Back to Journals » International Journal of General Medicine » Volume 17
Can We Predict the Grade of Clear Cell Renal Cell Carcinoma from Houns-Field Unit of Renal Lesion on Computerized Tomography Scan, a Retrospective Cross-Sectional Study
Authors Al-Zubi M, Al-Shami K, Sawalha L, Alguzo HM, Al Demour S , Al-Mnayyis AM , Alazab R, Al-Rawashdah SF, Alzoubi LT, Al-khawaldeh SR
Received 18 January 2024
Accepted for publication 16 April 2024
Published 23 April 2024 Volume 2024:17 Pages 1571—1577
DOI https://doi.org/10.2147/IJGM.S452754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohammad Al-Zubi,1 Khayry Al-Shami,2 Leen Sawalha,3 Heyam Mahmoud Alguzo,2 Saddam Al Demour,4 Asma’a Mohammad Al-Mnayyis,2 Rami Alazab,5 Samer Fathi Al-Rawashdah,6 Lana Talal Alzoubi,7 Sawsan Radi Al-khawaldeh2
1Department of Surgery, Division of Urology, Yarmouk University MEdical SChool, Irbid, 21110, Jodan; 2Department of Clinical Medical Sciences, Yarmouk University Medical school, Irbid, Jordan; 3Department of Clinical Medical Sciences, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Special Surgery, Division of Urology, the University of Jordan medical School, Amman, 11972, Jordan; 5Department of Surgery & Urology, Jordan University of Science & Technology, Irbid, 21110, Jordan; 6Department of Special Surgery, School of Medicine, Mutah University medical School, Karak, 61710, Jordan; 7Department of Dentistry, Private Sector, Amman, Jordan
Correspondence: Mohammad Al-Zubi, Tel +962 789724264, Email [email protected]
Introduction: Renal cell carcinoma (RCC) is a type of urological malignancy that affects approximately 2% of the global population. Imaging modalities, especially computed tomography (CT) scanning, play a critical role in diagnosing RCC. In this study, we investigated whether there is a relationship between tumour grade of clear cell RCC and HU values of renal lesions on CT scan performed before operation.
Materials and Methods: We conducted a retrospective analysis of 123 patients who underwent radical or partial (open or laparoscopic) nephrectomy for clear cell RCC between January 2017 and January 2021. Post-operation histopathological grades were recorded according to World Health Organization (WHO)/International Society of Urological Pathology (ISUP) 2016 grading system and divided into low grade (includes grade 1 and 2) and high grade (grade 3 and 4), and their links to age, sex, smoking habits, tumour size, and HUs of renal lesions were evaluated.
Results: The mean age of the patients studied was 63.02 years old. About 56.9% of the patients were low grade (grade 1 or grade 2), while 43.1% were high grade (grade 3 or 4). The mean tumour size was 6.31 cm. There were no significant differences in tumour grade according to age, sex, or smoking habits. We found a significant relation between tumour grade and HU in the pre-contrast and nephrogenic phases, with p values of 0.001 and 0.037, respectively. On the other hand, there was no significant relation linking the tumour grade to the difference in HU between these phases, where there was a p value of 0.641.
Conclusion: HU in the pre-contrast and nephrogenic phases in addition to tumour size on CT scan have a significant relation to clear cell RCC grade.
Keywords: renal cell carcinoma, grade, stage, Hounsfield unit, CT scan
Introduction
Renal cell carcinoma (RCC) is a kind of urological cancer that affects around 2% of people globally.1 It is more common in developed regions, especially in North America and Europe, with incidence rates 15 times higher than in Africa and Southeast Asia. RCC, which represents over 90% of kidney cancers and originates from renal tubular epithelial cells, is the ninth most common cancer in the United States.2,3 The World Health Organization classified RCC in 2016 into various subtypes based on their histological characteristics, including clear cell (70% of cases), papillary (15%), and chromophobe carcinoma (5%). Subtype identification is vital for prognosis and treatment. Key risk factors for RCC are hypertension, obesity, chronic/end-stage kidney disease, and smoking.4,5 Often RCC is incidentally diagnosed, sometimes revealing metastases at diagnosis. While most RCCs are small, low-grade tumors, up to 17% can have distant metastases, commonly in the liver, bones, lymph nodes, lungs, and brain.3,6,7 Since 1990, multislice computerised tomography (CT scans) has been crucial in staging, preoperative planning, and follow-up of RCC.8 The stage of cancer is determined through physical exams, biopsies, and imaging, guides treatment options and prognosis.9 The TNM staging system, by the Union for International Cancer Control and AJCC, classifies RCC based on tumor size (T), lymph node involvement (N), and distant metastasis (M). Survival rates vary significantly with the disease stage at diagnosis: 93% at stage I, 72.5% at stage II/III, and 12% at stage IV.1,10 Tumor grading, assessing cancer differentiation using light microscopy, is also crucial. The WHO/ISUP grading system applies to clear cell and papillary RCC, but grading systems for chromophobe RCC are not widely accepted. Collected duct carcinomas, aggressive and high-grade, are not graded.11,12 Treatment for localized RCC includes nephrectomy, radio ablation, and surveillance; advanced cases often require surgery and systemic therapy.13
This study aims to predict the grade of clear cell RCC post-operatively using pre-operative tumor size and HU values of renal lesions in various CT scan phases. Although some studies evaluate the association of nuclear grade with HU lesion attenuation of RCC on CT scan, but up to our knowledge, no previous studies have investigated this association for clear cell subtype of RCC.
Materials and Methods
Study Design and Approval
After obtaining Institutional Review Board approval (#10/2020/16049) from at King Abdullah University Hospital (KAUH), which is affiliated with Jordan University of Science and Technology (JUST), a retrospective analysis was conducted of 123 patients who underwent either partial or radical nephrectomy (open or laparoscopic) for clear cell RCC between January 2017 and January 2021. Patient informed consent was obtained.
Post-operative RCC histopathology grades were reviewed according to WHO/ ISUP 2016 grading classification for clear and papillary RCC, and their relationships to HU (pre-contrast, post-contrast, and the difference between the two), age, sex, smoking habits, preoperative CT scan results, and tumour size as measured on pre-operative CT scan were evaluated. These links were considered significant when the p value was less than 0.05.
Inclusion and Exclusion Criteria
All patients who underwent radical or partial nephrectomy (laparoscopic or open) for clear cell RCC between January 2017 and January 2021 were included in the study. Patients with histological subtypes other than clear cell RCC of the kidney, such as papillary, chromophobe, or sarcomatoid cancer, or with secondary renal cancer (metastatic cancer spread to the kidney from other organs) were excluded, as were cases with no available preoperative CT scan and patients lost in follow-up. In the end, 123 patients were included in the study.
Patient Assessment
Each patient’s age, sex, smoking habits, and medical and surgical history were obtained from their records. In addition to tumour size, the increase in HU of the renal mass between the pre-contrast CT scan and the post-contrast nephrogenic phase was measured pre-operatively. Post-operative histopathological grade was also reviewed and assessed.
Intervention
At the hospital in question, following the discovery of a renal mass on a CT scan, the tumour size in centimetres and HU of the mass were recorded. Radical or partial nephrectomy (laparoscopic or open) was performed under general anaesthesia. At the end of the procedure, all resected tissues were sent for histopathological examination. The grade of each histopathologically confirmed RCC was recorded.
The HU measured in CT scans reflect the linear transformation of the attenuation coefficient measured, which is based on the assigned densities of air and pure water.14
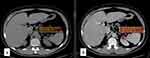
HU were measured on the axial image of the renal lesion by placing the oval region of interest over the middle part of the renal mass. We tried to avoid the margins of the lesion to exclude normal renal tissue. HU were measured in the pre-contrast and post-contrast nephrographic phases. The figures were recorded, and the difference between the pre- and post-contrast HU measurement was calculated (see Figure 1).
Image Analysis (Related to Figure 1)
Postcontrast HU-Precontrast HU = 47.59
Outcome Measures and Assessment Tools
The primary outcome was whether there was any correlation between the tumour grade detected on the histopathology report for clear cell RCC, the HU of the renal mass as measured using CT scanning (pre-contrast, post-contrast HU, and difference in HU between the phases), and the pre-operative tumour size. This would tell us whether we could predict the grade of clear cell RCC depending on these parameters before the operation.
Statistical Analysis
A statistical analysis was performed using IBM SPSS statistics 25.0 software. The dependent value was the tumour grade (low and high). We used Kolmogorov Smirnov test to confirm that the data align to the normal distribution before statistical analysis. For the independent samples, t-tests were used for the numerical variables (age, tumour size, HU in the pre-contrast phase, HU in the nephrogenic phase, and difference in HU between these phases), and chi-square tests were used for the categorical variables (smoking and gender). Differences were considered statistically significant at p < 0.05.
Results
The study population included 123 individuals who underwent radical or partial nephrectomy (open or laparoscopic) for clear cell RCC between January 2017 and January 2021 at our institution and met all inclusion criteria. The post-operative histopathological grade of each tumour was recorded as low (grade 1 or grade 2) or high (grade 3 or grade 4), and we assessed its relation to age, sex, smoking habits, tumour size, and the HU of the renal mass according to CT scanning (the pre-contrast phase, nephrogenic phase, and the difference in HU between these phases).
The mean age of the patients in the study was 63.02 years old, with a range from 39 to 83 years. About 56.9% of the patients were low grade, compared to 43.1% who were high grade. Most of the patients presented with stage 1 (59%). Marking an important risk factor for RCC15,16, most of the patients in our study were smokers (79.7%). Of the patients, 82 were male (66.7%) and 41 were female (33.3%). Tumour sizes on pre-operative CT scan ranged from 2 cm to 19 cm (mean: 6.31 cm). The mean value for the HU of the renal lesions as reviewed via CT scan in the pre-contrast phase, the mean value in the nephrogenic phase following contrast injection, and the difference between in HU between the phases were 29.14 HU, 87.78 HU, and 58 HU, respectively.
Table 1 presents the patients’ demographic information as well as smoking habits, tumour sizes and grades, and HU based on pre- and post-contrast scan results. There was no significant difference in histopathological tumour grade according to age, sex, or smoking status, with p values of 0.794, 0.607, and 0.056, respectively. We found that the high-grade tumours were generally larger than those with a low grade (p value: 0.001). We found a significant correlation between tumour grade and the tumour’s HU mean and range in the pre-contrast and nephrogenic phases, with p values of 0.001 and 0.037, respectively. On the other hand, there was no significant correlation between tumour grade and difference in HU between the phases (p value: 0.641). Table 2 shows the different variables and their p values.
 |
Table 1 Illustration for Demographics, Smoking Status, Tumor Size, Grades and HU in Pre-Contrast and Nephrogenic Phase |
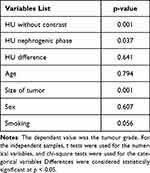 |
Table 2 Different Variables and Their P values |
Discussion
Renal cell carcinomas (RCCs) are the primary type of kidney tumors, making up 90% of such cases. These tumors originate in the renal cortex, unlike transitional cell carcinomas, which develop in the renal pelvis and account for about 8% of kidney cancers.17,18
The exact causes of RCC are not fully understood, but several risk factors have been identified. These include older age, obesity, hypertension, chronic renal failure, dialysis treatment, polycystic kidney disease, African American ethnicity, and sickle cell disease. Genetic factors also play a role. Occupational exposure to substances like cadmium, herbicides, asbestos, and trichloroethylene is also associated with increased RCC risk.15,16
RCC comprises different subtypes with unique features. As clear cell RCC is the most common subtype of RCC, it was chosen as our research object. It is marked by cells rich in glycogen and lipids, often linked to a 3p chromosome deletion. Chromophilic RCCs are usually bilateral and associated with trisomy of chromosomes 7 and 17. Chromophobic RCCs have large polygonal cells.19
When RCC is suspected, a renal and bladder ultrasound is typically the first diagnostic step. If this ultrasound reveals a solid mass or complex cyst, a CT scan of the kidneys, ureters, bladder, and the entire abdomen and pelvis, both before and after contrast, is usually performed.20 The renal mass CT protocol typically includes multiple phases: non-contrast, corticomedullary, nephrogenic, and excretory, each providing specific insights into the renal mass.21
The CT characteristics of RCC include significant enhancement post-contrast, usually with a Hounsfield Unit (HU) value increase of over 20. However, some subtypes like papillary and chromophobe RCC may show less enhancement. CT scanning is crucial for staging RCC, helping to detect lymph node involvement, vascular invasion, adjacent organ invasion, and distant metastases, including bone and lung metastases.22
Papillary RCC (pRCC), a subtype of RCC, often shows hypo-enhancement on CT scans, with HU values that may be indeterminate (10–20 HU) or even low (less than 10 HU). This is attributed to its hypovascular nature. A study noted that the mean attenuation of pRCCs was around 35 HU on unenhanced scans.23–25 Some studies investigate if we can predict the overall survival of clear cell RCC using an integrated nomogram combining both immune scores and clinicopathologic factors.26
The AJCC TNM staging system is widely used for RCC. It assesses the primary tumor size (T), regional lymph node involvement (N), and distant metastasis (M), providing a stage that correlates with prognosis. Survival rates vary with the stage, ranging from 80% to 95% for stage I to less than 10% in the cytokine era for stage IV, although newer therapies have improved survival for advanced stages.27–29
Treatment depends on the RCC stage, varying from nephrectomy in the early stages to palliative treatments in stage IV. For localized tumors, options include surgery, radiofrequency ablation, cryotherapy, or surveillance. Advanced cases may require radical nephrectomy, immunotherapy, chemotherapy, and treatments to manage bone metastases.30,31
This study’s retrospective nature and small sample size are limitations, suggesting the need for larger studies to confirm the relation between pre- and post-contrast HU differences and clear cell RCC grade.
Conclusion
HU in the pre-contrast phase and the nephrogenic phase following contrast injection in addition to tumour size on CT scan correlate significantly with clear cell RCC grade, in contrast to the difference in HU between the two phases, which is not significantly correlated with grade. However, further research with a larger patient sample needs to be conducted in this field.
Acknowledgment
The authors would like to thank Dr. Saleh Abuorouq and Dr. Manar Alshami from the Department of Clinical Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid, Jordan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79. doi:10.14740/wjon1279
2. Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Yrol. 2018;36:1341–1353. doi:10.1007/s00345-018-2286-7
3. Bahadoram S, Davoodi M, Hassanfadeh S, Bahadoram M, Barahman M, Mafakher L. Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol. 2022;39(3):1.
4. Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8(5):484. doi:10.4329/wjr.v8.i5.484
5. Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Europ urol. 2022;82(5):529–542. doi:10.1016/j.eururo.2022.08.019
6. Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906. doi:10.1016/S0140-6736(15)00046-X
7. Edge SB. Cancer AJCo. AJCC Cancer Staging Manual. Springer; 2010.
8. Sankineni S, Brown A, Cieciera M, Choyke PL, Turkbey B, editors. Imaging of Renal Cell Carcinoma. Urologic Oncology: Seminars and Original Investigations. Elsevier; 2016.
9. Deng S-P, Cao S, Huang D-S, Wang Y-P. Identifying stages of kidney renal cell carcinoma by combining gene expression and DNA methylation data. IEEE/ACM Transactions Computational Biol Bioinformatics. 2016;14(5):1147–1153. doi:10.1109/TCBB.2016.2607717
10. Capitanio U, Bedke J, Albiges L, et al. A renewal of the TNM staging system for patients with renal cancer to comply with current decision-making: proposal from the European Association of Urology Guidelines Panel. Europ urol. 2022;S0302-2838(22):02703.
11. Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019;74(1):4–17. doi:10.1111/his.13735
12. Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World j Urol. 2018;36(12):1913–1926. doi:10.1007/s00345-018-2447-8
13. Pontes O, Oliveira-Pinto S, Baltazar F, Costa M. Renal cell carcinoma therapy: current and new drug candidates. Drug Discovery Today. 2022;27(1):304–314. doi:10.1016/j.drudis.2021.07.009
14. Hounsfield GN. Computed medical imaging. Nobel lecture, December 8, 1979. J Computer Assisted Tomography. 1980;4(5):665–674. doi:10.1097/00004728-198010000-00017
15. Mikhail MI, Singh AK. Von Hippel Lindau Syndrome. Int J Med. 2017.
16. Ganguly S, Chandra A, Chatterjee IB. Pathobiology of cigarette smoke-induced invasive cancer of the renal pelvis and its prevention by vitamin C. Toxicol Rep. 2018;5:1002–1010. doi:10.1016/j.toxrep.2018.10.005
17. Kuroda N, Sugawara E, Kusano H, Yuba Y, Yorita K, Takeuchi K. A review of ALK-rearranged renal cell carcinomas with a focus on clinical and pathobiological aspects. Pol J Pathol. 2018;69(2):109–113. doi:10.5114/pjp.2018.76693
18. Grünwald V. Risk-adapted (immuno)therapy for renal cell carcinoma. Der Urologe. 2018;57(11):1326–1333. doi:10.1007/s00120-018-0791-3
19. Garfield K, LaGrange CA. Renal cell cancer. Hematol Oncol Clin. 2017.
20. Ward RD, Tanaka H, Campbell SC, Remer EM. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics. 2018;38(7):2021–2033. doi:10.1148/rg.2018180127
21. Lang EK, Hanano A, Rudman E, et al. The fate of small renal masses, less then 1 cm size: outcome study. Int Braz J urol. 2012;38(1):40–48. doi:10.1590/S1677-55382012000100006
22. Liaw CW, Winoker JS, Mehrazin R. Imaging protocols for active surveillance in renal cell carcinoma. Curr Urol Reports. 2018;19(10):1–7. doi:10.1007/s11934-018-0830-z
23. Herts BR, Coll DM, Novick AC, et al. Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. Am J Roentgenol. 2002;178(2):367–372. doi:10.2214/ajr.178.2.1780367
24. Dilauro M, Quon M, McInnes M, et al. Comparison of contrast-enhanced multiphase renal protocol CT versus MRI for diagnosis of papillary renal cell carcinoma. AJR Am J Roentgenol. 2016;206(2):319–325. doi:10.2214/AJR.15.14932
25. Schieda N, Vakili M, Dilauro M, Hodgdon T, Flood TA, Shabana WM. Solid renal cell carcinoma measuring water attenuation (− 10 to 20 HU) on unenhanced CT. Am J Roentgenol. 2015;205(6):1215–1221. doi:10.2214/AJR.15.14554
26. Wu Z, Ouyang C, Peng L. An immune scores-based nomogram for predicting overall survival in patients with clear cell renal cell carcinoma. Medicine. 2020;99(34):e21693. doi:10.1097/MD.0000000000021693
27. Elmore JM, Kadesky KT, Koeneman KS, Sagalowsky AI. Reassessment of the 1997 TNM classification system for renal cell carcinoma: a 5‐cm T1/T2 cutoff is a better predictor of clinical outcome. Cancer. 2003;98(11):2329–2334. doi:10.1002/cncr.11806
28. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi:10.1200/JCO.1999.17.8.2530
29. Mekhail TM, Abou-Jawde RM, BouMerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832–841. doi:10.1200/JCO.2005.05.179
30. Jonasch E. Updates to the management of kidney cancer. J National Compr Cancer Network. 2018;16(5S):639–641. doi:10.6004/jnccn.2018.0039
31. Dabestani S, Marconi L, Kuusk T, Bex A. Follow-up after curative treatment of localised renal cell carcinoma. World j Urol. 2018;36(12):1953–1959. doi:10.1007/s00345-018-2338-z
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.