Back to Journals » Cancer Management and Research » Volume 14
Preoperative Free Ferrous Protoporphyrin and Reactive Oxygen Species Status of Voided Urine Predicts Potential Recurrence Risk in NMIBC
Authors Li S , Chen Z , Chen R , Xue N , Shen X, Zhu H , Peng Y
Received 2 May 2022
Accepted for publication 22 July 2022
Published 3 August 2022 Volume 2022:14 Pages 2291—2297
DOI https://doi.org/10.2147/CMAR.S371974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shuaishuai Li,1 Zeyu Chen,1 Rui Chen,1 Ning Xue,1 Xihao Shen,2 Haitao Zhu,1 Yunpeng Peng1
1Department of Urology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China; 2The First Clinical Medical College of Nanjing Medical University, NanJing, People’s Republic of China
Correspondence: Haitao Zhu; Yunpeng Peng, Department of Urology, The Affiliated Hospital of Xuzhou Medical University, No. 99 Huaihai West Road, Quanshan District, Xuzhou, 221100, People’s Republic of China, Tel +8615055521680 ; +8617826444501, Email [email protected]; [email protected]
Purpose: This study aimed to assess the relationship between the preoperative reactive oxygen species and free ferrous protoporphyrin (ROS and FH) combined test and the risk of recurrence in a pathologically confirmed non-muscular invasive bladder cancer (NMIBC) patients.
Patients and Methods: The retrospective study included 218 patients, newly diagnosed with NMIBC between January 2019 and February 2022. According to the results of FH and ROS combined test of voided urine, all patients were classified as FH(-)/ROS(-), FH(+)/ROS(-), or FH(+) /ROS(+). We reviewed demographic information, pathological results, and the FH and ROS combined test status. The clinicopathological characteristics were evaluated, and the survival rates of each group were compared. Finally, we also analyzed the association between preoperative free ferrous protoporphyrin and reactive oxygen species status and the tumor stage and grade.
Results: This study included 218 NMIBC patients with a median age of 68 years (interquartile range [IQR] 60– 76 years). The number and proportion of patients in FH(-)/ROS(-), FH(+)/ROS(-) and FH(+) /ROS(+) were 95(43.6%), 79(36.2%) and 44(20.2%), respectively. And the pathological stages for those with FH(+) and ROS(+), FH(+) and ROS(-), FH(-) and ROS(-) at diagnosis were 0.5% Tis, 6.4% Ta, 13.3% T1; 2.3% Tis, 20.6% Ta, 13.3% T1; 5.5% Tis, 28.9% Ta, 9.2% T1, respectively. After adjusting for clinical factors, including tumor grade, tumor stage and FH/ROS status were independent risk factors for RFS In the multivariate Cox regression analysis. Through logistics regression analysis, FH(+)/ROS(+) were found to be corelated with high grade and more high stage (T1). Kaplan–Meier analysis showed that 1-year RFS of FH(+)/ROS(+), FH(+)/ROS(-) and FH(-)/ROS(-) were 46.0%, 87.8% and 93.4%, respectively (P=0.000).
Conclusion: In newly diagnosed NMIBC patients, the status of FH(+)/ROS(+) has an association with a higher risk in recurrence. Furthermore, FH(+)/ROS(+) at diagnosis was correlated with high grade and higher stage (T1). Hence, the FH/ROS combined test can help specify treatment options for patients diagnosed with NMIBC.
Keywords: non-muscular invasive bladder cancer, free ferrous protoporphyrin, reactive oxygen species, recurrence-free survival, stage, grade
Introduction
Bladder cancer (BC) ranks as the ninth most common cancer worldwide, estimated 500,000 new cases and 200 000 deaths worldwide, and in the US alone there are more than 80,000 new cases and 17,000 deaths each year.1–3 BC is a carcinoma of the urothelial, or “umbrella”, cells that line the lumen of the urinary bladder. Histologically, BC comprises 75% pure urothelial carcinoma and 25% “variant” histology, adding complexity to the management of this disease.4 BC can be broadly divided into non-invasive muscular bladder cancer (NMIBC) and invasive muscular bladder cancer (MIBC). On average, NMIBC represents approximately 70% of BC.5 Among the NMIBC, approximately 70% present as Ta lesions, 20% as T1 lesions, and 10% as carcinoma in situ (CIS, or Tis lesions).6 NMIBC is a heterogeneous disease with high rate of recurrence and progression.7 The AUA stratified patients with NMIBC into low-, intermediate-, and high-risk categories. Although survival is favorable, patients with low- and intermediate-risk NMIBC experience 5-year recurrence-free survival rates of 43% and 33%, respectively, and up to 21% with high-risk diseases will progress to MIBC.8,9 These patients are often monitored by cystoscopy and multiple transurethral resection of bladder tumor (TURBT) over many years.
Thus, non-invasive urine analysis can improve monitoring, reducing the morbidity and costs associated with cystoscopy and multiple TURBT. Chinese researchers have developed reactive oxygen species and free ferrous protoporphyrin (ROS and FH) combined detection kits based on the characteristics of ROS and FH. You et al demonstrated high sensitivity and specificity of this detection method, which has been employed as a new detection method for cervical cancer screening and delivered good results.10 In a recent study, Zhao et al proposed that FH(+) and ROS(-) at diagnosis predict a higher pathological stage in patients with Urothelial Carcinoma.11 Therefore, the potential of ROS and FH expression status in predicting survival in patients with NMIBC was further explored in our study, whether combining ROS and FH test add value to BC diagnosis.
Patients and Methods
In this single-center study, we retrospectively included 218 patients diagnosed with NMIBC between January 2019 and February 2022. The inclusion criteria were 1. Patients presented for the first time with hematuria or urinary tract irritation symptoms; 2. Patients who underwent transurethral resection of bladder tumor (TURBT); 3. Patients with NMIBC confirmed by postoperative pathological results. The exclusion criteria were: 1. Patients with a history of malignancy; 2. Patients with a prior history of radiotherapy and chemotherapy; 3. The pathological results were non-TCC (transitional cell carcinoma). The median age of all patients was 68 years ([IQR] 60–76 years). We obtained informed consent from all the patients. Clinical and pathological information (including sex, age, smoking history, T stage, size of the tumor and status of the FH and ROS at initial diagnosis) were collected from the medical records to assess their influence on the recurrence. In this study, all procedures involving human participants were performed following the ethical standards of the institutional and national research committee and complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This institutional review board of the Affiliated Hospital of Xuzhou Medical University approved this study.
After the malignant transformation of urinary tract epithelial cells, Intracellular FH and ROS levels increased. The test materials used by us are composed of reagents (including ROS reagent, FH reagent, sample preservation solution, color developing agent, sampling swab, dropper, test result recording sheet) and analyzer (epithelial CELL ROS+FH automatic staining analyzer). The kit produced color changes by combining the dye solution with FH and ROS in the sample for a specific reaction, and the result was obtained through a colorimetric card. After an adequate reaction, the analyzer can automatically recognize the color and give the final result within 3 minutes. Then, we divided all patients into three groups according to the test results: FH(+)/ROS(+), FH(+)/ROS(-), and FH(-)/ ROS(-). No patient presented with FH(-) and ROS(+) due to the sensitivity of FH being higher than that of ROS. A highly experienced urological pathologist with 15 years of clinical experience was invited to reexamine the original diagnoses of all pathological specimens. The tumor stage and grade were evaluated according to the UICC 2009 TNM classification of malignant tumors and the WHO classification 2004 grading system.
Statistical Analysis
The continuous variables were compared by Kruskal-Wallis test or one-way ANOVA test. The 1-year recurrence-free survival (RFS) was estimated by Kaplan-Meier survival curve analysis, and the differences among the three groups were compared by log-rank test. RFS was defined as from the date of surgery to the date of recurrence of BC. We analyzed the independent prognostic factors of RFS using univariate and multivariate Cox proportional hazards models. Logistics regression analysis was used to analyze the correlation between FH/ROS status and the tumor’s high grade and more advanced stage (T1). P<0.05 represents statistical significance. The SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) were used to conduct all statistical analysis.
Results
The study included 218 patients diagnosed with NMIBC, of whom 181 were male and 37 were female. The clinical and pathological information is shown in Table 1. The median age was 68 years (IQR 60–76 years). The number and proportion of patients in FH(-)/ROS(-), FH(+)/ROS(-) and FH(+) /ROS(+) were 95(43.6%), 79(36.2%) and 44(20.2%), respectively. The pathological stages for those with FH(+) and ROS(+), FH(+) and ROS(-), FH(-) and ROS(-) at diagnosis were 0.5% Tis, 6.4% Ta, 13.3% T1; 2.3% Tis, 20.6% Ta, 13.3% T1; 5.5% Tis, 28.9% Ta, 9.2% T1, respectively. The median body mass index (BMI) was 24.6 (kg/m2) (IQR 22.5–27.0) (kg/m2). The median tumor size was 2.5 cm (IQR 1.5–3.5 cm). There was no significant difference in the distribution of patients with a smoking history among the three groups (P=0.358). After surgery, there was no significance in the chemotherapy regimen among the groups (BCG, Epirubicin, Pirarubicin, Gemcitabine).
 |
Table 1 Clinical Characteristics of All Patients |
Kaplan-Meier analysis showed that 1-year RFS of FH(+)/ROS(+), FH(+)/ROS(-) and FH(-)/ROS(-) were 46.0%, 87.8% and 93.4%, respectively (P=0.000). Figure 1 shows the comparison of RFS curves between patients in different groups. Analysis of multivariate Cox regression indicated that FH/ROS status, tumor grade and tumor stage were the independent risk factors for RFS. (Table 2). Through logistics regression analysis, FH(+)/ROS(+) were found to be correlated with high grade and higher stage (T1) (Table 3).
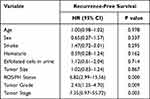 |
Table 2 Multivariable Cox Model for Recurrence-Free Survival |
 |
Table 3 Multivariate Logistic Regression Analysis of Factors Predicting High Grade or Stage (T1) |
 |
Figure 1 Comparison of RFS curves between patients with different status of ROS and FH was drawn by the author Shuaishuai Li. |
Discussion
At present, cystoscopy with biopsy and urine cytology are the gold standards for BC diagnosis. However, cystoscopy is invasive, cost-intensive, has an 85–90% sensitivity and involves a low risk of urinary tract infection, hematuria and suboptimal compliance with management recommendations.12 Furthermore, for patients with NMIBC, multiple postoperative cystoscopic follow-ups are sometimes difficult to achieve. The ideal situation is to screen patients with BC through urine. Urine cytology is non-invasive, has low sensitivity in low-grade tumors, and has a variable interpretation among pathologist.13 Some urine-based biomarkers, like ImmonoCyt (sensitivity 67–86%, specificity 75–79%), BTAstat (sensitivity 58–71%, specificity 73%), BTA-track (sensitivity 69–71%, specificity 66–90%), NMP22 (sensitivity 71–73%, specificity 73–78%), UroVysion (sensitivity 72%, specificity 72%) have been developed as potential alternatives or adjuncts to standard tests for the initial diagnosis of BC or for the identification of recurrent disease.14 Nevertheless, these biomarkers lack randomized controlled trials to determine efficacy.14 Moreover, these biomarkers miss in a significant proportion of patients with BC, and false-positive results may occur in other patients.
The potential role of novel urine tests in diagnosing and following up patients with BC is increasingly being explored. From an epigenetic perspective, Georgopoulos et al proposed that gene promoter hypermethylation could be a promising urine biomarker for BC diagnosis based on their study’s results.15 A systematic review and meta-analysis revealed that 8-hydroxy-2’-deoxyguanosine (8-OHdG) plays an essential role in the diagnosis and prognosis of lower urinary tract symptoms (LUTS) in patients with vascular injury or bladder outlet obstruction as a urinary biomarker.16
Zhao et al investigated the free ferrous protoporphyrin and reactive oxygen species (FH and ROS) status to evaluate the relationship between pathological stage and grade of Urothelial Carcinoma for the first time.11 The combined test aims to evaluate the prognosis and formulate further treatment plans. The reactive oxygen species (ROS) that are generated by mitochondrial respiration are potent inducers of oxidative damage and mediators of aging.17 ROS concentrations rise beyond antioxidant levels, and ROS damages cellular structures, including phospholipid bilayer, DNA, protein, and nucleic acid.18 The cumulative effect of oxidative stress is an central cause of many degenerative diseases, including tumors.19 Many studies have shown that ROS is involved in the occurrence and development of tumors.20 Free ferrous protoporphyrin (FH) is widely distributed in human cells, mainly in mitochondria.21 FH performs certain physiological functions by binding to specific proteins to form oxygen sensors or peroxidase.22 Studies have shown that activation of oxygen and hypoxia sensors in tumor cells enhances ROS activity, changes intracellular polarity quantification, and releases FH from hydrophobic nucleoproteins into free FH.23 Free FH can cause oxidative stress, disrupt tissue/cell stability, and inhibit cell proliferation, differentiation and apoptosis.24 During the occurrence and development of malignant tumors, membrane damage is aggravated, cell autolysis is serious, and FH infiltration is increased. Studies have shown that FH detection is highly valued in diagnosing cervical cancer and precancerous lesions.25 Chinese researchers have developed a ROS/FH combined assay kit based on these characteristics. This method has been used to screen for cervical cancer and has achieved good clinical results. In our study, we investigated the ability of ROS/FH combined assays to predict the recurrence risk of NMIBC. In combination with other diagnosis techniques, FH and ROS status may help distinguish high-risk populations.
The results of our study indicated that the FH(+) and ROS(+) group’s RFS was significantly shorter than the other two groups. Analysis of multivariate Cox regression also indicated that FH/ROS status is a risk factor for RFS. By further logistics regression analysis, FH(+)/ROS(+) were found be correlated with high grade and higher stage (T1). These results demonstrate the feasibility of this cost-effective combined test which may help distinguish between patients at high risk of recurrence. The status of FH(+) /ROS(+) indicates a high probability of recurrence in the future. Furthermore, in the FH(+)/ROS(+) group, we found a higher proportion of patients with high grade and higher stage (T1) of tumors than the other two groups. Thus, we further analyzed whether the status of FH(+)/ROS(+) was associated with higher grade and stage tumors. Moreover, as it turns out, the correlation does exist. It is worth mentioning that our results are different from those of Zhao et al.11 Because our results show that the status of FH(+) /ROS(+) is associated with high-grade and higher stage (T1) pathologic outcomes.
It must be emphasized that there are some shortcomings in this study. First, this study is a single-center, retrospective study, selection bias and data losses are unavoidable. Second, the numbers of patients in various subgroups were relatively small, and there were only 44 patients in FH(+)/ROS(+) group. Third, no patient presented with FH(-)/ROS(+) due to ROS sensitivity being weaker than FH’s. Thus, further experiments are needed to confirm our conclusions.
Conclusion
Our results suggested that FH(+) and ROS(+) are associated with higher recurrence risk in newly diagnosed patients with NMIBC. Furthermore, FH(+) and ROS(+) at diagnosis were correlated with high grade and higher stage (T1). This combined detection method may help specify treatment options for patients with NMIBC.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by The Affiliated Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Consent for Publication
Informed consent to publish was obtained.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Among these authors, Shuaishuai Li, Zeyu Chen, Rui Chen and Ning Xue contributed equally to this work.
Funding
There is no funding for this clinical trial.
Disclosure
The authors declare no conflict of interest.
References
1. Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. doi:10.1016/j.eururo.2016.06.010
2. Richters A, Aben K, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38(8):1895–1904. doi:10.1007/s00345-019-02984-4
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Lobo N, Shariat SF, Guo CC, et al. What is the significance of variant histology in urothelial carcinoma? Eur Urol Focus. 2020;6(4):653–663. doi:10.1016/j.euf.2019.09.003
5. Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. JAMA. 2020;324(19):1980–1991. doi:10.1001/jama.2020.17598
6. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(Suppl 6):4–34. doi:10.1016/j.urology.2005.07.062
7. Soukup V, Čapoun O, Cohen D, et al. Risk stratification tools and prognostic models in non-muscle-invasive bladder cancer: a critical assessment from the European association of urology non-muscle-invasive bladder cancer guidelines panel. Eur Urol Focus. 2020;6(3):479–489. doi:10.1016/j.euf.2018.11.005
8. Ritch CR, Velasquez MC, Kwon D, et al. Use and validation of the AUA/SUO risk grouping for nonmuscle invasive bladder cancer in a contemporary cohort. J Urol. 2020;203(3):505–511. doi:10.1097/JU.0000000000000593
9. van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60(3):493–500. doi:10.1016/j.eururo.2011.05.045
10. You M, Li H, Gao Y, et al. Clinical application value of FH/ROS combined test in cervical cancer screening. Hans J Biomed. 2019;9(4):149–153. doi:10.12677/HJBM.2019.94022
11. Zhao F, Qi N, Shen X, et al. Free ferrous protoporphyrin and reactive oxygen species status of voided urine predicts higher stage in urothelial carcinoma. Cancer Manag Res. 2022;14:615–621. doi:10.2147/CMAR.S352127
12. Karaoglu I, van der Heijden AG, Witjes JA. The role of urine markers, white light cystoscopy and fluorescence cystoscopy in recurrence, progression and follow-up of non-muscle invasive bladder cancer. World J Urol. 2014;32(3):651–659. doi:10.1007/s00345-013-1035-1
13. Yafi FA, Brimo F, Steinberg J, et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. 2015;33(2):
14. Chung W, Bondaruk J, Jelinek J, et al. Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1483–1491. doi:10.1158/1055-9965.EPI-11-0067
15. Georgopoulos P, Papaioannou M, Markopoulou S, et al. DNA hypermethylation of a panel of genes as an urinary biomarker for bladder cancer diagnosis. Urol J. 2021;19(3):214–220. doi:10.22037/uj.v18i.6743
16. Papaefstathiou E, Papaioannou M, Papaefstathiou E, et al. Do we have enough evidence to propose a urinary biomarker of bladder ischemia? A systematic review and meta-analysis. Low Urin Tract Symptoms. 2022. doi:10.1111/luts.12443
17. Giorgio M, Trinei M, Migliaccio E, et al. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8(9):722–728. doi:10.1038/nrm2240
18. Ryter SW, Kim HP, Hoetzel A, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89. doi:10.1089/ars.2007.9.49
19. Kostyuk VA, Potapovich AI. Mechanisms of the suppression of free radical overproduction by antioxidants. Front Biosci. 2009;1(1):179–188. doi:10.2741/E17
20. Buico A, Cassino C, Ravera M, et al. Oxidative stress and total antioxidant capacity in human plasma. Redox Rep. 2009;14(3):125–131. doi:10.1179/135100009X392557
21. Chiabrando D, Vinchi F, Fiorito V, et al. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014;5:61. doi:10.3389/fphar.2014.00061
22. Lipinski P, Starzyński RR, Styś A, et al. [Heme metabolism as an integral part of iron homeostasis]. Postepy Hig Med Dosw. 2014;68:557–570. Polish. doi:10.5604/17322693.1102284
23. Marengo B, Nitti M, Furfaro AL, et al. Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxid Med Cell Longev. 2016;2016:6235641. doi:10.1155/2016/6235641
24. Orino K. Functional binding analysis of human fibrinogen as an iron- and heme-binding protein. Biometals. 2013;26(5):789–794. doi:10.1007/s10534-013-9657-8
25. Zhou X, Zuo JH, Wu ZY, et al. Clinical diagnostic value of free body of reduced iron protoporphyrin in uterus epithelial cells on cervical carcinoma and precancerous lesion. Eur Rev Med Pharmacol Sci. 2017;21(9):2145–2149.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
