Back to Journals » Journal of Pain Research » Volume 15
Preoperative Lymphocyte to Monocyte Ratio as a Predictive Biomarker for Disease Severity and Spinal Fusion Failure in Lumbar Degenerative Diseases Patients Undergoing Lumbar Fusion
Authors Guo Y, Zhao H, Lu J, Xu H, Hu T, Wu D
Received 28 June 2022
Accepted for publication 6 September 2022
Published 13 September 2022 Volume 2022:15 Pages 2879—2891
DOI https://doi.org/10.2147/JPR.S379453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Youfeng Guo, Haihong Zhao, Jiawei Lu, Haowei Xu, Tao Hu, Desheng Wu
Department of Spine Surgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, 200092, People’s Republic of China
Correspondence: Desheng Wu; Tao Hu, Email [email protected]; [email protected]
Objective: This study was designed to determine whether lymphocyte to monocyte ratio (LMR) correlated with the intervertebral disc degeneration (IDD) severity and the postoperative spinal fusion rate in patients with lumbar disc disease.
Methods: 303 patients undergoing posterior lumbar decompression and fusion were retrospectively analyzed. An examination of the blood count was performed before surgery. The cumulative grade was calculated by summing the pfirrmann grades of all lumbar discs. Grouping was based on the 50th percentile of cumulative grade and spinal fusion. The relationship between LMR and IDD severity and spinal fusion was explored using correlation analyses and logistic regression models. The receiver operating characteristic (ROC) curve was performed to measure model discrimination, and Hosmer-Lemeshow (H-L) test was used to measure calibration. Meanwhile, the ROC curve evaluated the discrimination ability of LMR in predicting severe degeneration and fusion failure.
Results: LMR was significantly lower in the severe degeneration group (cumulative grade > 18) than in the mild to moderate degeneration group (cumulative grade ≤ 18). Furthermore, the LMR of the fusion group was significantly higher than that of the non-fusion group. The multivariate binary logistic models revealed that LMR was an independently influencing factor of the severe degeneration and fusion failure (OR: 0.793, 95% CI: 0.638– 0.987, p = 0.038; OR: 0.371, 95% CI: 0.258– 0.532, p < 0.001). The models showed excellent discrimination and calibration. The area under the curve (AUC) of severe degeneration and fusion failure identified by LMR were 0.635 and 0.643, respectively, and the corresponding cut-off values were 3.16 and 3.90.
Conclusion: LMR is significantly associated with the risk of severe disc degeneration and spinal fusion failure.
Keywords: intervertebral disc degeneration, spinal fusion, lymphocyte to monocyte ratio, prognostic factor
Introduction
Lumbar disc degenerative (LDD) predisposes to cause secondary disorders such as lumbar disc herniation, lumbar spondylolisthesis, and lumbar spinal stenosis, the main cause of lower back pain and neuralgia. Intervertebral disc degeneration (IDD) is a complex and multifactorial process influenced by aging, lifestyle, high mechanical load, infection, obesity, and heredity.1–6 These initialization factors, such as annulus fibrosus rupture and nucleus pulposus protrusion, lead to morphological changes. With the rapid development of molecular biology and molecular immunology and the in-depth study of cytokines and inflammatory mediators, more and more attention has been paid to the role of cytokines and inflammatory mediators in IDD.7 Many studies have shown that degenerative disc tissue can produce potent inflammatory mediators such as nitric oxide, interleukin-1, and interleukin-6, and their concentration in degenerative intervertebral disc is increased.8,9 Although the mechanisms have not yet been elucidated, the role of these factors in disc degeneration is prominent. As we all know, inflammation is related to the occurrence and development of many diseases. It is characterized by the synergistic activation of various signal pathways and regulates the expression of proinflammatory and anti-inflammatory mediators of recruited leukocytes in tissue cells and blood.10 IDD is associated with increased proinflammatory cytokines secreted by infiltrating leukocytes (including macrophages, neutrophils, and T cells).11 Chronic inflammation induced by these proinflammatory cytokines will lead to irreversible structural and biochemical changes in the disc.12 In addition, the release of proinflammatory cytokines induces macrophage differentiation and activates lymphocytes to help mediate the inflammatory response and up-regulates the expression of degrading enzymes to decompose proteoglycan and collagen in the extracellular matrix, resulting in abnormal metabolism of nucleus pulposus cells.13
For patients with lumbar disc degeneration, conservative therapies, such as oral anti-inflammatory and analgesic drugs or physical therapy, are advocated for mild-to-moderate symptoms. However, some patients need surgical treatment because conservative treatments fail to alleviate the symptoms adequately. These surgical modalities include spinal fusion or non-fusion surgery. Many studies have confirmed that posterior lumbar fusion surgery has achieved good clinical results in treating lumbar degenerative diseases.14 However, clinical trials have found that some patients have fusion failure after spinal fusion surgery.15 At the same time, some studies have shown that the high inflammatory state of the fusion site will reduce the osteogenic ability, which is not conducive to spinal fusion.16,17 For example, the increased expression of IL-1 and IL-6 at the fusion site will reduce the spinal fusion rate.18 Moreover, Zhang et al found that M1 macrophages gathered locally and mediated inflammatory response, reducing the fusion rate after spinal fusion.19
Collectively, the inflammatory response is not only involved in the occurrence of disc degeneration but also affects the clinical efficacy after spinal fusion. Lymphocyte to monocyte ratio (LMR) has recently been reported as a potential new biomarker of the baseline inflammatory process in cerebrovascular disease.20 In previous studies, lymphocytes or monocytes were only used as predictors of infection after spinal fusion,21,22 which could not be associated with spinal fusion rate and disease severity. More importantly, studies have shown that the ratio of compound inflammatory cells may have a higher predictive ability than traditional inflammatory factors.23 Nevertheless, the relationship between lymphocyte to monocyte ratio and the severity of disc degeneration and postoperative clinical outcome is still uncertain and worth exploring.
This study aimed to investigate the association of LMR before lumbar fusion with the degeneration severity and postoperative fusion rate in patients with lumbar disc degeneration. At the same time, according to the previous literature, we hypothesize that low LMR is associated with severe disc degeneration and fusion failure.
Material and Methods
Study Design and Participants
In this study, 303 lumbar disc herniation or lumbar spinal stenosis patients with low back pain as the main complaint and planning lumbar fusion surgery were retrospectively analyzed between April 2019 and March 2020 in the department of spine surgery of our hospital. Blood samples were collected for routine examination at admission. The study was conducted in accordance with the Helsinki Declaration. The study was approved by the Shanghai East Hospital Ethics Committee and obtained the informed consent of the participants or their legal representatives.
Inclusion criteria: (1) degenerative disorders of the lower lumbar spine such as lumbar disc herniation and symptomatic lumbar spinal stenosis with severe lower back pain and radiculopathy; (2) the presence of nerve root stimulation (a positive straight leg elevation test) or neurological disorders (motor weakness, numbness in the lower extremities, or lack of corresponding reflexes); (3) MRI findings of single-level herniated discs or spinal stenosis were also required for all participants in agreement with their presentation; (4) patients who intend to receive single-level posterior lumbar decompression and fusion surgery.
Exclusion criteria: (1) previous spinal infections, injuries, or tumors; (2) multilevel disc lesions observed on MRI; (3) The corresponding disc segment has a previous history of spine surgery; (4) known history of chronic diseases of the lungs, kidneys, or liver; (5) known inflammatory status (eg, osteomyelitis, systemic lupus erythematosus, ankylosing spondylitis, rheumatoid arthritis, and others); (6) lost to follow-up or incomplete follow-up data.
Data Acquisition
Each participant completed a standard demographic assessment (such as age, sex, BMI, and current smoking) on the day of admission. The VAS (visual analog scale) score is used to assess lower back pain. A VAS score of 0 indicates no pain, and a VAS score of 10 indicates the most severe pain. According to the Pfirrmann grading system, lumbar disc levels were graded from 1 to 5 based on T2-weighted mid-sagittal MRI images. Pfirrmann grades were combined for all lumbar discs to calculate the cumulative grade. If there are discrepancies, the third spine surgeon will be consulted, as needed, to resolve them. In the routine clinical diagnosis and treatment process, not every patient will receive a bone mineral density examination. Therefore, the diagnosis of osteoporosis was measured by lumbar CT value (L1 CT value ≤ 110 HU) as described in prior literature.24,25
The decompression and fusion surgery was performed on the patients using a conventional posterior surgical method. All resected discs were found to be responsible during intraoperative fluoroscopy prior to removal. A record of the length of hospitalization was kept. Follow-up radiography was prescribed for the patients after discharge. The imaging system collected lumbar CT data from patients two years after spinal fusion surgery to assess the spinal fusion rate. The included population was divided into fusion and non-fusion groups according to whether the spinal column of the surgical segment was fused during the follow-up period. Spinal fusion was evaluated by an experienced radiologist without prior knowledge of clinical information through CT images according to the evaluation system proposed by Siepe.26
Measurement of LMR from Blood Cell Counts
Serum samples were collected from patients before the surgical procedure. The monocytes and lymphocyte counts were retrieved from patients’ medical records. LMR was calculated by dividing the lymphocyte counts by the monocyte counts.
Statistical Analysis
The statistical analysis was performed with SPSS software. Participants were divided into two groups according to their median cumulative grade (high score and low score groups). We define a group with a cumulative grade of less than or equal to 18 as the low score group and one with a higher grade of 18 as the high score group. We also divided the subjects into fusion and non-fusion groups based on the presence or absence of fusion. Shapiro–Wilk normality test was used to evaluate the normality of data prior to performing statistical tests. Normal data are described as mean values ± SD and non-normal data as median [interquartile range]. Categorical variables were presented as n (%). Comparisons between groups were made using the one-way ANOVA test, the t-test (for normally distributed data), or the Mann–Whitney U-test (for non-normally distributed data) and the chi-square test or Fisher exact test (for categorical variables). Multiple testing was corrected by the Bonferroni correction. Correlation analyses were performed on the Kendall test for categorical variables and the Spearman test for continuous variables. The risk factors for severe disc degeneration and spinal fusion were also identified using logistic regression analysis. Eight multivariable logistic regression models were obtained for different indicators (including lymphocyte, monocyte, combination index, or LMR), with a binary indicator of severe degeneration/fusion failure as the dependent variable and clinical characteristics as the candidate independent variables. Hosmer-Lemeshow goodness of fit test was used to assess the model fit (Hosmer-Lemeshow statistic ≥ 0.05). LMR was tested to discriminate between severe degeneration and spinal fusion using receiver operating characteristic (ROC) curves. Multiple ROC curves were compared using the DeLong test. Youden is defined as the sum of specificity + sensitivity - 1. Significant values were two-tailed with a p-value of less than 0.05.
Results
The Baseline Characteristics of Patients
The baseline characteristics of study participants are shown in Table 1. The results of the normality tests are shown in Supplementary Table S1. There were 175 women (57.8%) among the patients with an average age of 63.7. The mean BMI of the patients was 24.83 kg/m2. 202 patients (66.7%) achieved successful spinal fusion during follow-up. There were significant differences in the following factors: age (p < 0.001), erythrocyte sedimentation rate (ESR) (p = 0.004), lymphocyte counts (p = 0.005), lumbar CT value (p < 0.001), LMR (p < 0.001) and the prevalence of hypertension (p = 0.001) and osteoporosis (p = 0.021) between the high score group (cumulative grade > 18) and the low score group (cumulative grade ≤ 18). No significant differences were observed among the two groups regarding gender distribution, smoking history, BMI, VAS, the length of hospital stay, postoperative fusion rate, or hematological indicators other than lymphocyte count.
 |
Table 1 Demographic Characteristics of Patients with Disc Degeneration Disease |
The Distribution of Disc Grades in the Target Population and Correlation Analysis
Table 2 illustrates the distribution of disc grades among the target population. Among the degeneration grades of L1/2, L2/3 and L3/4 of all the included population, the vast majority were 2, 3 and 3, accounting for 44.6%, 31.0%, and 30.0% respectively. For L4/5 and L5/S1, however, the majority (35.6% and 45.2%) were equal to or greater than 4. At the same time, the low score group showed the same trend as the whole population. On the other hand, all intervertebral discs except L1/2 scored more than or equal to 4 in the high score group.
 |
Table 2 The Pfirrmann Grading System for Lumbar Disc Degeneration |
Average pfirrmann grades < 4 indicate mild to moderate degeneration, and grades ≥ 4 indicate severe degeneration in terms of an individual disc. As shown in Table 3, the mean levels of LMR were substantially lower in the severe degeneration group (pfirrmann grade ≥ 4) compared with the mild to moderate degeneration group (pfirrmann grade < 4) in all lumbar discs except L4/5 and L5/S1. In addition, correlation analysis showed that LMR was significantly correlated with age (p < 0.001), gender (p < 0.001), neutrophil count (p < 0.001), hemoglobin (p = 0.007), hypertension (p = 0.015), and the length of hospital stay (p = 0.037) in all demographic and clinical parameters in Table 4. It is worth mentioning that there is a borderline positive correlation between LMR and serum albumin (p = 0.053). The LMR did not show any significant correlation with VAS.
 |
Table 3 The Relationship Between the Severity of Individual Disc Degeneration and LMR |
 |
Table 4 Correlation of LMR with Demographic and Clinical Parameters |
Analysis of Risk Factors Associated with Severe Degeneration
The correlation analysis revealed that cumulative grade was correlated with LMR (r = −0.231, p < 0.001). The ROC curve was then generated to determine whether LMR can predict disc degeneration severity. The area under the curve (AUC) was found to be 0.635. Furthermore, as indicated by its larger area under the ROC curve than lymphocytes (AUC = 0.593) or monocytes (AUC = 0.535), LMR has a superior capability of detecting severe disc degeneration in Figure 1A. The Youden index reaches its maximum value when the LMR cut-off is set to 3.16. This corresponds to a sensitivity of 0.77 and a specificity of 0.49. Univariable binary logistic regression analysis showed that each additional unit of age (p < 0.001), hypertension (p = 0.001), osteoporosis (p = 0.021), ESR (p = 0.003), and LMR (p = 0.001) were significantly associated with severe degeneration (Table 5). Multivariable binary logistic regression in model 1 built on clinical parameters further demonstrated that every one unit increase in age (OR: 1.081; 95% CI: 1.044–1.119; p < 0.001), LMR (OR: 0.793; 95% CI: 0.638–0.987; p = 0.038), and the occurrence of CHD (OR: 0.199; 95% CI: 0.078–0.506; p = 0.001) were determined to be independent predictors of severe degeneration. Moreover, LMR did not interact significantly with age or CHD in the one-way ANOVA. Lymphocytes and monocytes were not predictive of severe degeneration in models 2, 3, and 4 (Supplementary Table S2). The model 1 was significant, p = 0.491 for Hosmer and Lemeshow goodness of fit test. At the same time, the observed vs predicted risk of severe disc degeneration in model 1 within risk deciles showed a good fit (Figure 2A). The area under the ROC curve of model 1 is 0.776 (Figure 2C). Therefore, model 1 has effective calibration and discrimination (p > 0.05 and p < 0.05; Figure 2A and C). The Delong test of the area under the curve shows that the test efficiency of model 1 is not significantly different from models 2 (AUC = 0.768), 3 (AUC = 0.768), and 4 (AUC = 0.772) in Supplementary Figure S1A.
 |
Table 5 Univariate and Multivariate Analysis Model 1 of Risk Factors for Severe Degeneration |
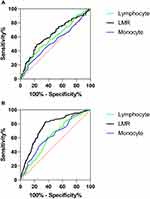 |
Figure 1 Receiver operating characteristic (ROC) curve to determine the predictive performance of lymphocyte, monocyte, and LMR for severe degeneration (A) and spinal fusion (B). |
 |
Figure 2 Actual versus predicted severe degeneration (A) and spinal fusion failure (B) by risk deciles for models 1 and 5 (see Tables 5 and 6 for included variables). ROC curve analysis of prognostic models 1 (C) and 5 (D). |
Analysis of Risk Factors Associated with Spinal Fusion Failure
A correlation analysis revealed a correlation between LMR and spinal fusion failure (t = −0.477, p < 0.001). Meanwhile, the level of LMR in the fusion group was much higher than that in the non-fusion group (p < 0.05). ROC curves were employed to determine the value of preoperative LMR in predicting spinal fusion in Figure 1B. It turns out that the area under the curve for preoperative LMR is 0.792. Moreover, the AUC of LMR is larger than that of lymphocytes (AUC = 0.635; p < 0.001) and monocytes (AUC = 0.654; p < 0.001), indicating its superior ability to predict fusion rate. A cut-off value of 3.90 in LMR raises the Youden index to its maximum value. Specificity was 0.92, while sensitivity was 0.64. Therefore, LMR > 3.90 is associated with mild to moderate degeneration as well as a greater likelihood of spinal fusion. There was a higher rate of disc degeneration and poor spinal fusion rate in patients with LMR < 3.16. Patients with LMR greater than 3.16 but less than 3.90 also had mild to moderate degeneration but poor fusion. Univariable binary logistic regression analysis revealed that each additional unit of age (p = 0.006), gender (male) (p = 0.003), alcohol abuse (p = 0.043), and LMR (p < 0.001) were significantly associated with postoperative spinal fusion failure, as shown in Table 6. After adjustment by all covariable estimates, multivariate binary logistic regression analysis showed that for every unit increase in LMR (OR: 0.371; 95% CI: 0.258–0.532; p < 0.001) and alcohol abuse (OR: 3.835; 95% CI: 1.066–13.792; p < 0.040) could be independent prognostic factors for spinal fusion failure in patients with lumbar disease undergoing lumbar fusion surgery. At the time, the one-way ANOVA showed no significant interactions between LMR and alcohol abuse. In models 6, 7, and 8, monocytes and lymphocytes may also be independent predictors of spinal fusion failure (Supplementary Table S3). The model 5 was significant, p = 0.811 for Hosmer and Lemeshow goodness of fit test. At the same time, the observed vs predicted risk of fusion failure in model 5 within risk deciles showed a good fit (Figure 2B). The area under the ROC curve is 0.825 (Figure 2D). Therefore, model 5 is also capable of calibration and discrimination (p > 0.05 and p < 0.05; Figure 2B and D). The Delong test of the area under the curve reveals that model 5ʹs test efficiency is much higher than models 6 (AUC = 0.766; p = 0.004) and 7 (AUC = 0.752; p = 0.003) in Supplementary Figure S1B. However, there is no significant difference between models 5 and 8 (AUC = 0.816; p = 0.185).
 |
Table 6 Univariate and Multivariate Analysis Model 5 of Risk Factors for Spinal Fusion Failure |
Discussion
In the current study, lower LMR was associated with more severe disc degeneration in patients undergoing spinal fusion surgery for lumbar spine diseases. Further, the fusion rate after spinal fusion surgery is significantly affected by LMR. According to multivariate analysis, a low LMR was an independent risk factor for fusion failure and severe disc degeneration.
A pro-inflammatory state is thought to be the cause of disc degeneration, accelerating the process further. The secretion of cytokines also recruits immune cells, causes an inflammatory cascade, and aggravates degeneration.27 Kim et al also found that the level of inflammatory factors was associated with discogenic low back pain.28 Furthermore, systemic inflammation can also affect the therapeutic effect and postoperative clinical outcomes of patients with lumbar spine diseases. Studies have shown that inflammation at the fusion site inhibits osteogenesis and does not facilitate spinal fusion.29
Research has recently focused on the importance of leukocyte proportions in various inflammatory conditions, including cancer, diabetes, and their complications.30 The inflammatory indexes, ie, platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and LMR, are calculated based on the neutrophil, lymphocyte, monocyte, and platelet counts that are routinely tested in most clinical settings worldwide. Lymphocytes and monocytes are also key immune cells in the inflammatory response. A low lymphocyte count and a high monocyte count may be relevant to inflammation and oxidative stress, associated independently with the prognosis of various diseases.31–34 For example, the most frequent abnormal blood tests among cancer patients were high inflammatory markers (C-reactive protein (CRP) or ESR), high monocyte count, low lymphocyte count, hypo-albuminaemia, and high alkaline phosphatase.35 The multivariate regression models of this study indicate that high monocyte count and low lymphocyte count are independent risk factors for fusion failure (Supplementary Table S2). Our findings echo with reports in the literature. The lymphocyte-to-monocyte ratio (LMR) is another inflammatory marker, reflecting the balance of change between innate and adaptive immunity and offering a simple indicator of immune status and inflammation level. Therefore, the potential of LMR as a prognostic biomarker is demonstrated by high lymphocyte count and low monocyte count. LMRs that are lower are associated with higher levels of systemic inflammation and vice versa.36 To our knowledge, this may be the first study to examine the relationship between preoperative LMR and the degree of intervertebral disc degeneration and spinal fusion rate. In the current study, LMR correlated independently with the degree of degeneration. Furthermore, patients with severe disc degeneration have a lower LMR (ie, a higher systemic inflammatory state) in comparison to people with mild to moderate disc degeneration. A possible explanation for our results is that as a composite indicator of the state of the systemic inflammatory response, low LMR means that high levels of local chronic inflammation and production of inflammatory factors may promote the progression of disc degeneration. Meanwhile, in model 1, LMR was a highly reliable predictor of severe degeneration, and the Hosmer-Lemeshow test and ROC curve of the prognostic model indicated that the model has effective discrimination and calibration. Additionally, the ROC curve of LMR prediction of severe degeneration showed that patients with LMR < 3.16 had severer degeneration. On the other hand, in this study, we also found that LMR is associated with the prognosis of spinal fusion, ie, those with higher LMR have a higher chance of fusion. It is possible that low levels of LMR (high inflammatory state) inhibit osteogenic differentiation and new bone formation, thereby slowing the fusion rate. Furthermore, as previously mentioned, LMR may significantly affect spinal fusion rate through the protective effect of high lymphocyte and low monocyte levels. Multivariate regression model 5 demonstrated that a high level of LMR protects against spinal fusion failure, and the model also had acceptable calibration and discrimination. Monocyte count is also a risk factor for spinal fusion failure in other regression models, but lymphocyte count is a protective factor. This may be related to the immune response regulated by the monocyte-macrophage system as described above. At the same time, the ROC curve of LMR prediction of spinal fusion failure showed that patients with LMR > 3.90 had a higher rate of spinal fusion. LMR > 3.90 is associated with mild to moderate degeneration as well as a greater likelihood of spinal fusion. There was a higher rate of disc degeneration and poor spinal fusion rate in patients with LMR < 3.16. Patients with LMR greater than 3.16 but less than 3.90 also had mild to moderate degeneration but poor fusion. Further, we should note that although the AUC of LMR in this study did not reach more than 0.7 in predicting severe disc degeneration and fusion failure, correlation analysis and multivariate regression analysis have shown that low LMR is significantly associated with severe disc degeneration and fusion failure. It is clinically significant to a certain extent that this correlation exists. The identification of risk factors for intervertebral disc degeneration and spinal fusion failure that are related to their disease progression and their long-term prognosis will allow patients to avoid or reduce the possibility of bearing a substantial economic burden.
The results also showed that the severity of disc degeneration was not significantly correlated with low back pain and length of hospital stay. Many patients with lumbar disc degeneration do not experience obvious symptoms during routine clinical diagnosis and treatment. Similarly, although LMR can reflect inflammatory status in vivo, it does not significantly affect low back pain. Despite a negative correlation trend between LMR and VAS, no significant statistical significance is found in this study, which may be related to the small sample size. Certainly, there are other demographic and clinical parameters except for LMR that may affect severe degeneration and spinal fusion failure. Study results have shown that intervertebral disc degeneration increases with age in terms of clinical and demographic parameters.37 In accordance with previous research, correlation analysis and multiple logistic regression have revealed that age is an independent risk factor for severe disc degeneration. In addition, we found that CHD was an independent predictor of severe degeneration in all logistic regression models. It may be explained by the significant correlation between coronary atherosclerotic heart disease and serum calcium levels.38 The serum calcium level was negatively correlated with disc degeneration and could be used as an indicator of disc degeneration’s prognosis, according to Zhao et al.39 Therefore, coronary heart disease may delay disc degeneration through this mechanism. On the other hand, there was no significant association between age and spinal fusion. It is also noteworthy that we found alcohol abuse to affect the success of spinal fusion. This may be related to alcohol inhibiting osteogenic differentiation and secondary osteopenia.40 Other clinical and demographic parameters did not significantly affect the degeneration severity and the prognosis of spinal fusion.
There are some challenges and limitations to the current study. It remains to be seen whether current preoperative LMR cut-off values in other cases are practicable. Secondly, we need to admit that the area under the ROC curve of the established model is not particularly ideal, which is one of the limitations of this study. This may relate to the small sample size. Thirdly, LMR may also vary considerably during hospitalization and follow-up depending on age, diet, and other factors.41,42 This study revealed a borderline positive correlation (p = 0.053) between LMR and albumin, an indicator of nutritional status. Therefore, our future research should be able to detect LMR dynamically rather than just preoperatively. The sample size could not be calculated because there were never previous studies on the LMR and the fusion rate postoperatively. More studies are needed to test our findings to determine whether they are statistically significant and whether the difference is clinically important. Moreover, it is meaningful and beneficial for future research to establish an independent validation queue. While we cannot completely overcome the aforementioned limitations, a single LMR preoperatively has proven to be a reliable predictor for other diseases, making our results credible.
Conclusions
The findings of our study indicate that preoperative LMR is associated with the severity of disc degeneration. The LMR is also correlated with the fusion rate after spinal fusion surgery.
Data Sharing Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Shanghai East Hospital Ethics Committee and obtained the informed consent of the participants or their legal representatives. At the same time, this study conforms to the principles outlined in the Declaration of Helsinki.
Author Contributions
Youfeng Guo: conceptualization, methodology, material preparation, data collection, analysis and original draft preparation. Haihong Zhao, Jiawei Lu, and Haowei Xu: investigation, data collection, and visualization. Desheng Wu and Tao Hu: supervision and writing-reviewing and editing. All authors contributed to data analysis, drafting or revising the article, have agreed on this journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Shanghai East Hospital Xuri Young Excellent Talents Program 2019xrrcjh04 and Key Laboratory of Inorganic Coating Materials, Chinese Academy of Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. doi:10.1016/j.arr.2019.100978
2. Vieira LA, Dos Santos AA, Peluso C, Barbosa CP, Bianco B, Rodrigues LMR. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: a case-control study. Eur J Med Res. 2018;23(1):11. doi:10.1186/s40001-018-0309-x
3. Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011;19(8):1011–1018. doi:10.1016/j.joca.2011.04.005
4. Granville Smith I, Danckert NP, Freidin MB, Wells P, Marchesi JR, Williams FMK. Evidence for infection in intervertebral disc degeneration: a systematic review. Eur Spine J. 2022;31(2):414–430. doi:10.1007/s00586-021-07062-1
5. Curic G. Intervertebral disc and adipokine leptin-loves me, loves me not. Int J Mol Sci. 2020;22(1):375. doi:10.3390/ijms22010375
6. Chang H, Yang X, You K, et al. Integrating multiple microarray dataset analysis and machine learning methods to reveal the key genes and regulatory mechanisms underlying human intervertebral disc degeneration. PeerJ. 2020;8:e10120. doi:10.7717/peerj.10120
7. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi:10.1038/nrrheum.2013.160
8. Zhang C, Gullbrand SE, Schaer TP, et al. Inflammatory cytokine and catabolic enzyme expression in a goat model of intervertebral disc degeneration. J Orthop Res. 2020;38(11):2521–2531. doi:10.1002/jor.24639
9. Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging. 2019;11(22):10499–10512. doi:10.18632/aging.102472
10. Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91(2):207–215. doi:10.1189/jlb.0811402
11. Xiao L, Ding M, Zhang Y, et al. A novel modality for functional imaging in acute intervertebral disk herniation via tracking leukocyte infiltration. Mol Imaging Biol. 2017;19(5):703–713. doi:10.1007/s11307-016-1038-6
12. Ruiz-Fernández C, Francisco V, Pino J, et al. Molecular relationships among obesity, inflammation and intervertebral disc degeneration: are adipokines the common link? Int J Mol Sci. 2019;20(8):2030. doi:10.3390/ijms20082030
13. Li XC, Luo SJ, Fan W, Zhou TL, Huang CM, Wang MS. M2 macrophage-conditioned medium inhibits intervertebral disc degeneration in a tumor necrosis factor-α-rich environment. J Orthop Res. 2022. doi:10.1002/jor.25292
14. Tokuhashi Y, Ajiro Y, Umezawa N. Follow-up of patients with delayed union after posterior fusion with pedicle screw fixation. Spine. 2008;33(7):786–791. doi:10.1097/BRS.0b013e31816956f7
15. Lee C, Dorcil J, Radomisli TE. Nonunion of the spine: a review. Clin Orthop Relat Res. 2004;419(419):71–75. doi:10.1097/00003086-200402000-00012
16. NaPier Z, Kanim LEA, Nelson TJ, et al. The effect of insulin dependent diabetes on bone metabolism and growth after spinal fusion. Spine J. 2020;20(5):800–808. doi:10.1016/j.spinee.2019.11.011
17. Hu T, Abbah SA, Toh SY, et al. Bone marrow-derived mesenchymal stem cells assembled with low-dose BMP-2 in a three-dimensional hybrid construct enhances posterolateral spinal fusion in syngeneic rats. Spine J. 2015;15(12):2552–2563. doi:10.1016/j.spinee.2015.08.063
18. Koerner JD, Markova DZ, Schroeder GD, et al. The local cytokine and growth factor response to recombinant human bone morphogenetic protein-2 (rhBMP-2) after spinal fusion. Spine J. 2018;18(8):1424–1433. doi:10.1016/j.spinee.2018.03.006
19. Zhang ZC, Yang YL, Li B, et al. Low-intensity pulsed ultrasound promotes spinal fusion by regulating macrophage polarization. Biomed Pharmacother. 2019;120:109499. doi:10.1016/j.biopha.2019.109499
20. Lux D, Alakbarzade V, Bridge L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17(1):60. doi:10.1186/s12974-020-01739-y
21. Iwata E, Shigematsu H, Koizumi M, et al. Lymphocyte count at 4 days postoperatively and CRP level at 7 days postoperatively: reliable and useful markers for surgical site infection following instrumented spinal fusion. Spine. 2016;41(14):1173–1178. doi:10.1097/BRS.0000000000001501
22. Yamamoto Y, Iwata E, Shigematsu H, et al. Comparison of neutrophil and lymphocyte at 1 and 4 days postoperatively: reliable and early detection markers for surgical site infection following instrumented spinal fusion. Spine Surg Relat Res. 2018;2(2):127–134. doi:10.22603/ssrr.2017-0052
23. Curbelo J, Luquero Bueno S, Galván-Román JM, et al. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS One. 2017;12(3):e0173947. doi:10.1371/journal.pone.0173947
24. Muraki S, Yamamoto S, Ishibashi H, et al. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int. 2004;15(9):724–728. doi:10.1007/s00198-004-1600-y
25. Zou D, Li W, Deng C, Du G, Xu N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur Spine J. 2019;28(8):1758–1766. doi:10.1007/s00586-018-5776-9
26. Siepe CJ, Stosch-Wiechert K, Heider F, et al. Anterior stand-alone fusion revisited: a prospective clinical, X-ray and CT investigation. Eur Spine J. 2015;24(4):838–851. doi:10.1007/s00586-014-3642-y
27. Schlundt C, Schell H, Goodman SB, Vunjak-Novakovic G, Duda GN, Schmidt-Bleek K. Immune modulation as a therapeutic strategy in bone regeneration. J Exp Orthop. 2015;2(1):1. doi:10.1186/s40634-014-0017-6
28. Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine. 2008;33(21):2253–2259. doi:10.1097/BRS.0b013e318182c35f
29. Bedair TM, Lee CK, Kim DS, et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J Tissue Eng. 2020;11:2041731420967591. doi:10.1177/2041731420967591
30. Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis. Clin Chim Acta. 2018;481:142–146. doi:10.1016/j.cca.2018.03.008
31. Chen J, Zhong Z, Shi D, et al. Association between monocyte count to high-density lipoprotein cholesterol ratio and mortality in patients undergoing peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2021;31(7):2081–2088. doi:10.1016/j.numecd.2021.03.014
32. Meikle PJ, Formosa MF, Mellett NA, et al. HDL phospholipids, but not cholesterol distinguish acute coronary syndrome from stable coronary artery disease. J Am Heart Assoc. 2019;8(11):e011792. doi:10.1161/JAHA.118.011792
33. Acanfora D, Scicchitano P, Carone M, et al. Relative lymphocyte count as an indicator of 3-year mortality in elderly people with severe COPD. BMC Pulm Med. 2018;18(1):116. doi:10.1186/s12890-018-0685-6
34. Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–3233. doi:10.2174/092986711796391633
35. Næser E, Møller H, Fredberg U, Frystyk J, Vedsted P. Routine blood tests and probability of cancer in patients referred with non-specific serious symptoms: a cohort study. BMC Cancer. 2017;17(1):817. doi:10.1186/s12885-017-3845-9
36. Ren H, Liu X, Wang L, Gao Y. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(11):2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
37. Ohnishi T, Sudo H, Tsujimoto T, Iwasaki N. Age-related spontaneous lumbar intervertebral disc degeneration in a mouse model. J Orthop Res. 2018;36(1):224–232. doi:10.1002/jor.23634
38. Shin S, Kim KJ, Chang HJ, et al. Impact of serum calcium and phosphate on coronary atherosclerosis detected by cardiac computed tomography. Eur Heart J. 2012;33(22):2873–2881. doi:10.1093/eurheartj/ehs152
39. Zhao B, Wang K, Zhao J, Luo Y. Serum calcium concentration as an indicator of intervertebral disk degeneration prognosis. Biol Trace Elem Res. 2013;154(3):333–337. doi:10.1007/s12011-013-9747-z
40. Chen Y, Yu H, Zhu D, et al. miR-136-3p targets PTEN to regulate vascularization and bone formation and ameliorates alcohol-induced osteopenia. FASEB j. 2020;34(4):5348–5362. doi:10.1096/fj.201902463RR
41. Koh YW, Park CS, Yoon DH, Suh C, Huh J. Should the cut-off values of the lymphocyte to monocyte ratio for prediction of prognosis in diffuse large B-cell lymphoma be changed in elderly patients? Eur J Haematol. 2014;93(4):340–348. doi:10.1111/ejh.12354
42. Raguzzini A, Poce G, Consalvi S, et al. Chocolate consumers and lymphocyte-to-monocyte ratio: a working hypothesis from a preliminary report of a pilot study in celiac subjects. Antioxidants. 2019;8(10):440. doi:10.3390/antiox8100440
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
