW, O'Gorman N
Received 24 August 2023
Accepted for publication 13 December 2023
Published 21 December 2023 Volume 2023:15 Pages 1981—1997
DOI https://doi.org/10.2147/IJWH.S436624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Lyndsay Creswell,1 Daniel Lorber Rolnik,2 Stephen W Lindow,1 Neil O’Gorman1
1Department of Obstetrics and Gynecology, The Coombe Hospital, Dublin, Ireland; 2Department of Obstetrics and Gynecology, Monash University, Melbourne, VIC, Australia
Correspondence: Lyndsay Creswell, Bernard Stuart Perinatal Ultrasound Fellow, Department of Obstetrics and Gynecology, The Coombe Hospital, Cork Street, Dublin, D08 XW7X, Ireland, Email [email protected]
Abstract: Preterm birth (PTB) affects approximately 10% of births globally each year and is the most significant direct cause of neonatal death and of long-term disability worldwide. Early identification of women at high risk of PTB is important, given the availability of evidence-based, effective screening modalities, which facilitate decision-making on preventative strategies, particularly transvaginal sonographic cervical length (CL) measurement. There is growing evidence that combining CL with quantitative fetal fibronectin (qfFN) and maternal risk factors in the extensively peer-reviewed and validated QUanititative Innovation in Predicting Preterm birth (QUiPP) application can aid both the triage of patients who present as emergencies with symptoms of preterm labor and high-risk asymptomatic women attending PTB surveillance clinics. The QUiPP app risk of delivery thus supports shared decision-making with patients on the need for increased outpatient surveillance, in-patient treatment for preterm labor or simply reassurance for those unlikely to deliver preterm. Effective triage of patients at preterm gestations is an obstetric clinical priority as correctly timed administration of antenatal corticosteroids will maximise their neonatal benefits. This review explores the predictive capacity of existing predictive tests for PTB in both singleton and multiple pregnancies, including the QUiPP app v.2. and discusses promising new research areas, which aim to predict PTB through cervical stiffness and elastography measurements, metabolomics, extracellular vesicles and artificial intelligence.
Keywords: preterm labor, screening, cervical length, fibronectin, QUiPP
Introduction
Preterm birth (PTB) is defined as a live birth occurring before 37 completed weeks of pregnancy by the World Health Organization (WHO).1 This can be further classified by the gestational age (GA) at the time of delivery; extremely preterm (<28 weeks), very preterm (28 to 32 weeks), moderately preterm (32 to 34 weeks) and late preterm (34 to 37 weeks).1 PTBs may be ‘spontaneous’, due to preterm labor (PTL; 40–45%) or preterm pre-labor rupture of membranes (PPROM; 30–35%), or be “provider-initiated” in response to an obstetric indication (30–35%).2 Estimates indicate a global PTB rate of approximately 10%1,3 equating to almost 15 million PTBs annually.1 Prematurity is the most significant direct cause of neonatal death worldwide and is responsible for approximately 35% of neonatal mortalities within the first 4 weeks of life.1 The risk of PTB-related complications is inversely proportional to GA, and subsequently extremely preterm infants born <28 weeks are at greatest risk of morbidity and mortality.4 The EPICure studies demonstrated improved survival for infants born between 22 and 25 weeks between 1996 and 2006 (40% in 1995 to 53% in 2006, p<0.001),4 and more recent UK studies have reported continued national improvement in the survival of very preterm infants, particularly between 22+0 to 23+6 weeks (annual percentage change 6.03%, 95% CI 2.47–3.53, p=0.02).5 However, no similar improvements have been noted in long-term neonatal morbidity, including bronchopulmonary dysplasia, major cerebral injury and retinopathy of prematurity.4 Discussions with parents anticipating a PTB must address not only survivability but include complications of prematurity (Table 1) as the continued improvements in survival of extremely preterm infants may lead to a growing number of children and adults with long-term morbidity. Indeed, there is significant evidence that even late preterm infants remain at increased risk of short- and long-term adverse outcomes when compared to their term counterparts including respiratory distress syndrome (RDS),6 hypothermia,7 hypoglycemia,6 jaundice,6,8 infection,8 neurodevelopmental delay9–11 and metabolic syndrome.12
 |
Table 1 Morbidities Resulting from Complications of Premature Birth.13 |
Screening for Preterm Birth
Spontaneous preterm labor (PTL) has been described as a complex “syndrome” reflecting the multiple aetiologies including infection,13 uteroplacental disorders,14,15 cervical disease16 and uterine overdistension, which lead to premature myometrial contractions, decidual activation and cervical ripening.17,18 The evolving clinical presentations, notably PTL or PPROM, depend on the nature and timing of underlying pathological processes, which then activate the common “parturition pathway”.17,19 The heterogeneity of causality in PTB explains the challenge in developing globally effective protocols to prevent, diagnose and treat the clinical problem. Screening asymptomatic high-risk women is, however, strongly recommended,20–22 and the most extensively investigated modalities include assessments of cervical length (CL) and fetal fibronectin (fFN), which will also be considered in tandem given the creation of the novel clinical assessment application QUantitative Innovation in Predicting Preterm Birth (QUiPP™).
Measurement of Cervical Length
Premature cervical ripening is considered as part of the common pathway to PTB, which may precede delivery by weeks or months.19 Effacement of the cervix can be assessed and quantified by measuring the CL, which is considered the most predictive23 and reproducible24 variable detectable by transvaginal ultrasound (TVUS). The shorter the CL, the greater the risk of PTB.25–28 The GA at cervical assessment is also important as the odds of delivery <35 weeks decrease by approximately 6% for each additional millimeter of CL (odds ratio (OR) 0.94, 95% CI 0.92–0.95, p=0.001) and by 5% for each additional week of pregnancy at which the CL is measured (OR 0.95; 95% CI 0.92–0.98, p=0.004).29 As pregnancy advances, the cervix shortens physiologically25,30 and thus a CL of 25mm corresponds to the 0.5th, 3rd, 10th and 20th percentiles at 16, 22, 28 and 33 weeks, respectively.30 Maternal characteristics including parity, weight and height have also been shown to correlate with CL.31 First-trimester assessment of CL is controversial and is not currently a recommended practice.22 Berghella et al found that a CL <25mm rarely occurs prior to 14 weeks, even in high-risk patients destined to deliver prematurely, hypothesizing that cervical changes predictive of PTB develop after 16 weeks.32 However, more recent studies have found associations between PTB and short CLs at 11–13 weeks.33 Multiple international and professional societies currently recommend initiating measurements of CL from the second trimester,20–22 and offering screening based upon factors, which increase PTB risk, including previous PTB,34,35 cervical surgery,36,37 congenital uterine anomalies38,39 and Cesarean sections at full cervical dilatation (FDCS).40,41 Whilst the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends universal screening with TVUS between 18 and 24 weeks in singleton pregnancies,22 the Royal College of Obstetricians and Gynaecologists (RCOG) advocate targeted screening for intermediate and high-risk women (Table 2) and recommend ultrasound-indicated transvaginal cerclage (TVC) if a CL <25mm is identified prior to 24 weeks.21 Whilst a meta-analysis has demonstrated that TVUS best predicts PTB <35 weeks when a CL threshold of 20mm is used (pooled sensitivity 22.1%, specificity 98.2%, area under the receiver-operating characteristics (ROC) curve (AUC) 0.89), increasing the CL cut-off to 25mm improves sensitivity to 33.3%, and maintains a high specificity of 95.9% (AUC 0.85).42 This increases the screen-positive rate from 1.8% to 4.1% but better identifies the women who, without intervention, will have a PTB.42
 |
Table 2 Women Identified as Intermediate or High Risk Should Have TVUS Surveillance to Assess the Need for an Ultrasound-Indicated TVC.21 |
Professional society guidelines recommend the use of TVUS for CL measurement (Figure 1).20–22 Whilst CL measurements may be performed using a transabdominal (TA) approach, the transvaginal route is preferred, as technical challenges are often encountered performing TAUS due to the greater distance between the probe and the cervix and acoustic shadowing from maternal and fetal structures.22 A full bladder is also required for adequate visualization of the cervix, and this appears to artificially elongate the CL.43,44 There is substantial evidence to show safety and patient acceptability of TVUS as a screening modality when effective counselling is provided, with women reporting the scan as significantly less difficult than a cervical smear (p<0.001).45 Indeed, following the introduction of a universal CL screening programme in a Chicago hospital, 99.9% of women offered a TVUS agreed to the examination.46 Within the context of a Preterm Birth Surveillance Clinic (PSC), a UK qualitative study reported that 95% of women found the TVUS examination acceptable, suggesting that vaginal examinations do not deter women from accepting PTB surveillance.47 TVUS measurements of CL should follow a sequential and standardised protocol, with opportunities for revalidation and quality assurance.22 The Fetal Medicine Foundation and CLEAR programme (Cervical Length Education and Review) are high-quality educational courses that offer certification to healthcare professionals who demonstrate competence in the recommended technique for TVUS cervical assessment.48,49
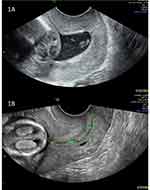 |
Figure 1 Images to show two described methods for measuring cervical length (CL). Image (A) shows the straight-line method in a short cervix. Image (B) shows the two-lines method in a normal cervix, with calipers between the external os and the point of maximum curvature, and from the endpoint of the first line to the internal os.22 Images courtesy of Dr Lyndsay Creswell and Dr Neil O’Gorman. |
Use of Cervical Length Measurement in Patients with Symptoms of Preterm Labor
The diagnosis of PTL is challenging as many women presenting to hospital with symptoms of preterm uterine contractions are not in PTL. However, given the burden of perinatal morbidity and mortality associated with PTB, patients are often admitted to hospital for magnesium sulphate, tocolysis and corticosteroid administration. The current UK National Institute of Clinical Excellence (NICE) guidelines recommend a “treat all” policy in preference to objective assessments of PTB risk at gestations less than 29+6 weeks.50 However, as 15% of attendances at emergency obstetric units are attributable to threatened PTL, there is a need to differentiate between women who will deliver a preterm infant from the majority who will have a term birth.51 This is of increasing importance, as the timing of steroid administration plays a large role in maximising their benefits and should only be reserved for women at high risk of delivering a preterm infant within 1 week of threatened PTL.52 In addition, there is recent observational-level evidence suggesting an association between antenatal corticosteroid use and childhood mental and behavioural disorders.53 Therefore, it is of paramount importance that the most accurate tests available are used in screening for preterm birth, to limit the exposure of antenatal steroids to those who are most likely to need them.
Measurements of CL in symptomatic women can detect a significant proportion of individuals who will deliver within 7 days and subsequently rationalise care. A large meta-analysis reported that for birth less than 7 days from presentation a TVUS CL <20mm had a sensitivity of 75.4% (95% CI 66.6–82.9%) specificity of 79.6% (95% CI 77.1–81.9%), positive likelihood ratio (LR+) of 3.74 (95% CI 2.77–5.05) and negative likelihood ratio (LR-) of 0.33 (95% CI 0.15–0.73).54 Comparatively, a 15mm threshold improves specificity and the likelihood of the event of PTB within 7 days (sensitivity 59.9%; 95% CI 52.7–66.8%, specificity 90.5%; 95% CI 89.0–91.9%, LR+ 5.71; 3.77–8.65, LR- 0.51; 0.33–0.80).54 Thus, a CL of <15mm at gestations over 30 weeks is advocated by NICE for initiating counselling on active management for PTL including corticosteroid and tocolytic treatment in symptomatic patients.50 A Cochrane review has further reviewed the effect of knowing the TVUS CL on prevention of PTB in symptomatic women and reported that whilst it could not confirm a clear effect on reducing deliveries <37 weeks (relative risk (RR) 0.59; 95% CI 0.26–1.32), it may prolong pregnancy by approximately 4 days.24
Cervical Length in Twin Pregnancies
Twin pregnancies are at greater risk of perinatal morbidity and mortality than singleton gestations, with MBRRACE-UK reporting a 2.3- and 3.5-fold increase in stillbirth and neonatal deaths, respectively, for twins compared to singletons in 2020.55 Notably, within this confidential enquiry, extreme prematurity was cited as the most common cause of perinatal loss in twin pregnancies.55 Chorionicity and amnionicity are significant determinants of adverse pregnancy outcomes in multiple pregnancies, including fetal loss <24 weeks’, perinatal death and PTB.56 Litwinska et al’s analysis of 6225 twin gestations reported fetal losses <24 weeks in 2.3% of dichorionic diamniotic (DCDA) twins, 7.7% of monochorionic diamniotic (MCDA) twins (RR 3.26; 95% CI 2.71–3.92) and 21.8% of monochorionic monoamniotic (MCMA) twins (RR 9.29; 95% CI, 6.38–13.53).56 PTB rates are similarly influenced by chorionicity and amnionicity, with births prior to 32 weeks occurring in 7.4% of DCDA twins, 14.2% of MCDA twins (RR 1.92; 95% CI, 1.62–2.28) and 26.8% MCMA twins (RR 3.64; 95% CI 2.17–6.09).56 Accurate determination of chorionicity and amnionicity by ultrasound examination prior to 14 weeks’ gestation is therefore essential to guide appropriate antenatal surveillance and patient counselling in multiple pregnancies.
Many studies have documented the utility of TVUS CL to predict spontaneous PTL in twin gestations, demonstrating that the rate of spontaneous PTB in twin pregnancies is inversely related to second trimester CL measurements.57–59 Despite the well-recognized increased risk of PTB in multiple pregnancies, the UK NICE guidelines do not recommend routine screening for PTB risk in unselected twin pregnancies.60 This is in contrast with FIGO, who advocate for routine second trimester CL screening in all twin pregnancies between 18 and 24 weeks utilizing a threshold of 20mm.61 This recommendation is largely based upon evidence from a large systematic review of 21 studies, which indicated that a CL ≤20mm between 20 and 24 weeks most accurately predicted PTB <32 and <34 weeks’ gestation in asymptomatic women (pooled sensitivities, specificities, LR+ and LR- of 39% and 29%, 96% and 97%, 10.1 and 9.0, and 0.64 and 0.74, respectively).62 The accuracy of CL measurement for the prediction of PTB <34 weeks and <37 weeks’ gestation was limited in symptomatic women (pooled LR+ and LR- between 1.2 and 1.9 and between 0.65 and 0.69, respectively),62 although more recent evidence has suggested that the performance of CL as a predictive test in PTL (measured by the examination-to-delivery interval) is not statistically different in twin pregnancies compared to singletons (Spearman correlation coefficient (r)=0.30 vs r=0.29, p=0.9).63
Further large systematic reviews have agreed that second trimester CL in asymptomatic women is a strong predictor of PTB, although Lim et al reported improved sensitivity at CLs <25mm (36%) compared to <20mm (30%), with no compromise in specificity (94% for both).64 Thus, in keeping with singletons, most publications tend to use a threshold of 25mm for screening between 18 and 24 weeks in twins.22 However, in addition to the CL measurement obtained, the gestational age at the time of screening is an important consideration in twin pregnancies, providing accurate prediction of spontaneous PTB probability. Kindinger et al’s individual participant data (IPD) meta-analysis reported that ≤18 weeks’ gestation, any CL measurement <30mm provided a greater risk of an extremely PTB (prior to 28 weeks) compared to singleton gestations (p<0.001) and that screening at or after 24 weeks best predicted later PTBs between 28+1 and 36+0 weeks (p<0.001).65 Thus, there appears to be a potential role for TVUS CL in the screening of twin pregnancies, however there is a paucity of prospective evidence on the benefits of serial CL scanning, and the accuracy and threshold for treatment utilizing CL measurements in cases of threatened PTL. Evidence to date has also not shown any proven benefit for the use of cervical cerclage, progesterone and cervical pessary to reduce the rates of PTB in twin gestations with a short cervix.66
Fetal Fibronectin
Fetal fibronectin (fFN) is an extracellular matrix glycoprotein produced by amniocytes and cytotrophoblasts, which is found in high concentrations at the maternal–fetal interface of the amniotic membrane, where it acts as a “glue” between the pregnancy and uterus.67,68 In early pregnancy, prior to the fusion of the decidua and fetal membranes, fFN is present in cervico-vaginal fluid, however after 22 weeks these levels are normally low (<50ng/mL).69 It is theorized that increased levels of fFN are released into cervico-vaginal secretions when the choriodecidual interface is disrupted by a mechanical or inflammatory-mediated injury, signifying a greater risk of PTB or PPROM.70 Research has consistently identified an association between qualitative detection of fFN and the risk of PTB in both asymptomatic high-risk women with singleton pregnancies71 and women attending hospital with signs or symptoms of PTL.7270,73 NICE advocate that a fFN cut-off of <50ng/mL can be utilized as an alternative to CL in symptomatic patients to rule out PTL if a TVUS is unacceptable to the patient or in the absence of a skilled operator.50 Indeed, the predictive value of fFN appears to be greatest amongst symptomatic women, with a large meta-analysis concluding 76% sensitivity and 88% specificity for delivery within 7 days of sampling.74 This has been echoed by Honest et al’s systematic review, which concluded that PTB was best predicted within 7–10 days of a positive fFN test in patients with symptoms of PTL (LR+ 5.42, 95% CI 4.36–6.74).75 Certainly, the greatest value of fFN in symptomatic patients is its high NPV for delivery within 14 days (99.2%), which can reduce interventions including hospitalisation, untimely corticosteroid use and maternal anxiety.76
The quantification of fFN (qfFN) allows the individual PTB risk to be defined; the EQUIPP study was the first trial to prospectively indicate the utility of qfFN for PTB <34 weeks in symptomatic women.73 Quantifying fFN at thresholds of 10, 50, 200, and 500 ng/mL increased the PPV from 19% to 32%, 61% and 75%, respectively, whilst maintaining a high negative predictive value (NPV) which has obvious clinical advantages in triaging patients with symptoms of PTL.73 Similarly, qfFN testing alone (AUC 0.82, 95% CI 0.76–0.89) appears to perform equally to the combination of CL and qualitative fFN (AUC 0.83, 95% CI 0.77–0.88) in the assessment of symptomatic women.77
A prospective masked cohort study of qfFN in asymptomatic women at high risk of PTB between 22 and 26+6 weeks also identified increased rates of spontaneous PTB <34 weeks with rising concentrations of cervicovaginal fFN (2.7% at <10ng/mL, 11.0% at 10–49, 14.9% at 50–199, 33.9% at 200–499 and 47.6% >500ng/mL).71, This study further demonstrated the diagnostic accuracy of increasing concentrations of qfFN to predict spontaneous PTB with an AUC of 0.78 (95% CI 0.73–0.84) compared to the original qualitative test (AUC 0.68 for birth <34 weeks, 95% CI 0.63–0.73), and aided risk discrimination amongst women with a short CL <25mm as 9.5% delivered preterm with qfFN <10ng/mL compared with 55.1% at concentrations >200ng/mL (p<0.001).71
Kuhrt et al developed the first version of the QUiPP app from a prospective dataset incorporating qfFN and a maternal history of previous PPROM/PTB to aid prediction of PTB in symptomatic women. This model showed an improvement in the prediction of PTB <34 and <37 weeks’ (AUC 0.83 and 0.77, respectively) when compared to previous literature, including Honest et al’s review that reported AUCs ranging between 0.71 and 0.77 for fFN’s ability to predict PTL at the same gestations.75,78, The study showed high NPVs for delivery at five different time points; delivery <30 weeks (97.2%; 95% CI 93.6–99.1%), <34 weeks (98.1%; 95% CI 94.6–99.6%), <37 weeks (94.9%; 95% CI 89.7–97.9%) and within 2 weeks (97.2%; 95% CI 93.5–99.1%) and 4 weeks (94.0%; 95% CI 89.2–97.1%).78 The performance accuracy of the QUiPP app was evaluated in a secondary analysis of prospectively collected data from 355 women presenting with symptoms of PTL. Each episode of threatened PTL was retrospectively assigned a risk of PTB within 7 days using the QUiPP app. Using a 5% risk as a threshold for treatment, all women who had a PTB would have been correctly identified, giving a sensitivity of 100% and a NPV of 100% for testing between 24 and 34 weeks.79 The positive predictive value (PPV) for delivery within 7 days was 30.0% (95% CI 11.9–54.3%) in those presenting at 24+0 to 29+6 weeks and 20.0% (95% CI 12.7–30.1%) between 30+0 and 34+0 weeks.79 Furthermore, when comparing women with a QUiPP low risk of delivery (<5%) with the NICE treat-all strategy for gestations <30 weeks, 89.4% of admissions would have been prevented and 168 women would have avoided unnecessary treatment including hospitalisation, antenatal corticosteroids and in-utero transfer.79
A Cochrane review has assessed the effect of a clinician’s knowledge of qfFN results in reducing PTB in symptomatic singleton pregnancies between 23 and 34+6 weeks. The evidence was of low quality, but management based upon awareness of the fFN results was felt to potentially reduce PTB before 37 weeks’ gestation (RR 0.72; 95% CI 0.52–1.01).69 Evidence for gestations less than 34 weeks was of very low quality, and the effect of fFN knowledge in this context is therefore uncertain. Similarly, effects on outcomes including birthweight, perinatal death, maternal hospitalisation, respiratory distress syndrome and neonatal intensive care unit (NICU) admission are unknown due to serious concerns in study design and inconsistencies between the included trials.69
The Hologic® Rapid fFN 10Q test manufacturers advise against test use with vaginal bleeding or ruptured membranes, due to the risk of a false-positive result, given the normal occurrence of fFN in maternal serum and amniotic fluid80. Contamination of the test swab with lubricants, soaps, or creams may also physically interfere with absorption of the cervicovaginal secretions or alter assay results. Lukes et al found three factors that significantly contributed to positive test results, including sexual activity within 24 hr of sample collection (p<0.001), vaginal bleeding (p<0.0001) and cervical examination within 24 hr of sample collection (p<0.001).81 A systematic review of six studies has further assessed the effect of TVUS, sterile vaginal examination and sexual intercourse on FFN results in high-risk asymptomatic patients or patients reporting signs and symptoms of PTL. It identified a high overall proportion agreement between positive fFN specimens obtained before and after TVUS (93.4%) and sterile vaginal examination (88.5%), suggesting a minimal effect of these interventions on positive test results.82 Conversely, women reporting coitus 24 to 48 hr prior to fFN collection were up to five times more likely to have a positive qualitative result (RR 5.6; 95% CI 3.0–10.6), suggesting a sound rationale for discouraging testing in this setting.82
A secondary analysis of data obtained in the multicentre APOSTEL-1 (Alleviation of Pregnancy Outcome by Suspending of Tocolysis in Early Labour-1) study showed that multiple factors including sexual intercourse and vaginal examinations contributed to invalid test results, but statistical significance was only observed for vaginal bleeding at the time of testing (OR 4.5; 95% CI 1.7–12).83 Hezelgrave et al’s study of high-risk asymptomatic women found that median fFN concentrations in blood-stained swabs were significantly greater than case-matched controls (66ng/mL vs 7.5ng/mL, p<0.001) and that these swabs had greater sensitivity but lower specificity for predicting PTB.84 As the ROC curve obtained was similar between the blood-stained and control groups (0.78 vs 0.84) there may still be predictive value in testing women who present with a small volume of vaginal bleeding given that low fFN levels have a high NPV and indicate a low risk of PTB, with limitations of testing discussed with the patient.84
Fetal Fibronectin in Twin Pregnancies
NICE discourages clinicians from the use of independent fFN testing to predict the risk of PTB in twin gestations, citing a lack of evidence for its accuracy in multiple pregnancies.60 Following the publication of the 2015 NICE guidelines, there have been studies supporting the use of fFN to predict spontaneous PTB in both symptomatic and asymptomatic twin pregnancies. Marleen et al performed a systematic review of biochemical markers in the prediction of PTB in twin pregnancies, demonstrating increased odds of PTB <34 weeks in both asymptomatic (OR 8.07, 95% CI 5.28–12.33 p=0.001) and symptomatic (OR 3.07, 95% CI 1.44–6.57 p=0.004) women with positive fFN tests.85 Kuhrt et al identified 130 eligible asymptomatic women with twin pregnancies in a prospective cohort study and reported that qfFN measured between 18 and 27+6 weeks was significantly related to the risk of delivery prior to 30 weeks’ gestation (AUC 0.8, 95% CI 0.7–1.0).86 For women with a short cervix <25mm, the spontaneous PTB rate <30 weeks increased from 0% for those with low qfFN concentrations <10ng/mL, to 86% for those with a qfFN ≥200ng/mL, and notably for patients with a qfFN >200ng/mL and a CL >25mm, the PPV for spontaneous PTB was 0%.86 Whilst the study is limited by its small size, these results suggest that combining qfFN with CL can improve the prediction of PTB in twin pregnancies, and this has since been incorporated into the QUiPP app v.2.87 A further systematic review including 1332 twin pregnancies has reported sensitivities ranging from 29% to 41% and specificities between 92% and 96% for fFN in the assessment of PTB risk. However, the authors have concluded that the accuracy of fFN in multiple pregnancies is inconclusive, and this is likely secondary to the limited and heterogeneous data included in the analysis.88 This highlights the need for prospective, high-quality research to determine the accuracy of fFN in twin gestations.
Preterm Birth Screening Clinics (PSCs) and QUiPP
The UK Saving Babies Lives Care Bundle Version 3 (SBLCBv3) supports the development of specialist PSCs to standardise care pathways for high-risk women, facilitate access to specialist care and encourage research through preterm birth networks.89 The efficacy of PSCs has been disputed in the literature; a Cochrane systematic review failed to show any evidence of benefit of PSCs in reducing rates of PTB.90 However, this analysis was limited by a lack of statistical power and large variation in outcomes measured by the included RCTs. Furthermore, the included studies were conducted in the 1980s, when currently recommended screening methods were not available, and the interventions performed, including repeat digital cervical assessments, have not been shown to be effective in the prevention of PTB.91 Between 2012 and 2017, there has been a 44% increase in the number of UK specialist PTB clinics.92 Although studies have shown variation in practice between maternity units internationally93, there appears to be an increasing consensus UK-wide in referral criteria to clinic, gestation at first appointment and CL thresholds for intervention.92 A recent prospective cohort study of singleton pregnancies between 23 and 28 weeks demonstrated the ability of PSCs measuring CL and qfFN in conjunction with maternal history to accurately triage asymptomatic high-risk women.94 Patients who screened positive and were admitted to hospital were significantly more likely to deliver before 30 weeks (RR 27.6; 95% CI 15.0–50.1).94 Patients who screened negative, despite their risk factors, had a low rate of PTB, comparable to the background population (1.3% vs 1.2%) and lower rates of neonatal death, stillbirth and NICU admission (p<0.001 for all).94 This predictive modelling utilizing qfFN and CL has been incorporated into a decision-support tool (QUiPP v.2), providing an individualized assessment of the risk of delivery within pre-specified time frames for both symptomatic and asymptomatic populations of women with singleton or twin pregnancies.87,95, QUiPP’s performance to triage women has been evaluated in a UK prospective, multicentre study of asymptomatic, high-risk women. This included 1667 singleton and 136 twin pregnancies in the training set and 887 singleton and 17 twin pregnancies in the validation set. The study demonstrated high accuracy for QUiPP’s ability to predict spontaneous PTB and is thus advocated for widespread use to assist in risk stratification and management decisions between 18+0 and 36+6 weeks’ gestation.87 For the convenience of clinicians, given local variations in resources, three different prediction models incorporating clinical risk factors have been created; 1) qfFN 2) CL, 3) both CL and qfFN. All three models have demonstrated good accuracy in PTB prediction at <30, <34 and <37 weeks with AUCs ranging, dependent on the algorithm chosen, between 0.76 and 0.83, 0.71 and 0.76, and 0.68 and 0.75, respectively.87 Whilst there were trends towards improved prediction when using the third model combining risk factors, CL and qfFN, there were no significant differences between algorithms. A low (<1%) risk on the QUiPP app is associated with an event rate of only 0.5%, whilst a high risk (>10%) is associated with a 26% risk of delivery within 4 weeks (Table 3).87 As accuracy is high for the prediction of PTB within 4 weeks (AUC >0.87 for all three combinations of tests), clinicians can reliably inform and manage women at high risk, and be reassured that less frequent surveillance appointments are appropriate for women at lower risk.87 Further research is required to identify if a specific QUiPP risk should prompt interventions including transvaginal cerclage (TVC), however, the authors stress the importance of interpreting results in consideration of the clinical context and women’s views.86
 |
Table 3 Prediction of PTB Within Four Weeks, and Prior to 34 Weeks as Calculated by the QUiPP App v.2 in Comparison with Observed Event Rates in Asymptomatic High-Risk* Women.87 |
Caution should be applied when managing patients not represented in the dataset, which generated and validated QUiPP. Women with a previous FDCS were not included in the training and validation cohorts and thus care must be applied when utilizing the QUiPP app in this population whilst awaiting the results from the CRAFT-OBS trial, which aims to assess the efficacy of CL and qfFN in risk stratification of women with previous a CS in labor.96
QUiPP App v.2 for Symptomatic Women
The new QUiPP algorithm has expanded the utility of the previous app by incorporating additional risk factors including twin pregnancy, second trimester miscarriage (STM) and cervical surgery, and the calculation of risk using qfFN, TVUS CL or a combination of both.95 The dataset utilized for QUiPP App v.2 was substantially larger than the original version (1538 vs 382 symptomatic women), included 41 and 33 twin pregnancies in the training and validation sets, respectively, and demonstrated a significant increase in sensitivity at all outcome time points with similar NPVs. All three algorithms have good accuracy for the prediction of PTB at <30, <34 and <37 weeks’ gestation and within 1 and 2 weeks’ post testing (For qfFN and CL AUC: 0.95 <30 weeks; 0.83 <34 weeks; 0.73 <37 weeks; 0.88 within 1 week; 0.89 within 2 weeks).95 The “Evaluation of the QUiPP app for Triage and Transfer” (EQUIPTT) multicentre cluster RCT has further allowed validation of the app’s real-time performance using qfFN to triage a large sample of symptomatic women, comparing the intervention with a control of standard emergency obstetric care for threatened PTL (AUC 0.90; 95% CI 0.85–0.95 for delivery within 7 days).97 As CL was only utilized in 5.5% of all visits, there was not enough data to draw conclusions on its validity in triaging symptomatic women with threatened PTL.97 Four women from the intervention sites utilizing QUiPP missed timely treatment including steroids, compared to 12 from the control sites, and no deliveries <30 weeks occurred outside of any hospital sites.97 However, there was no statistically significant reduction in hospital admissions using QUiPP compared to the control arm (7.4% vs 9.9%, OR 0.72; 95% CI 0.45–1.16).97 This may have occurred due to difficulties implementing the QUiPP app in the intervention group hospitals. It may also highlight that the management of threatened PTL in many control sites already varies considerably from the NICE recommended “treat-all strategy” for women prior to 30 weeks, but this may not reflect wider UK practice.
Other Tests
Other biochemical tests can assist in the prediction of PTB including placental alpha-microglobulin-1 (PAMG-1) and phosphorylated insulin-like growth factor-binding protein-1 (phlGFBP-1). A systematic review and meta-analysis of 65 cohort studies assessed the accuracy and performance of fFN, PAMG-1 and phlGFBP-1 for PTB prediction in low- and high-risk symptomatic women within 7 days of testing. The AUCs were 0.87, 0.91 and 0.80 for fFN, PAMG-1 and phlGFBP-1, respectively, with PAMG-1 also achieving a high NPV of 97% and statistically superior PPV across all risk groups (p<0.05), two to six-fold higher than those of fFN and phlGFBP-1.98 However, the predictive performance of PAMG-1 comparative to the QUiPP app has not yet been evaluated.
Saade et al have developed a novel serum test utilizing a log ratio of insulin-like growth factor-binding protein 4 (IBP4) and sex hormone-binding globulin (SHBG) to predict spontaneous PTB in asymptomatic women. A multicenter prospective US study of 5501 women enrolled between 17+0 and 28+6 weeks demonstrated an AUC of 0.75 for the prediction of spontaneous PTB <37 weeks, with a sensitivity and specificity of 75% and 74%, respectively.99 However, this study was not sufficiently powered to assess the performance of IBP4/SHBG in improving neonatal outcomes, or in the prediction of extreme or very PTBs <32 weeks. Subsequently, Markenson et al have published a US multicenter prospective observational study between 19+1 and 20+6 weeks, confirming that the IBP4:SHBG ratio significantly predicts PTB <32 weeks’ gestation with an AUC of 0.71 (95% CI 0.55–0.87, p=0.016).100 The IBP4:SHBH ratio was also predictive of severe neonatal morbidity with an AUC of 0.67 (95% CI 0.57–0.77, p=0.005) and neonatal mortality with an AUC of 0.78 (95% CI 0.63–0.93, p=0.026).100 This test is commercially available as PreTRM® (Sera Prognostics, Inc. Salt Lake City, UT). Evidence on the test’s clinical and cost-effectiveness101 is limited, and further prospective, preferably randomized studies are required to assess its utility in current clinical practice. A recent prospective study of 1191 low-risk women, randomized to the PreTRM test or usual care, showed no significant benefit of the PreTRM mid-trimester screening test to reduce births <37 weeks, when coupled with a spontaneous PTB risk reduction protocol in screen-positive patients (2.7% vs 3.5%, p=0.41). This study had multiple limitations, as it excluded high-risk women, and was prematurely terminated due to inadequate funding, and hence underpowered. However, as infants in the screened group had a significantly shorter NICU length of stay, this should prompt further study.102
Future Considerations: Metabolomics, Extracellular Vesicles, Cervical Elastography, Cervical Stiffness and Artificial Intelligence
Metabolomics
Metabolomics is a comprehensive study of the altered metabolic states of substrates and their metabolites within cells, bio-fluids, tissues or organisms.103 Specific metabolites produced or modified by the vaginal metabolome are theorized to provide a quantitative representation of local inflammatory processes present in the cervico-vaginal microbiota. Alterations in the vaginal microbiome have repeatedly been associated with spontaneous PTB104,105 and adverse pregnancy outcomes including PPROM and early-onset neonatal sepsis.106
Identification of metabolites including specific endogenous amino acids, lipids, nucleotides, and carbohydrates within the vaginal metabolome may act as potential early biomarkers for intra-amniotic inflammation, infection and spontaneous PTB, aiding identification of high-risk women.107,108 Studies have shown unique metabolic profiles in women who experience PTBs compared to those who experience a term delivery. Similar to the vaginal microbiome structure, significant differences have also been demonstrated between the metabolome of Black and White women (p<0.001),109 which may contribute to the racial disparity seen in PTB rates between these groups.107,110,
Additionally, several xenobiotics of a suspected, exogenous source including diethanolamine (DEA), ethyl-glucoside, tartrate and Ethylenediaminetetraacetic acid (EDTA) have also been reported to be significantly associated with PTB (p<0.05), raising concerns of possible harm to women in pregnancy from environmental exposures.107
These findings suggest the biological importance of the cervicovaginal metabolome in pregnancy.111 Whilst data demonstrate promise for the study of metabolomics in women who experience PTB, its current use remains within the context of clinical research. Future research is required to further characterise the contribution of these individual metabolic differences in those who have spontaneous PTBs and ascertain the utility and viability of this as a biomarker in routine clinical practice.
Extracellular Vesicles
“Extracellular vesicle” (EV) is the collective term for anucleated lipid bilayer-delimited particles that are released by cells in response to physiological and metabolic changes.112 EVs contribute to cell-to-cell communication, transporting signalling molecules or “cellular cargo” including proteins, lipids and nucleic acids between cells in extracellular spaces, plasma and bio-fluids.113 The cargo contained within these vesicles appears to reflect the physiological or pathological state of the releasing cell, and this has the capacity to influence the biological functions of recipient cells.,113 In normal early pregnancy development, endometrial, embryonic and placental-derived EVs have a well-recognized role in influencing successful implantation, immunomodulation, and spiral artery remodelling.114,115 At term, fetal-derived EVs also appear to have a role in inducing functional progesterone withdrawal in placental trophoblasts, initiating spontaneous labour.116 With increasing recognition of the role of EVs in trans-placental feto-maternal communication, there is growing interest in them as predictive and diagnostic markers of obstetric diseases including PTB.
Cantonwine et al first identified statistically significant EV proteins, which were uniquely expressed in the first-trimester plasma samples of women who delivered spontaneously prior to 34 weeks. A panel of three proteins (A2MG, HEMO and MBL2) was specifically reported to have a specificity of 83% (95% CI 0.65–0.94%) at fixed sensitivity of 80% for birth <34 weeks and AUC of 0.89 (0.83–0.95).117 A larger, multicenter study validated these findings and identified that a panel of five EV proteins isolated from maternal plasma in first-trimester singleton pregnancies may predict spontaneous PTB before 35 weeks with an AUC of 0.74 (95% CI 0.63–0.81).118 Performance utilizing a separate panel of four markers observed a marginally improved AUC of 0.77 (95% CI 0.61–0.90) in nulliparous women.118
Menon et al have also reported significant changes in the micro RNA (miRNA) and protein content of circulating EVs amongst term and preterm gestation births. 167 and 153 miRNAs were found to significantly change as a function of gestational age across term and PTBs, respectively. The functional roles reported for many of the miRNAs overrepresented in the PTB cohort were related to cell growth, cellular senescence, and inflammation, suggesting potential disturbances in the normal cell cycle in this group.119
Similarly, the placental EV protein cargo appears to significantly differ between women who experience PTB and term birth. A comparative analysis revealed 96 proteins differing significantly across gestations (p<0.05), with lower levels seen amongst the PTB cohort.120 A further study by Menon et al has additionally shown significant differences between the levels of individual EV proteins isolated in maternal serum between four cohorts of patients; term not in labour, term in labour, PPROM, and PTL (p<0.001).121 Differences in proteins associated with inflammation, coagulation and oxidative stress were identified even between PPROM and PTL groups (p<0.001),121 highlighting the heterogeneous nature of preterm birth syndrome. Further research within this field could shed even further light on the physiological process of parturition and potentially improve the early identification of women who will go on to deliver prematurely. This exciting topic is likely to play a more important role in this subject as more and more studies are added to the existing small body of literature.
Cervical Elastography
A promising future approach to screening for preterm birth by examining the integrity of cervical tissue is gaining traction in the research literature. While cervical elastography is not a novel idea, there have been significant developments in this field. Ultrasound elastography is an imaging technique that can show alterations in the stiffness of an examined tissue structure and this has been applied to the uterine cervix. Several different approaches have been described such as strain elastography (SE) and shear wave elastography (SWE). In the prediction of preterm birth, SE has a sensitivity and specificity of 84% (95% CI 68–100%) and 80% (95% CI 60–100%), respectively.122 The detection rates of PTB using SWE and SE are not statistically different (p=0.94).122 In a meta-analysis, Wang et al conclude that cervical elastography’s diagnostic capability is superior to CL alone in the prediction of spontaneous PTB.122 Despite these encouraging data, cervical elastography has not yet been refined enough to be translated into routine practice.
Pregnolia® Cervical Stiffness
There is also another method of assessing cervical consistency, or cervical stiffness, which appears to be an effective and easily reproducible method of PTB prediction.123,124 Parra-Saavedra et al, in a study of 1115 women, reported a statistically significant inverse relationship between cervical consistency and CL, with this association being demonstrably stronger for CL measurements below 28mm.124 This could suggest that the well-recognized association between a short cervix and PTB is mediated through changes in cervical stiffness, and as cervical softening appears to precede cervical shortening, early identification of changes in cervical consistency could facilitate more prompt identification and treatment of high-risk patients.124 Utilizing a cervical consistency index (CCI), the prediction of PTB before 32, 34 and 37 weeks in this study outperformed CL; the AUC <32 weeks was 0.947 vs 0.631, <34 weeks was 0.943 vs 0.645 and <37 weeks was 0.907 vs 0.643.124
The Pregnolia® system (Schlieren, Switzerland) has been developed to enable an objective and quantitative measurement of cervical stiffness.125 The device consists of a control unit and a single-use sterile probe, placed in contact with the anterior lip of the cervix following visualisation with a Cusco bivalve speculum (Figure 2). The aspiration device applies negative pressure on the cervical epithelium as the operator, utilizing a foot pedal, creates a small vacuum inside the probe tip, gently pulling on the cervical tissue to a predefined safe level of deformation. The greater the stiffness of the cervical tissue, the higher the tension required to displace the tissue into the probe tip. The obtained value of the closing pressure (mbar) is displayed by the control unit and gives a direct measurement of ectocervical stiffness (CSI).
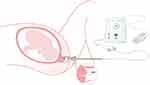 |
Figure 2 An illustration demonstrating the use of the Pregnolia system, with the probe tip in contact with the anterior lip of the cervix, the need for a foot pedal to initiate the vacuum and the control unit to display the cervical stiffness index (CSI).125 Image courtesy of Dr Sabrina Badir. |
A prototype of the device was tested in a feasibility study to describe the evolution of cervical stiffness in normal pregnancies, comparing cervical stiffness measurements in 50 pregnant and 50 non-pregnant women. CLs were also obtained by ultrasound examination for all participants. The CSI in the first trimester was reported to be significantly lower than in the non-pregnant group, at less than half of the corresponding value of the non-pregnant cervix.126 The mean value (mbar) further decreased as pregnancy advanced with significant differences found between the first (153 ± 78) and second trimesters (77 ± 37) (p<0.001).126 No differences were demonstrable in CSI between the second and third trimesters, in direct contrast with CL measurements, which significantly decreased between these times.126 This study supports the idea that cervical softening is initiated earlier than cervical shortening and poses the research question of whether the Pregnolia® system can predict significant cervical changes more accurately or earlier than what is currently achievable by TVUS CL, qfFN or other biochemical tests. Further observational studies investigating this are awaited.
Artificial Intelligence
Artificial intelligence (AI) refers to the ability of a machine or software program to perform tasks that are typically associated with human intelligence, including reasoning, learning, adaptation, understanding and interaction.127 There is significant potential for the use of AI in obstetric and gynaecological imaging, facilitating the training of ultrasound sonographers and providing instant image quality assurance.127 It is also hoped that AI technologies will emerge as a transformative tool in PTB screening, revolutionising the prediction of spontaneous PTL and promoting individualized care of high-risk women by enabling more precise, data-driven decision-making.
Lacerda de Andrade Júnior et al performed a retrospective observational study of 524 unselected singleton pregnancies, who underwent a second trimester CL assessment, with the objective of creating an AI screening algorithm for PTB. An AI preterm birth predictive model was created based on a stacking-based ensemble learning method (SBELM) utilizing a Neural Network approach, multivariate logistic regression (LR) and the best AI algorithm. Eight variables were considered significant including: previous spontaneous PTB <37 weeks, smoking history, maternal weight, cervical funnelling on TVUS, previous curettage, CL <30.9mm, use of antibiotics during pregnancy, and index of the straight CL/internal angle inside the cervix. Fixing a 10% false-positive rate, results of the algorithm for the prediction of spontaneous PTB <35 weeks’ gestation were directly compared to a CL of less than 25mm, respectively; sensitivity 33.3% vs 47.3%, specificity 91.8% vs 92.8%, PPV 23.1% vs 32.7%, NPV 94.9% vs 96.0%, AUC 0.32 vs 0.81.128 Larger, multicenter, prospective studies utilizing AI computer technology and ultrasound findings are recommended to confirm these results, particularly in high-risk cohorts, and provide statistical power to the findings.
Conclusion
PTB is an important public health priority with long-term implications for individuals, families and healthcare systems. Accurate prediction in high-risk asymptomatic women and symptomatic patients is essential to guide clinicians to offer necessary preventative interventions and treatment to reduce morbidity and mortality. There is an abundance of evidence supporting the utility of CL and qfFN in both clinical settings, and subsequently, there is clear prospective, multicenter and “real-world” evidence validating the QUiPP prediction models in both singleton and twin gestations. Further research is required, including the predictive efficacy of the QUiPP model in women with previous FDCS, multiple pregnancies and in countries outside of the UK. Large, prospective studies investigating the new exciting research area of second trimester CSI, amongst other technologies, are awaited.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Howson CP, Kinney MV, Lawn JE. March of dimes, PMNCH, save the children, WHO. In: Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012. Available from: https://iris.who.int/bitstream/handle/10665/44864/9789241503433_eng.pdf?sequence=1.
2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi:10.1016/S0140-6736(08)60074-4
3. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi:10.1016/S2214-109X(18)30451-0
4. Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345(dec04 3):e7976. doi:10.1136/bmj.e7976
5. Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F208–F215. doi:10.1136/archdischild-2017-312748
6. Boyle JD, Boyle EM. Born just a few weeks early: does it matter? Arch Dis Child Fetal Neonatal Ed. 2013;98(1):F85–F88. doi:10.1136/archdischild-2011-300535
7. Chang HY, Sung YH, Wang SM, et al. Short- and long-term outcomes in very low birth weight infants with admission hypothermia. PLoS One. 2015;10(7):e0131976. doi:10.1371/journal.pone.0131976
8. Natarajan G, Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33(3):305–317. doi:10.1055/s-0035-1571150
9. Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154(2):169–176. doi:10.1016/j.jpeds.2008.08.020
10. Cheong JL, Doyle LW, Burnett AC, et al. Association between moderate and late preterm birth and neurodevelopment and social-emotional development at age 2 years. JAMA Pediatr. 2017;171(4):e164805. doi:10.1001/jamapediatrics.2016.4805
11. Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3):e622–e629. doi:10.1542/peds.2009-3598
12. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. 2019;210:69–80.e5. doi:10.1016/j.jpeds.2019.02.041
13. Platt MJ. Outcomes in preterm infants. Public Health. 2014;128(5):399–403. doi:10.1016/j.puhe.2014.03.010
14. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi:10.1055/s-2006-956773
15. Giorgione V, Quintero Mendez O, Pinas A, Ansley W, Thilaganathan B. Routine first‐trimester pre‐eclampsia screening and risk of preterm birth. Ultrasound Obstet Gynecol. 2022;60(2):185–191. doi:10.1002/uog.24915
16. Chiu CPH, Feng Q, Chaemsaithong P, et al. Prediction of spontaneous preterm birth and preterm prelabor rupture of membranes using maternal factors, obstetric history and biomarkers of placental function at 11–13 weeks. Ultrasound Obstet Gynecol. 2022;60(2):192–199. doi:10.1002/uog.24917
17. Roman A, Suhag A, Berghella V. Overview of cervical insufficiency: diagnosis, etiologies, and risk factors. Clin Obstet Gynecol. 2016;59(2):237–240. doi:10.1097/GRF.0000000000000184
18. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. doi:10.1111/j.1471-0528.2006.01120.x
19. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. doi:10.1126/science.1251816
20. Shennan A, Story L, Jacobsson B, Grobman WA; The FIGO Working Group for Preterm Birth. FIGO good practice recommendations on cervical cerclage for prevention of preterm birth. Int J Gynaecol Obstet. 2021;155:19–22. doi:10.1002/ijgo.13835
21. Shennan A, Story L. Cervical Cerclage. BJOG. 2022;129(7):1178–1210. doi:10.1111/1471-0528.17003
22. Coutinho CM, Sotiriadis A, Odibo A, et al. ISUOG practice guidelines: role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obstet Gynecol. 2022;60(3):435–456. doi:10.1002/uog.26020
23. Souka AP, Pilalis A. Reproducibility of cervical length measurement throughout pregnancy. J Matern Fetal Neonatal Med. 2021;34(13):2185–2191. doi:10.1080/14767058.2019.1660765
24. Berghella V, Saccone G. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst Rev. 2019;9(9):CD007235. doi:10.1002/14651858.CD007235.pub4
25. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. national institute of child health and human development maternal fetal medicine unit network. N Engl J Med. 1996;334(9):567–572. doi:10.1056/NEJM199602293340904
26. Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003;46(4):947–962. doi:10.1097/00003081-200312000-00026
27. To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18(3):200–203. doi:10.1046/j.1469-0705.2001.00437.x
28. Celik E, To M, Gajewska K, Smith GCS, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31(5):549–554. doi:10.1002/uog.5333
29. Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110(2):311–317. doi:10.1097/01.AOG.0000270112.05025.1d
30. Salomon LJ, Diaz-Garcia C, Bernard JP, Ville Y. Reference range for cervical length throughout pregnancy: non-parametric LMS-based model applied to a large sample. Ultrasound Obstet Gynecol. 2009;33(4):459–464. doi:10.1002/uog.6332
31. Gudicha DW, Romero R, Kabiri D, et al. Personalized assessment of cervical length improves prediction of spontaneous preterm birth: a standard and a percentile calculator. Am J Obstet Gynecol. 2021;224(3):288.e1–288.e17. doi:10.1016/j.ajog.2020.09.002
32. Berghella V, Talucci M, Desai A. Does transvaginal sonographic measurement of cervical length before 14 weeks predict preterm delivery in high-risk pregnancies? Ultrasound Obstet Gynecol. 2003;21(2):140–144. doi:10.1002/uog.28
33. Greco E, Lange A, Ushakov F, Calvo JR, Nicolaides KH. Prediction of spontaneous preterm delivery from endocervical length at 11 to 13 weeks. Prenat Diagn. 2011;31(1):84–89. doi:10.1002/pd.2640
34. Ferrero DM, Larson J, Jacobsson B, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS One. 2016;11(9):e0162506. doi:10.1371/journal.pone.0162506
35. Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7(6):e015402. doi:10.1136/bmjopen-2016-015402
36. Khalid S, Dimitriou E, Conroy R, et al. The thickness and volume of LLETZ specimens can predict the relative risk of pregnancy-related morbidity. BJOG. 2012;119(6):685–691. doi:10.1111/j.1471-0528.2011.03252.x
37. Kyrgiou M, Athanasiou A, Kalliala IEJ, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev. 2017;11(11):CD012847. doi:10.1002/14651858.CD012847
38. Akhtar M, Saravelos S, Li T, Jayaprakasan K. Reproductive implications and management of congenital uterine anomalies. BJOG. 2020;127(5). doi:10.1111/1471-0528.15968
39. Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet Gynecol. 2011;38(4):371–382. doi:10.1002/uog.10056
40. Watson HA, Carter J, David AL, Seed PT, Shennan AH. Full dilation cesarean section: a risk factor for recurrent second-trimester loss and preterm birth. Acta Obstet Gynecol Scand. 2017;96(9):1100–1105. doi:10.1111/aogs.13160
41. Levine LD, Sammel MD, Hirshberg A, Elovitz MA, Srinivas SK. Does stage of labor at time of cesarean delivery affect risk of subsequent preterm birth? Am J Obstet Gynecol. 2015;212(3):360.e1–360.e7. doi:10.1016/j.ajog.2014.09.035
42. Domin CM, Smith EJ, Terplan M. Transvaginal ultrasonographic measurement of cervical length as a predictor of preterm birth. Ultrasound Q. 2010;26(4):241–248. doi:10.1097/RUQ.0b013e3181fe0e05
43. To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol. 2000;15(4):292–296. doi:10.1046/j.1469-0705.2000.00094.x
44. Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. J Matern Fetal Neonatal Med. 2012;25(9):1682–1689. doi:10.3109/14767058.2012.657278
45. Clement S, Candy B, Heath V, To M, Nicolaides KH. Transvaginal ultrasound in pregnancy: its acceptability to women and maternal psychological morbidity. Ultrasound Obstet Gynecol. 2003;22(5):508–514. doi:10.1002/uog.893
46. Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1–365.e5. doi:10.1016/j.ajog.2015.12.020
47. Carlisle N, Carter J, Radford S, Shennan A. Women’s experiences of tests and procedures carried out at a preterm birth surveillance clinic. Br J Midwifery. 2018;26(1):31–34. doi:10.12968/bjom.2018.26.1.31
48. Perinatal Quality Foundation. Cervical Length Education and Review (CLEAR). Available from: https://clear.perinatalquality.org.
49. The Fetal Medicine Foundation. FMF certification: cervical assessment. Available from: https://fetalmedicine.org/education/cervical-assessment.
50. National Institute for Health and Care Excellence (NICE). Preterm Labour and Birth NICE Guideline [NG25]; 2015. Available from: www.nice.org.uk/guidance/ng25.
51. Guinn DA, Goepfert AR, Owen J, Brumfield C, Hauth JC. Management options in women with preterm uterine contractions: a randomized clinical trial. Am J Obstet Gynecol. 1997;177(4):814–818. doi:10.1016/S0002-9378(97)70274-6
52. Norman J, Shennan A, Jacobsson B, Stock SJ; FIGO Working Group for Preterm Birth. FIGO good practice recommendations on the use of prenatal corticosteroids to improve outcomes and minimize harm in babies born preterm. Int J Gynecol Obstet. 2021;155(1):26–30. doi:10.1002/ijgo.13836
53. Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323(19):1924. doi:10.1001/jama.2020.3937
54. Sotiriadis A, Papatheodorou S, Kavvadias A, Makrydimas G. Transvaginal cervical length measurement for prediction of preterm birth in women with threatened preterm labor: a meta-analysis. Ultrasound Obstet Gynecol. 2010;35(1):54–64. doi:10.1002/uog.7457
55. Draper ES, Gallimore ID, Kurinczuk JJ, Kenyon S; On behalf of MBRRACE-UK. MBRRACE-UK 2019 perinatal confidential enquiry: stillbirths and neonatal deaths in twin pregnancies. In: The Infant Mortality and Morbidity Studies. Leicester: Department of Health Sciences, University of Leicester; 2021. Available from: https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/perinatal-report-2020-twins/MBRRACE-UK_Twin_Pregnancies_Confidential_Enquiry.pdf.
56. Litwinska E, Syngelaki A, Cimpoca B, Frei L, Nicolaides KH. Outcome of twin pregnancy with two live fetuses at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2020;55(1):32–38. doi:10.1002/uog.21892
57. Skentou C, Souka AP, To MS, Liao AW, Nicolaides KH. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound Obstet Gynecol. 2001;17(1):7–10. doi:10.1046/j.1469-0705.2001.00357.x
58. To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol. 2006;194(5):1360–1365. doi:10.1016/j.ajog.2005.11.001
59. Heath VCF, Daskalakis G, Zagaliki A, Carvalho M, Nicolaides KH. Cervicovaginal fibronectin and cervical length at 23 weeks of gestation: relative risk of early preterm delivery. BJOG. 2000;107(10):1276–1281. doi:10.1111/j.1471-0528.2000.tb11620.x
60. National Institute for Health and Care Excellence (NICE). Twin and Triplet Pregnancy NICE Guideline [NG137]; 2019. Available from: https://www.nice.org.uk/guidance/ng137.
61. FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: management of twin pregnancy. Int J Gynecol Obstet. 2019;144(3):330–337. doi:10.1002/ijgo.12742
62. Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203(2):128.e1–128.e12. doi:10.1016/j.ajog.2010.02.064
63. Melamed N, Hiersch L, Gabbay-Benziv R, et al. Predictive value of cervical length in women with twin pregnancy presenting with threatened preterm labor. Ultrasound Obstet Gynecol. 2015;46(1):73–81. doi:10.1002/uog.14665
64. Lim AC, Hegeman MA. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta-analysis. Ultrasound Obstet Gynecol. 2011;38(1):10–17. doi:10.1002/uog.9013
65. Kindinger L, Poon L, Cacciatore S, et al. The effect of gestational age and cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta-analysis. BJOG. 2016;123(6):877–884. doi:10.1111/1471-0528.13575
66. D’Antonio F, Berghella V, Di Mascio D, et al. Role of progesterone, cerclage and pessary in preventing preterm birth in twin pregnancies: a systematic review and network meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;261:166–177. doi:10.1016/j.ejogrb.2021.04.023
67. Berghella V, Saccone G. Fetal fibronectin testing for prevention of preterm birth in singleton pregnancies with threatened preterm labor: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(4):431–438. doi:10.1016/j.ajog.2016.04.038
68. Feinberg RF, Kliman HJ, Lockwood CJ. Is oncofetal fibronectin a trophoblast glue for human implantation? Am J Pathol. 1991;138(3):537–543.
69. Berghella V, Saccone G. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev. 2019;7(7):CD006843. doi:10.1002/14651858.CD006843.pub3
70. Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325(10):669–674. doi:10.1056/NEJM199109053251001
71. Abbott DS, Hezelgrave NL, Seed PT, et al. Quantitative fetal fibronectin to predict preterm birth in asymptomatic women at high risk. Obstet Gynecol. 2015;125(5):1168–1176. doi:10.1097/AOG.0000000000000754
72. Goldenberg R, Mercer B, Meis P, Copper R, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. Obstet Gynecol. 1996;87:643–648. doi:10.1016/0029-7844(96)00035-X
73. Abbott DS, Radford SK, Seed PT, Tribe RM, Shennan AH. Evaluation of a quantitative fetal fibronectin test for spontaneous preterm birth in symptomatic women. Am J Obstet Gynecol. 2013;208(2):122.e1–122.e6. doi:10.1016/j.ajog.2012.10.890
74. Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 1999;180(5):1169–1176. doi:10.1016/S0002-9378(99)70612-5
75. Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325(7359):301. doi:10.1136/bmj.325.7359.301
76. Peaceman AM, Andrews WW, Thorp JM, et al. Fetal fibronectin as a predictor of preterm birth in patients with symptoms: a multicenter trial. Am J Obstet Gynecol. 1997;177(1):13–18. doi:10.1016/S0002-9378(97)70431-9
77. Bruijn MMC, Kamphuis EI, Hoesli IM, et al. The predictive value of quantitative fibronectin testing in combination with cervical length measurement in symptomatic women. Am J Obstet Gynecol. 2016;215(6):793.e1–793.e8. doi:10.1016/j.ajog.2016.08.012
78. Kuhrt K, Hezelgrave N, Foster C, Seed PT, Shennan AH. Development and validation of a tool incorporating quantitative fetal fibronectin to predict spontaneous preterm birth in symptomatic women. Ultrasound Obstet Gynecol. 2016;47(2):210–216. doi:10.1002/uog.14894
79. Watson HA, Carter J, Seed PT, Tribe RM, Shennan AH. The QUiPP app: a safe alternative to a treat-all strategy for threatened preterm labor. Ultrasound Obstet Gynecol. 2017;50(3):342–346. doi:10.1002/uog.17499
80. Hologic I. Rapid FFN® 10Q cassette kit package insert; 2016. Available from: https://www.hologic.com/sites/default/files/package-insert/AW-09189-002_004_02.pdf.
81. Lukes AS, Thorp JM, Eucker B, Pahel-Short L. Predictors of positivity for fetal fibronectin in patients with symptoms of preterm labor. Am J Obstet Gynecol. 1997;176(3):639–641.d. doi:10.1016/S0002-9378(97)70561-1
82. Levy AT, Quist-Nelson J, Berghella V. The effect of transvaginal ultrasound, vaginal examination, or coitus on fetal fibronectin results: individual participant data from 6 cohort studies. Am J Obstet Gynecol. 2020;2(4):100170. doi:10.1016/j.ajogmf.2020.100170
83. Bruijn M, Hermans F, Vis J, et al. Which factors contribute to false-positive, false-negative, and invalid results in fetal fibronectin testing in women with symptoms of preterm labor? Am J Perinatol. 2016;34(03):234–239. doi:10.1055/s-0036-1585466
84. Hezelgrave NL, Kuhrt K, Cottam K, Seed PT, Tribe RM, Shennan AH. The effect of blood staining on cervicovaginal quantitative fetal fibronectin concentration and prediction of spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol. 2017;208:103–108. doi:10.1016/j.ejogrb.2016.11.027
85. Marleen S, Dias C, MacGregor R, et al. Biochemical predictors of preterm birth in twin pregnancies: a systematic review involving 6077 twin pregnancies. Eur J Obstet Gynecol Reprod Biol. 2020;250:130–142. doi:10.1016/j.ejogrb.2020.04.015
86. Kuhrt K, Hezelgrave‐Elliott N, Stock SJ, Tribe R, Seed PT, Shennan AH. Quantitative fetal fibronectin for prediction of preterm birth in asymptomatic twin pregnancy. Acta Obstet Gynecol Scand. 2020;99(9):1191–1197. doi:10.1111/aogs.13861
87. Watson HA, Seed PT, Carter J, et al. Development and validation of predictive models for QUiPP App v.2: tool for predicting preterm birth in asymptomatic high‐risk women. Ultrasound Obstet Gynecol. 2020;55(3):348–356. doi:10.1002/uog.20401
88. Dos Santos F, Daru J, Rogozińska E, Cooper NAM. Accuracy of fetal fibronectin for assessing preterm birth risk in asymptomatic pregnant women: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(6):657–667. doi:10.1111/aogs.13299
89. NHS England. Saving babies’ lives version three: a care bundle for reducing perinatal mortality; 2023. Available from: https://www.england.nhs.uk/long-read/saving-babies-lives-version-3/.
90. Whitworth M, Quenby S, Cockerill RO, Dowswell T. Specialised antenatal clinics for women with a pregnancy at high risk of preterm birth (excluding multiple pregnancy) to improve maternal and infant outcomes. Cochrane Database Syst Rev. 2011;9:CD006760.
91. Alexander S, Boulvain M, Ceysens G, Haelterman E, Zhang WH. Repeat digital cervical assessment in pregnancy for identifying women at risk of preterm labour. Cochrane Database Syst Rev. 2010;6:CD005940.
92. Care A, Ingleby L, Alfirevic Z, Sharp A. The influence of the introduction of national guidelines on preterm birth prevention practice: UK experience. BJOG. 2019;126(6):763–769. doi:10.1111/1471-0528.15549
93. Dawes L, Groom K, Jordan V, Waugh J. The use of specialised preterm birth clinics for women at high risk of spontaneous preterm birth: a systematic review. BMC Pregnancy Childbirth. 2020;20(1):58. doi:10.1186/s12884-020-2731-7
94. Min J, Watson HA, Hezelgrave NL, Seed PT, Shennan AH. Ability of a preterm surveillance clinic to triage risk of preterm birth: a prospective cohort study. Ultrasound Obstet Gynecol. 2016;48(1):38–42. doi:10.1002/uog.15925
95. Carter J, Seed PT, Watson HA, et al. Development and validation of predictive models for QUiPP app v.2: tool for predicting preterm birth in women with symptoms of threatened preterm labor. Ultrasound Obstet Gynecol. 2020;55(3):357–367. doi:10.1002/uog.20422
96. Carlisle N et al . (2020). CRAFT (Cerclage after full dilatation caesarean section): protocol of a mixed methods study investigating the role of previous in-labour caesarean section in preterm birth risk. BMC Pregnancy Childbirth, 20(1), 10.1186/s12884-020-03375-z
97. Watson HA, Carlisle N, Seed PT, et al. Evaluating the use of the QUiPP app and its impact on the management of threatened preterm labour: a cluster randomised trial. PLoS Med. 2021;18(7):e1003689. doi:10.1371/journal.pmed.1003689
98. melchor JC, Khalil A, Wing D, Schleussner E, Surbek D. Prediction of preterm delivery in symptomatic women using PAMG-1, fetal fibronectin and phIGFBP-1 tests: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52(4):442–451. doi:10.1002/uog.19119
99. Saade GR, Boggess KA, Sullivan SA, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214(5):633.e1–633.e24.
100. Markenson GR, Saade GR, Laurent LC, et al. Performance of a proteomic preterm delivery predictor in a large independent prospective cohort. Am J Obstet Gynecol. 2020;2(3):100140. doi:10.1016/j.ajogmf.2020.100140
101. Grabner M, Burchard J, Nguyen C, et al. Cost-effectiveness of a proteomic test for preterm birth prediction. Clinicoecon Outcomes Res. 2021;13:809–820. doi:10.2147/CEOR.S325094
102. Branch DW, VanBuren JM, Porter TF, et al. Prediction and prevention of preterm birth: a prospective, randomized intervention trial. Am J Perinatol. 2023;40(10):1071–1080. doi:10.1055/s-0041-1732339
103. Beger RD, Dunn W, Schmidt MA, et al. Metabolomics enables precision medicine: “a white paper, community perspective”. Metabolomics. 2016;12(9):149. doi:10.1007/s11306-016-1094-6
104. Gudnadottir U, Debelius JW, Du J, et al. The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci Rep. 2022;12(1):7926. doi:10.1038/s41598-022-12007-9
105. Kosti I, Lyalina S, Pollard KS, Butte AJ, Sirota M. Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front Microbiol. 2020;11:476. doi:10.3389/fmicb.2020.00476
106. Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16(1):9. doi:10.1186/s12916-017-0999-x
107. Kindschuh WF, Baldini F, Liu MC, et al. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat Microbiol. 2023;8(2):246–259. doi:10.1038/s41564-022-01293-8
108. Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23(12):1344–1359. doi:10.3109/14767058.2010.482618
109. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl 1(Suppl 1):4680–4687. doi:10.1073/pnas.1002611107
110. Schaaf JM, Liem SMS, Mol BWJ, Abu-Hanna A, Ravelli ACJ. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2013;30(6):433–450. doi:10.1055/s-0032-1326988
111. Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. Am J Obstet Gynecol. 2015;212(6):776.e1–776.e12. doi:10.1016/j.ajog.2015.03.052
112. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
113. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi:10.3402/jev.v4.27066
114. Chen K, Liang J, Qin T, Zhang Y, Chen X, Wang Z. The role of extracellular vesicles in embryo implantation. Front Endocrinol. 2022;13:809596. doi:10.3389/fendo.2022.809596
115. Morelli AE, Sadovsky Y. Extracellular vesicles and immune response during pregnancy: a balancing act*. Immunol Rev. 2022;308(1):105–122. doi:10.1111/imr.13074
116. Palomares KT, Parobchak N, Ithier MC, et al. Fetal exosomal platelet-activating factor triggers functional progesterone withdrawal in human placenta. Reprod Sci. 2021;28(1):252–262. doi:10.1007/s43032-020-00283-7
117. Cantonwine DE, Zhang Z, Rosenblatt K, et al. Evaluation of proteomic biomarkers associated with circulating microparticles as an effective means to stratify the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2016;214(5):631.e1–631.e11. doi:10.1016/j.ajog.2016.02.005
118. McElrath TF, Cantonwine DE, Jeyabalan A, et al. Circulating microparticle proteins obtained in the late first trimester predict spontaneous preterm birth at less than 35 weeks’ gestation: a panel validation with specific characterization by parity. Am J Obstet Gynecol. 2019;220(5):488.e1–488.e11. doi:10.1016/j.ajog.2019.01.220
119. Menon R, Debnath C, Lai A, et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a Longitudinal Study. Endocrinology. 2019;160(2):249–275. doi:10.1210/en.2018-00836
120. Menon R, Debnath C, Lai A, et al. Protein profile changes in circulating placental extracellular vesicles in term and preterm births: a Longitudinal Study. Endocrinology. 2020;161(4). doi:10.1210/endocr/bqaa009
121. Menon R, Dixon CL, Sheller-Miller S, et al. Quantitative proteomics by SWATH-MS of maternal plasma exosomes determine pathways associated with term and preterm birth. Endocrinology. 2019;160(3):639–650. doi:10.1210/en.2018-00820
122. Wang B, Zhang Y, Chen S, et al. Diagnostic accuracy of cervical elastography in predicting preterm delivery. Medicine. 2019;98(29):1.
123. Badir S, Bernardi L, Feijó Delgado F, Quack Loetscher K, Hebisch G, Hoesli I. Aspiration technique-based device is more reliable in cervical stiffness assessment than digital palpation. BMC Pregnancy Childbirth. 2020;20(1):391. doi:10.1186/s12884-020-03080-x
124. Parra-Saavedra M, Gómez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol. 2011;38(1):44–51. doi:10.1002/uog.9010
125. Pregnolia AG. Pregnolia system cervical stiffness assessment: instructions for use; 2022. Available from: https://en.pregnolia.com/_files/ugd/bb77a3_8d51603c300d45f3b638a74dedaffecf.pdf.
126. Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn. 2013;33(8):737–741. doi:10.1002/pd.4116
127. Drukker L, Noble J A, Papageorghiou A T. Introduction to artificial intelligence in ultrasound imaging in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2020;56(4): 498–505. doi:10.1002/uog.22122
128. Andrade Júnior V L, França MS, Santos RAF, et al. A new model based on artificial intelligence to screening preterm birth. The Journal of Maternal-Fetal & Neonatal Medicine. 2023;36(2): 2241100. doi:10.1080/14767058.2023.2241100
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
