Back to Journals » OncoTargets and Therapy » Volume 16
Predictive Significance of Systemic Immune-Inflammation Index in Patients with Breast Cancer: A Retrospective Cohort Study
Authors Zhou Y , Guo X, Shen L, Liu K, Sun Q, Wang Y, Wang H, Fu W, Yao Y, Wu S, Chen H, Qiu J, Pan T, Deng Y
Received 6 August 2023
Accepted for publication 9 November 2023
Published 16 November 2023 Volume 2023:16 Pages 939—960
DOI https://doi.org/10.2147/OTT.S434193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Yunxiang Zhou,1,2,* Xianan Guo,1,2,* Lu Shen,1,2 Kexin Liu,1,2 Qunan Sun,3 Yali Wang,4 Hui Wang,5 Wenyu Fu,6 Yihan Yao,7 Shijie Wu,2 Huihui Chen,1 Jili Qiu,1 Tao Pan,1 Yongchuan Deng1
1Department of Breast Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Department of Medical Oncology, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 4Department of Breast Surgery, Fujian Medical University Union Hospital, Fuzhou, People’s Republic of China; 5Department of Pathology, Cancer Hospital of the University of Chinese Academy of Science (Zhejiang Cancer Hospital), Hangzhou, People’s Republic of China; 6Department of Surgery, Hangzhou Fuyang Hospital of Traditional Chinese Medicine, Hangzhou, People’s Republic of China; 7Institute of Immunology, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongchuan Deng; Tao Pan, Email [email protected]; [email protected]
Background: Peripheral blood inflammation indices, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), have become research hotspots in the diagnosis, treatment, and prognosis prediction of breast cancer, whereas existing research findings remain controversial.
Methods: Data pertaining to 1808 breast cancer patients were collected retrospectively to analyze the predictive value of NLR/PLR/SII for breast cancer clinicopathological characteristics, chemotherapy response, and relapse. 1489, 258, and 53 eligible breast cancer patients entered into the three analyses, respectively. Logistic regression analyses were used to assess the correlation between these indices and poor response to chemotherapy. A predictive scoring model was established to predict chemotherapeutic responses based upon the odds ratio values of significant variables identified in logistic regression analyses.
Results: Higher pretherapeutic NLR/PLR/SII values were significantly correlated with higher tumor stage, triple-negative breast cancer, premenopausal status, and younger age. Logistic regression analyses indicated that pretherapeutic high SII (as a continuous variable or with a cut-off value of 586.40) and HER2-negative status were independent predictors of poor response to neoadjuvant chemotherapy. A first-in-class SII-based predictive scoring model well distinguished patients who might not benefit from neoadjuvant chemotherapy, with an area under the curve of 0.751. In HR-positive cancers, SII was more strongly associated with clinicopathological features and chemotherapy response. In addition, a receiver operating characteristic curve analysis indicated that the specificity of follow-up SII in identifying cancer relapse was greater than 98.0% at a cut-off value of 900.
Conclusion: As a predictor of breast cancer, especially in the HR-positive subtype, SII may eclipse NLR/PLR. SII-high patients are more likely to have a worse chemotherapy response and a higher risk of recurrence.
Keywords: breast cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, chemotherapy response, predictor
Introduction
Breast cancer has overtaken lung cancer as the global most popular cancer, and the mortality rate also ranks first among all female malignancies.1 Although the survival rate of breast cancer has improved with the progress, standardization, and individualization of breast cancer treatment, the prognosis of breast cancer patients could be improved still further.2 Early prediction of the clinicopathological characteristics and treatment response of patients has the potential to provide precise treatment and ameliorate the overall prognosis of breast cancer.
Immuno-inflammatory cells play pivotal roles in tumorigenesis and progression.3 Increasing evidence demonstrates that peripheral blood inflammation indices reflect the level of local immune inflammation in the tumor microenvironment.4,5 These parameters may aid in precision medicine in patients with cancer (Figure 1). Correspondingly, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have become popular topics in the diagnosis, treatment, follow-up management, and prognosis prediction of various solid tumors, including colon cancer,6 gastric cancer,7 lung cancer,8 ovarian cancer,9 bladder cancer,10 as well as breast cancer,11,12 due to their accessibility, reproducibility, non-invasiveness, and low cost. In addition, the systemic immune-inflammation index (SII), despite the rare research in breast cancer, may better reflect the immune-inflammatory state of the body and provide superior predictive information, considering the integration of the three cells.10,13 Unfortunately, the current predictive and prognostic value of these peripheral blood inflammation indices in breast cancer remains controversial.14–18 Additionally, few studies have attempted to compare their differences in value across subtypes of breast cancer.
This study investigated the value of NLR, PLR, and SII on diagnosis, prognosis, and follow-up monitoring of breast cancer patients by analyzing the baseline levels and dynamic changes of these indicators before, during, and after initial treatment. This study is a large one to explore the predictive value of NLR, PLR, and SII in breast cancer, as well as the first to propose a predictive scoring model for breast cancer chemotherapy efficacy based on SII.
Patients and Methods
Patients and Study Design
We retrospectively collected data from 1808 primary breast cancer patients who were admitted to the Department of Breast Surgery of the Second Affiliated Hospital of Zhejiang University School of Medicine between June 2010 and October 2020. A patient flowchart was shown in Figure 2. Detailed eligibility criteria for clinicopathological characteristic, neoadjuvant chemotherapy response, and relapse analyses are described in Supplementary Table 1. Eventually, 1489, 258, and 53 eligible breast cancer patients entered into the three analyses, respectively.
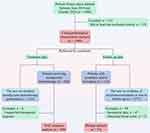 |
Figure 2 Patient flowchart. Abbreviation: NAC, neoadjuvant chemotherapy. |
The study was approved by the Ethics Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine, which waived the informed consent requirement due to the retrospective design of the study.
Data Collection
Clinicopathological and laboratory data were collected retrospectively from patient medical records and entered into a dedicated anonymized database.
Peripheral complete blood count was performed before any treatment modality was initiated and during the follow-up period. NLR was calculated by dividing the neutrophil count by the lymphocyte count; PLR was the platelet to lymphocyte count ratio. SII was calculated as the product of the neutrophil and platelet counts divided by the lymphocyte count.
Pathological Assessment
Tumor staging was defined as per the 8th edition of staging criteria issued by the American Joint Committee on Cancer (AJCC). Response evaluation of neoadjuvant chemotherapy was determined according to the postoperative pathological MP grading system by pathologists. MP grade 1–2 referred to poor chemotherapy response, while Miller-Payne (MP) grade 3–5 was identified as a good chemotherapy response. HER2 was considered positive when scored 3 via immunohistochemistry or confirmed amplification via fluorescent in situ hybridization. Tumors were considered hormone receptor (HR)-positive when they were positive for either or both estrogen receptor (ER) and progesterone receptor (PR) (determined by an immunohistochemistry score ≥ 1%). Based upon HR and HER2 status, breast cancers were separated into four subtypes: HR+HER2-, triple-negative breast cancer (TNBC), HR-HER2+, or HR+HER2+. Pathological complete response (pCR) was defined as the absence of invasive cancer cells in the breast and axilla (ypT0/is ypN0).
Statistical Analysis
Kolmogorov–Smirnov test was utilized to distinguish the distribution pattern. Continuous variables with normal distributions were expressed as mean ± standard deviation, while other continuous variables were shown as median [interquartile range (IQR)]. Categorical variables were presented as numbers (proportion). When assessing the associations between NLR/PLR/SII and clinicopathological characteristics, Student’s t‐test or Mann‐Whitney U‐test was used for continuous variables, while Pearson’s chi‐square or Fisher’s exact test was used for categorical variables. Friedman rank-sum test and paired Wilcoxon signed-rank test were applied when determining the value of peripheral blood inflammation indices as indicators for monitoring tumor recurrence and metastasis.
An exploratory evaluation for optimal cut-off values of NLR, PLR, and SII to predict chemotherapeutic response was undertaken using the receiver operating characteristic (ROC) curve analysis. Logistic regression models were applied for univariate and multivariate analyses. Candidate variables with P < 0.1 in univariate analysis were subsequently entered into multivariable models, and those resulting variables with P < 0.05 were considered independent risk factors of poor chemotherapy response.
A predictive scoring model was established to predict patients’ response to neoadjuvant chemotherapy based upon the odds ratio (OR) values of significant variables identified by univariate analysis: (a) if the corresponding OR value > 1, the number was divided by 2 and rounded to the nearest integer to create a score; (b) if the OR value < 1, its reciprocal was divided by 2 and rounded to the nearest integer, and the negative of the calculation was used as the final score. The constant 3 and the individual scores for each variable were summed together to generate a total risk score. The optimal cut-off value of the predictive scoring model was calculated using the maximum Youden’s index of the ROC curve, and the chosen cut-off value was used to evaluate the area under the curve (AUC), sensitivity, and specificity values of the prediction scoring model. All statistical analyses were performed using SPSS version 26.0 (IBM). Two-tailed significance values were used, and P<0.05 was considered statistically significant.
Results
Relationship Between Pretherapeutic NLR/PLR/SII and Clinicopathological Characteristics
Clinicopathological Characteristics of the 1489 Patients
A total of 1489 patients (median age, 51 years, range: 23–89) were included in the clinicopathological characteristic analysis. HR+HER2- breast cancers represented over half of the patients (52.0%), followed by HR+HER2+ (19.6%), HR-HER2+ (13.4%), and TNBC (13.0%) subtypes, while 2% had unknown molecular subtype. Cases classified as invasive ductal carcinoma reached 67.4%, which might be underestimated because the histologic patterns of 260 patients were incompletely specified invasive carcinoma. Other clinicopathological features of the patients were shown in Table 1.
 |
Table 1 Clinicopathological Characteristics of All 1489 Patients with Breast Cancer |
Relationship Between Peripheral Blood Inflammation Indices and Clinicopathological Characteristics
The median neutrophil, platelet, and lymphocyte counts of the 1489 patients were 3.81×109/L (IQR, 3.03–4.80×109/L), 219×109/L (IQR, 181–258×109/L), and 1.75× 109/L (IQR, 1.39–2.12×109/L), respectively. Since no validated NLR, PLR, and SII cut-off values have been reported in the literature, we performed ROC curve analyses for these parameters and most of the clinicopathological characteristics, which undesirably yielded AUCs<0.6. Therefore, the medians of the three peripheral blood inflammation indices were chosen as cut-off values for grouping: NLR, 2.18 (≤2.18 vs >2.18); PLR, 124.10 (≤124.10 vs >124.10); and SII, 475.00 (≤475.00 vs >475.00); patients were categorized into either low or high groups.
Table 2 showed the characteristics of patients grouped according to the three peripheral blood inflammation indices. At least one of the three parameters was significantly correlated with lymph node stage, tumor AJCC stage, T stage, Ki-67 expression, HER2 status, and histological grade, among others. For instance, AJCC stage 0–1 vs 2–4 were significantly different between different NLR groups, and stage 0–3b vs 3c-4 were significantly different between different PLR and SII groups. Importantly, these blood parameter values increased in parallel with the grade and stage of breast cancer. Although obesity (BMI≥25) appeared not related to parameter values, a significant value difference for low-weight patients was identified by further analysis as more patients with high NLR were underweight (BMI<18.5) (P<0.05). In addition, a Mann–Whitney U-test revealed that patients with high PLR had significantly lower BMIs than patients with lower PLR (P=0.001). These findings suggested some relationship between BMI and peripheral blood inflammation indices, especially NLR and PLR. No correlation was found between the three peripheral blood inflammation indices and lymph node involvement, ER status, PR status, lesion number, lymphovascular invasion (LVI), histologic grade, or tumor location.
 |
Table 2 The Correlation Between Pretherapeutic NLR/PLR/SII and Clinicopathological Characteristics Among All 1489 Patients with Breast Cancer |
The respective correlation between pretherapeutic NLR/PLR/SII and clinicopathological characteristics across subtypes of breast cancer was roughly in line with the overall population. Notably, these peripheral parameters appeared to have better potential to predict the characteristics of HR+ breast cancer (Supplementary Table 2).
Predictive Value of Pretherapeutic NLR/PLR/SII for Chemotherapy Response
Best Discriminating Value of NLR/PLR/SII for Predicting Poor Chemotherapy Response
For therapeutic response analysis, 258 patients undergoing neoadjuvant chemotherapy were included. Pathologists determined the response evaluation of neoadjuvant chemotherapy according to the postoperative pathological MP grading system. MP grades 1–2 indicated a poor chemotherapy response, while MP grades 3–5 indicated a good chemotherapy response. Accordingly, patients were classified into two groups: poor responders (n=43) and good responders (n=215). Clinicopathological characteristics of these 258 patients were shown in Table 3.
 |
Table 3 Clinicopathological Characteristics of 258 Patients Undergoing Neoadjuvant Chemotherapy |
Since there were no established cut-off values for the three peripheral blood parameters, ROC analysis was performed, and cut-off points were calculated using the maximum Youden’s index of the curve. The “optimal” cut-off values were thus determined as NLR, 1.777; PLR, 137.31 or 139.75; and SII 586.40; the corresponding AUCs of the ROC curves were 0.545 [95% confidence interval (CI) 0.450–0.641; P=0.347], 0.560 (95% CI 0.461–0.658; P=0.215), and 0.602 (95% CI 0.503–0.700; P=0.035), respectively. Remarkably, the first two AUCs were small and did not achieve statistical significance. Hence, we calculated variable ORs ranging from 1.60 to 2.20 for NLR and 127 to 155 for PLR in the cohort, and minimum P-values were obtained at 1.77 for NLR and 137 for PLR. Based on this analysis, we categorized patients into high (≥ 1.77, n=185) and low NLR (< 1.77, n=73), high PLR (≥ 137, n=105), and low PLR (< 137, n=153), and high (≥ 586.40, n=103) and low SII (< 586.40, n=155) groups.
Relationship Between Clinicopathological Characteristics and Response to Neoadjuvant Chemotherapy
Univariate analysis showed that patients with higher pretherapeutic SII (as a continuous variable or with a cut-off value of 586.40), obesity (BMI≥25), T4 tumor, invasive ductal carcinoma, PR-positive status, lower Ki-67, HER2-negative status (all P<0.05), ER-positive status and AJCC stage-high tumors (both P<0.1) tended to be resistant to neoadjuvant chemotherapy. A subsequent multivariate logistic regression analysis revealed high SII and HER2-negative status to be independent predictors of poor neoadjuvant chemotherapy response (Table 4).
 |
Table 4 Univariate and Multivariable Analysis for Risk Factors of Poor Chemotherapy Response |
Interestingly, although NLR and PLR as categorical variables were not significantly correlated with poor chemotherapy response, univariate analysis suggested that NLR and PLR as continuous variables were significantly associated with neoadjuvant chemotherapy resistance (P=0.008, P=0.050, respectively), and NLR remained significant in multivariate analysis (OR=1.383, 95% CI 1.049–1.823, P=0.021, adjusted for PLR, BMI, AJCC stage, histological type, ER status, PR status, Ki-67 expression, and HER2 status). Furthermore, HR+HER2- breast cancer was an independent predictor of poor chemotherapy response (OR=2.534, 95% CI 1.220–5.263, P=0.013), with adjustment for SII, BMI, AJCC stage, histological type, Ki-67 expression.
Factors affecting pCR were analyzed in patients with stage II–III breast cancer (n=251, 87 patients with pCR). Univariate analysis revealed that higher AJCC stage, non-IDC histological type, ER-negative status, PR-negative status, high Ki-67 expression, HER2-positive status, and ≥6 chemotherapy cycles were significantly associated with increased pCR possibility. Notably, SII was unlikely to predict pCR in this study (OR=0.999, 95% CI 0.998–1.000) and was unquestionably rejected after multivariate analysis, while AJCC stage, HER2 status, and histological type were the only factors remaining with a significant correlation with pCR (Figure 3).
Development of an SII-Based Predictive Scoring Model for Breast Cancer Chemotherapy Efficacy
To determine in advance who may not benefit from chemotherapy, we developed a novel predictive scoring model capable of predicting chemotherapy efficacy in breast cancer patients based on variables with univariate associations of P<0.05. Notably, histological type was not included in the model as this variable was not usually determined by core needle biopsy pathology. We converted OR values for each variable into corresponding scores, and individual scores were summed together to generate a total risk score of 0 to 8. The specific process of assignment can be found in the Statistical analysis section, and full details were shown in Table 5.
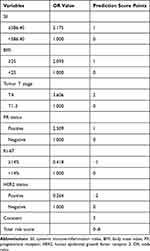 |
Table 5 The Predictive Scoring System for Breast Cancer Chemotherapy Efficacy |
Next, we constructed ROC curves for predicting chemotherapy resistance using patient risk scores. The AUC of this curve was 0.751 (95% CI 0.675–0.827; P=0.000; Figure 4a). Hosmer-Lemeshow Goodness-of-Fit test further confirmed the model had a good fit (P=0.202). The optimal cut-off value of the model was 2.5 according to the maximum Youden’s index of the ROC curve (sensitivity, 86.0%; specificity, 58.6%). Therefore, neoadjuvant chemotherapy efficacy was more likely to be poor for patients with a risk score ≥ 3. Subsequently, we conducted stratification analysis according to the four molecular subtypes to demonstrate that, compared to HR- subtypes, this model had a better predictive ability for chemotherapy response for HR+HER2- and HR+HER2+ breast cancer (AUC: 0.657 and 0.763, respectively, all P < 0.05, Supplementary Table 3).
We tested our SII-based model with an independent cohort and achieved good results in predicting chemotherapeutic responses, with an accuracy of 88.9% (Table 6 and Supplementary Table 4). When SII was removed from the model, the AUC value dropped to 0.727. Moreover, we established another scoring model, which accounted for only those variables with multivariate associations of P < 0.05 (SII and HER2), and it yielded a lower AUC of 0.695 (Figure 4b).
 |
Table 6 The Accuracy of the Predictive Scoring Model Tested by an Independent Cohort |
Potential of NLR, PLR, and SII for Monitoring Recurrence and Metastasis in Breast Cancer Patients
Fifty-three eligible patients were enrolled in this cohort. Dynamic peripheral blood inflammation index changes before treatment and during follow-up were summarized in Table 7. Peripheral blood parameters were collected before treatment (NLR0, PLR0, and SII0), 3–6 months after (neo)adjuvant chemotherapy and surgery completion (NLR1, PLR1, and SII1), and within 3 days before and after recurrence/metastasis (NLR2, PLR2, and SII2). Friedman rank-sum test was applied to analyze the respective dynamic changes of NLR, PLR, or SII at different time points, but no significant differences were observed. A paired Wilcoxon signed-rank test was subsequently used to further analyze NLR/PLR/SII changes between any two time points. PLR1 and PLR0 were significantly different (P=0.026), but PLR2 and PLR1 were not (P=0.465), suggesting that PLR is not suitable for monitoring recurrence. SII1 and SII0 were comparable and statistically nonsignificant; nonetheless, SII2 had a nominal upward trend compared with SII1 (493.99 [337.93–791.49] vs 430.45 [279.18–659.70], P=0.073). ROC curve analysis indicated that the specificity of SII2 in identifying recurrence/metastasis was greater than 98.0% when its cut-off value was set at 900.
 |
Table 7 Dynamic Changes of NLR/PLR/SII Before, During, and After Initial Treatment |
Discussion
Peripheral blood inflammation indices, which were originally developed to reflect the systemic inflammation and stress intensity in critical patients but have since proven to possess predictive and prognostic values across a wide range of diseases,19,20 have the advantages of being easily accessible, cost-effective, repeatable, and noninvasive. Herein, we retrospectively analyzed the correlation of NLR/PLR/SII with clinicopathological characteristics, chemotherapy response, and relapse in breast cancer, revealing the potential of these peripheral blood inflammation indices for clinical application.
Specifically, we found that NLR, PLR, and SII were significantly higher for TNBC/HER2-enriched cancers or those with higher tumor stage, Ki-67 expression, and histological grade, which is in line with previous research findings.21,22 However, we did not find a significant correlation between the three peripheral blood inflammation indices and ER status, PR status, the number of lesions, lymphovascular invasion, and histological type. Consistent with the findings of Zhu et al,23 the higher pretherapeutic peripheral blood inflammation indices in our study were significantly related to young age (<50 years) and premenopausal status. Inversely, there are studies showing that elderly patients are more likely to have high NLR,22 in which 437 cases were included, and the average age of patients reached 63.6 years. Therefore, different sample characteristics may explain the different findings. Indeed, there is always controversy in the results of various studies regarding the relationship between peripheral blood parameters and the clinicopathological characteristics of patients. For instance, the studies by Ulas et al,24 Pistelli et al,25 and Jiang et al13 found that the level of NLR, PLR, and SII could not reflect any clinicopathological characteristics of breast cancer patients. Importantly, in addition to the differences in the research population, the optimal cut-off values of these peripheral blood inflammation indices remain unclear,12,21 possibly contributing to the inconsistent results. In this study, the median 2.18, 124.10, and 475.00 of the NLR, PLR, and SII were used as cut-off values when we investigate the predictive value of these indicators on the clinicopathological characteristics. Although not based on the maximum Youden index, these cut-off points demonstrated clinically meaningful results in the succeeding statistical analysis, especially in HR+ cancers.
Many studies have shown that patients with lower NLR, PLR, and SII are more likely to achieve pCR after chemotherapy.17,26 In contrast, some studies have shown that NLR and PLR cannot independently predict pCR.15–18 Here, we revealed the independent predictive power of SII/NLR for poor chemotherapy response, but not pCR. Patients with an SII beyond a cut-off value of 586.40 were more likely to have a poor chemotherapeutic response. Notably, this cut-off value is similar to those reported in the related literature.21 Unfortunately, we failed to find an optimal NLR cut-off value. The meta-analysis of Ethier et al showed that the cut-off value of NLR predicting the prognosis of breast cancer patients in previous studies was in the interval of 1.9–5.0, with a median of 3.11 Therefore, many studies exploring the prognosis of breast cancer defined the NLR cut-off point as 3,27–29 but some scholars28,29 and we cannot obtain statistically significant results by using this cut-off value (data not shown). In future studies, we will recruit more cases and improve the experimental scheme, further explore the optimal cut-off value of NLR and verify the SII cut-off value proposed here. Additionally, the molecular HR+HER2- subtyping was found to act as an independent predictor of poor chemotherapeutic response, which is highly consistent with previous studies.17,30 Previous evidence shows that histological type of IDC,31 obesity,32 high degree of tumor invasion,33 positive PR status,30 and low Ki-6734 may be related factors of poor response to chemotherapy, which is also consistent with our findings, but these relationships no longer exist in the multivariate analysis. Some literature also reveals that age,15 neoadjuvant chemotherapy cycle,15 lymph node involvement,35 neoadjuvant chemotherapy regimen,35,36 combined targeted therapy37 and among others are related to the efficacy of chemotherapy, but we did not confirm the correlation between them in our research. Since the core needle biopsy of our clinical center did not determine the histological grade of the tumor most of the time, this factor was not included in the logistic regression analysis of poor chemotherapy response, although the histological grade has been showed by some scholars to independently predict the chemotherapy response.33 However, some studies asserted that histological grade is not an independent predictor of chemotherapy response,17 which therefore, needs to be further clarified in future studies. Pretherapeutic PLR was not significantly correlated with poor chemotherapeutic response, regardless of its use as a continuous variable or with a cut-off value of 137 for univariate and multivariate binary logistic regression. Indeed, PLR, which is the “twin brother” of NLR, has generally been found inferior to NLR in predicting pCR or prognosis,33,38,39 which is also consistent with our findings.
At present, some studies have used models based on peripheral blood inflammation indices to predict the efficacy of neoadjuvant chemotherapy in breast cancer patients23 or prognosis.40,41 Zhu et al23 established a model based on NLR, tumor size, hormone receptor status, and Ki-67 expression level, with an AUC of 0.705. In this study, a new predictive scoring model based on SII was established to predict the likelihood of a poor chemotherapy response in breast cancer patients, with an AUC of 0.751 (95% CI 0.675–0.827; P=0.000). When we removed SII from this model, the AUC dropped to 0.727, indicating the importance of SII to the model. In addition to SII, BMI, tumor T stage, Ki-67, and HER2 status were included in the model. Further stratified analysis of the four molecular subtypes revealed that the model has a better predictive ability for HR+ breast cancer, which may be attributed to a previous finding that the causality between high BMI and a worse outcome is stronger in HR+ breast cancer, but not other subtypes.42 These results are clinically important, as this breast cancer subtype has a low pCR rate. Accordingly, neoadjuvant chemotherapy should not be the first choice for HR+ patients with a risk score ≥ 3, and the surgery could be performed first. Certainly, although this study only involved patients receiving neoadjuvant chemotherapy, the results of the study can be analogized to all patients who need chemotherapy, especially considering we even included the patients with oligometastasis in the analysis.
Evidence indicates that the dynamic changes of peripheral blood inflammation indices have important predictive and monitoring significance for breast cancer patients.27,43 Our results revealed that SII tended to increase during the follow-up period. In addition, ROC curve analysis showed a specificity of recognizing cancer relapse greater than 98.0% at an SII cut-off value of 900. Therefore, we recommend that patients suspected of relapse with a peripheral SII value below 900 undergo further examination to assess whether the tumor has indeed recurred. Notably, many factors can alter peripheral blood indicators, for example, tamoxifen also has side effects related to neutropenia.44 However, endocrine therapy has little effect on peripheral blood count in practice, so it is generally recognized that these parameters continue to reflect the immune microenvironment even if endocrine therapy is started.45 Nevertheless, the effect of radiotherapy on peripheral blood cannot be ignored.46 Correspondingly, we set the second time for blood sampling to 3–6 months after the finish of surgery and (neo)chemotherapy, to dodge the influence of radiotherapy on peripheral blood inflammation indices. Moreover, we also excluded cases with abnormal values of the neutrophil count, lymphocyte count, or platelet count during the second blood collection. In future studies, we will further control variables that affect these indices and increase the blood sampling frequency to obtain better results.
Our research shows that the value of SII in the prediction, prognosis, and monitoring of breast cancer patients is more valuable than NLR and PLR, which is also coincident with the results of Jiang et al47 and Jiang et al13 Surely, the value of NLR, PLR, and SII in the diagnosis and treatment of breast cancer is not limited to this. Evidence suggests that these indicators may also have predictive value for the treatment response of endocrine therapy,48 eribulin,49 and everolimus,50 as well as the necessity of axillary lymph node dissection.51 Furthermore, these parameters are reported to have the potential to differentially diagnose breast cancer and benign breast diseases.15,52 Actually, in addition to NLR, PLR, and SII, there are many other inflammatory parameters derived from complete blood counts, including neutrophil count, lymphocyte count, platelet count, and derived NLR [neutrophil count/(white blood cell count minus neutrophil count) ratio], lymphocyte/monocyte ratio, neutrophil/monocyte ratio, red blood cell volume distribution width, platelet distribution width/platelet ratio,38,53,54 and other systemic inflammation parameters derived from the blood such as albumin/globulin ratio,55 C-reactive protein,56 and prognostic nutritional index,57 have all been found to be related to the diagnosis, prognosis, and management of breast cancer. Similarly, in our study, a significant difference in platelet count between the poor chemotherapy response group and the sensitive chemotherapy response group was also observed (P<0.01), but there was no significant difference in neutrophil count and lymphocyte count between the two groups. Most scholars believe that the combined use of multiple indicators can often provide better and more valuable information for the diagnosis and treatment of breast cancer.17,55,56 In this study, when we tried to combine NLR and PLR as a joint indicator, not only ROC curve analysis but also regression analysis of poor chemotherapy response had better predictive power than either alone. When we further combined another inflammation-related indicator—BMI (body mass index), a more statistically significant result was achieved (data not shown). Therefore, the combined utilization of a number of systemic inflammation parameters with no collinearity-ship will also be a key direction of our next research.
Two main limitations must be acknowledged. Firstly, as a single-center retrospective study, the results of the study are not necessarily applicable to other patient populations. Secondly, the sample sizes analyzed in the latter two analyses are relatively small (n=258, n=53, respectively), which may have an impact on some research results. Notwithstanding these limitations, our study provides evidence support for better predicting the clinicopathological characteristics, therapeutic response, and relapse of breast cancer patients, but it needs to be further verified in prospective studies with larger sample sizes in the future.
Conclusions
Pretherapeutic NLR, PLR, and SII were correlated with multiple clinicopathological characteristics of patients with breast cancer, especially in HR+ cancers. Higher peripheral blood inflammation indices indicate higher risk factors for breast cancer. Breast cancer patients with higher pretherapeutic SII and/or NLR were more likely to have a worse result of neoadjuvant chemotherapy, and an SII cut-off point of 586.40 was observed to be reasonable, feasible, and effective. Besides, SII has a certain value in monitoring the recurrence and metastasis of breast cancer. Collectively, SII may be the superior predictor among the three indices.
Abbreviations
AJCC, American Joint Committee on Cancer; AUC, area under the curve; BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; IDC, invasive ductal carcinoma; IL, interleukin; ILC, invasive lobular carcinoma; IQR, interquartile range; LVI, lymphovascular invasion; MP, Miller-Payne; NLR, neutrophil-to-lymphocyte Ratio; OR, odds ratio; pCR, pathological complete response; PLR, platelet-to-lymphocyte ratio; PLT, platelet; PR, progesterone receptor; ROC, receiver operating characteristic; ROS, reactive oxygen species; SII, systemic immune-inflammation index; TNBC, triple-negative breast cancer.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Ethics Approval and Consent to Participate
This study was reviewed and approved by Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine (ethical approval NO. 2021-0295), which waived the informed consent requirement due to the retrospective design of the study. This study complied with the Declaration of Helsinki. The patient data was maintained with confidentiality.
Acknowledgments
The authors wish to thank all patients who participated in this study.
Funding
This work was funded by Natural Science Foundation of Zhejiang Province (LQ19H160045).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
3. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
4. Yoon CI, Park S, Cha YJ, et al. Associations between absolute neutrophil count and lymphocyte-predominant breast cancer. Breast. 2020;50:141–148. doi:10.1016/j.breast.2019.09.013
5. Romero-Cordoba S, Meneghini E, Sant M, et al. Decoding immune heterogeneity of triple negative breast cancer and Its association with systemic inflammation. Cancers. 2019;11(7):911. doi:10.3390/cancers11070911
6. Mazaki J, Katsumata K, Kasahara K, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20(1):922. doi:10.1186/s12885-020-07429-5
7. Wang G, Tan Y, Jiang Y, et al. Prognostic model of D2 radical gastrectomy combined with neoadjuvant chemotherapy for gastric cancer. Risk Manag Healthc Policy. 2023;16:1259–1271. doi:10.2147/RMHP.S413052
8. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
9. Prodromidou A, Andreakos P, Kazakos C, et al. The diagnostic efficacy of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ovarian cancer. Inflamm Res. 2017;66(6):467–475. doi:10.1007/s00011-017-1026-6
10. Liu P, Chen S, Gao X, et al. Preoperative sarcopenia and systemic immune-inflammation index can predict response to intravesical Bacillus Calmette-Guerin instillation in patients with non-muscle invasive bladder cancer. Front Immunol. 2022;13:1032907. doi:10.3389/fimmu.2022.1032907
11. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi:10.1186/s13058-016-0794-1
12. Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–4148. doi:10.1002/cam4.2281
13. Jiang C, Lu Y, Zhang S, Huang Y. Systemic immune-inflammation index is superior to neutrophil to lymphocyte ratio in prognostic assessment of breast cancer patients undergoing neoadjuvant chemotherapy. Biomed Res Int. 2020;2020:7961568. doi:10.1155/2020/7961568
14. Xu T, Zhang SM, Wu HM, et al. Prognostic significance of prognostic nutritional index and systemic immune-inflammation index in patients after curative breast cancer resection: a retrospective cohort study. BMC Cancer. 2022;22(1):1128. doi:10.1186/s12885-022-10218-x
15. Peng Y, Chen R, Qu F, et al. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol Ther. 2020;21(2):189–196. doi:10.1080/15384047.2019.1680057
16. Muñoz-Montaño W, Cabrera-Galeana P, Alvarado-Miranda A, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in different phenotypes of locally advanced breast cancer During neoadjuvant systemic treatment. Clin Breast Cancer. 2020;20(4):307–316.e1. doi:10.1016/j.clbc.2019.12.011
17. Graziano V, Grassadonia A, Iezzi L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019;44:33–38. doi:10.1016/j.breast.2018.12.014
18. Qian Y, Tao J, Li X, et al. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 2018;11:1423–1432. doi:10.2147/OTT.S148496
19. Bhikram T, Sandor P. Neutrophil-lymphocyte ratios as inflammatory biomarkers in psychiatric patients. Brain Behav Immun. 2022;105:237–246. doi:10.1016/j.bbi.2022.07.006
20. Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta-analysis. Int J Surg. 2018;55:73–80. doi:10.1016/j.ijsu.2018.05.022
21. Zhang Y, Sun Y, Zhang Q. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int. 2020;20(1):224. doi:10.1186/s12935-020-01308-6
22. Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. doi:10.1007/s12032-012-0432-4
23. Zhu J, Jiao D, Zhao Y, et al. Development of a predictive model utilizing the neutrophil to lymphocyte ratio to predict neoadjuvant chemotherapy efficacy in early breast cancer patients. Sci Rep. 2021;11(1):1350. doi:10.1038/s41598-020-80037-2
24. Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J buon. 2015;20(3):714–722.
25. Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15(1):195. doi:10.1186/s12885-015-1204-2
26. Corbeau I, Jacot W, Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers. 2020;12(4):958. doi:10.3390/cancers12040958
27. Iwase T, Sangai T, Sakakibara M, et al. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol Clin Oncol. 2017;6(2):266–270. doi:10.3892/mco.2016.1101
28. Che YQ, Zhang Y, Wang D, et al. Baseline lymphopenia: a predictor of poor outcomes in HER2 positive metastatic breast cancer treated with trastuzumab. Drug Des Devel Ther. 2019;13:3727–3734. doi:10.2147/DDDT.S212610
29. Watanabe J, Saito M, Horimoto Y, Nakamoto S. A maintained absolute lymphocyte count predicts the overall survival benefit from eribulin therapy, including eribulin re-administration, in HER2-negative advanced breast cancer patients: a single-institutional experience. Breast Cancer Res Treat. 2020;181(1):211–220. doi:10.1007/s10549-020-05626-1
30. Lips EH, Mulder L, de Ronde JJ, et al. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat. 2013;140(1):63–71. doi:10.1007/s10549-013-2620-0
31. Nagao T, Kinoshita T, Hojo T, et al. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 2012;21(3):289–295. doi:10.1016/j.breast.2011.12.011
32. Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26(25):4072–4077. doi:10.1200/JCO.2007.14.4527
33. Eren T, Karacin C, Ucar G, et al. Correlation between peripheral blood inflammatory indicators and pathologic complete response to neoadjuvant chemotherapy in locally advanced breast cancer patients. Medicine (Baltimore). 2020;99(22):e20346. doi:10.1097/MD.0000000000020346
34. Chen X, He C, Han D, et al. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. Future Oncol. 2017;13(9):843–857. doi:10.2217/fon-2016-0420
35. Bae SJ, Cha YJ, Yoon C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in human epidermal growth factor receptor 2-negative breast cancer patients who received neoadjuvant chemotherapy. Sci Rep. 2020;10(1):13078. doi:10.1038/s41598-020-69965-1
36. Pathak M, Dwivedi SN, Deo SVS, et al. Neoadjuvant chemotherapy regimens in treatment of breast cancer: a systematic review and network meta-analysis protocol. Syst Rev. 2018;7(1):89. doi:10.1186/s13643-018-0754-1
37. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi:10.1016/S1470-2045(11)70336-9
38. Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014;7:1743–1752. doi:10.2147/OTT.S69657
39. Liu C, Huang Z, Wang Q, et al. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in hormone-receptor-negative breast cancer. Onco Targets Ther. 2016;9:4653–4660. doi:10.2147/OTT.S106017
40. Xu Y, Ju L, Tong J, Zhou C, Yang J. Supervised machine learning predictive analytics for triple-negative breast cancer death outcomes. Onco Targets Ther. 2019;12:9059–9067. doi:10.2147/OTT.S223603
41. Polley MC, Leon-Ferre RA, Leung S, et al. A clinical calculator to predict disease outcomes in women with triple-negative breast cancer. Breast Cancer Res Treat. 2021;185:557–66.
42. Ballinger TJ, Jiang G, Kassem N, Radovich M, Schneider BP. Impact of body mass index on presence of ctDNA and disease recurrence after neoadjuvant chemotherapy for triple-negative breast cancer: analysis from BRE12-158. Clin Cancer Res. 2021;27(4):1195–1199. doi:10.1158/1078-0432.CCR-20-3341
43. Liu J, Ma F, Sun B, et al. Predictive value of lymphocyte-related blood parameters at the time point of lymphocyte Nadir during radiotherapy in breast cancer. Onco Targets Ther. 2020;13:151–161. doi:10.2147/OTT.S233244
44. Miké V, Currie VE, Gee TS. Fatal neutropenia associated with long-term tamoxifen therapy. Lancet. 1994;344(8921):541–542. doi:10.1016/S0140-6736(94)91929-1
45. Takada K, Kashiwagi S, Asano Y, et al. Clinical evaluation of dynamic monitoring of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in primary endocrine therapy for advanced breast cancer. Anticancer Res. 2019;39(10):5581–5588. doi:10.21873/anticanres.13752
46. Rotstein S, Blomgren H, Petrini B, Wasserman J, Baral E. Long term effects on the immune system following local radiation therapy for breast cancer. I. cellular composition of the peripheral blood lymphocyte population. Int J Radiat Oncol Biol Phys. 1985;11(5):921–925. doi:10.1016/0360-3016(85)90114-2
47. Jiang L, Fang J, Ding J. High systemic immune-inflammation index predicts poor survival in patients with human epidermal growth Factor receptor-2 positive breast cancer receiving adjuvant trastuzumab. Cancer Manag Res. 2020;12:475–484. doi:10.2147/CMAR.S231444
48. Iimori N, Kashiwagi S, Asano Y, et al. Clinical significance of the neutrophil-to-lymphocyte ratio in endocrine therapy for stage IV breast cancer. In Vivo (Brooklyn). 2018;32(3):669–675. doi:10.21873/invivo.11292
49. Miyagawa Y, Araki K, Bun A, et al. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. 2018;18(5):400–409. doi:10.1016/j.clbc.2018.03.002
50. Schettini F, Sobhani N, Ianza A, et al. Immune system and angiogenesis-related potential surrogate biomarkers of response to everolimus-based treatment in hormone receptor-positive breast cancer: an exploratory study. Breast Cancer Res Treat. 2020;184(2):421–431. doi:10.1007/s10549-020-05856-3
51. Ibrahim A, Serkan YF, Tuba A, Erol B, Lütfi P. Can neutrophil to lymphocyte ratio be a predictor tool for the non-sentinel lymph node metastasis in breast cancer? Chirurgia (Bucur). 2019;114(1):83–88. doi:10.21614/chirurgia.114.1.83
52. Fang Q, Tong YW, Wang G, et al. Neutrophil-to-lymphocyte ratio, obesity, and breast cancer risk in Chinese population. Medicine (Baltimore). 2018;97(30):e11692. doi:10.1097/MD.0000000000011692
53. Chen L, Kong X, Yan C, Fang Y, Wang J. The research progress on the prognostic value of the common hematological parameters in peripheral venous blood in breast cancer. Onco Targets Ther. 2020;13:1397–1412. doi:10.2147/OTT.S227171
54. Pivatto Júnior F, Santos ÂBS, Englert EF, et al. Monocyte-to-lymphocyte ratio as predictor of cancer therapy-related cardiotoxicity in patients with breast cancer: a pilot cohort study. Breast Cancer Res Treat. 2023;200(3):355–362. doi:10.1007/s10549-023-06979-z
55. Xuan Q, Yang Y, Ji H, et al. Combination of the preoperative albumin to globulin ratio and neutrophil to lymphocyte ratio as a novel prognostic factor in patients with triple negative breast cancer. Cancer Manag Res. 2019;11:5125–5131. doi:10.2147/CMAR.S195324
56. Sata A, Fukui R, Miyagawa Y, et al. C-reactive protein and absolute lymphocyte count can predict overall survival of patients treated with eribulin. Anticancer Res. 2020;40(7):4147–4156. doi:10.21873/anticanres.14414
57. Oba T, Maeno K, Ono M, et al. Prognostic nutritional index is superior to neutrophil-to-lymphocyte ratio as a prognostic marker in metastatic breast cancer patients treated with eribulin. Anticancer Res. 2021;41(1):445–452. doi:10.21873/anticanres.14794
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



