Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Risk Factors of Pancreatic Exocrine Insufficiency Developed After Acute Pancreatitis: A Retrospective Cohort Study
Authors Guo Y , Wang X, Wang S, Li A, Cao F , Li F
Received 10 October 2022
Accepted for publication 25 February 2023
Published 15 March 2023 Volume 2023:16 Pages 1157—1167
DOI https://doi.org/10.2147/JIR.S392932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yulin Guo,1,2,* Xiaohui Wang,1,2,* Shuo Wang,1,2,* Ang Li,1,2 Feng Cao,1,2 Fei Li1,2
1Department of General Surgery, Xuanwu Hospital of Capital Medical University, Beijing, People’s Republic of China; 2Acute Pancreatitis Clinical Center of Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Li; Feng Cao, Department of General Surgery, Xuanwu Hospital, Capital Medical University, No. 45 Changchun Street, Beijing, 100053, People’s Republic of China, Tel/Fax +86-10-83198835, Email [email protected]; [email protected]
Purpose: The aim of this study was to compare the clinical characteristics of acute pancreatitis (AP) patients between those who developed pancreatic exocrine insufficiency (PEI) and those who did not, and to investigate the predictive factors of PEI.
Patients and Methods: From October 1st 2019 to July 30th 2021, AP patients admitted at our center were included. The fecal elastase-1 assay was adopted for PEI diagnosis. The clinical characteristics, treatments, and outcomes between the patients with and without PEI were analyzed.
Results: In total, 63 males and 42 females were included. There were 27 patients with mild AP, 54 with moderately severe AP, and 24 with severe AP. The median modified computed tomography severity index (MCTSI) was 6.000(4.000, 8.000). During the follow-up, 38 patients developed PEI after AP. The univariate analysis showed that higher ASA grade (P = 0.006), more severe AP (P = 0.000), the presence of multiple organ dysfunction syndrome (P = 0.030), higher MCTSI (P = 0.000), the development of infected pancreatic necrosis (P = 0.002) and local complications (P = 0.000), higher levels of triacylglycerol (P = 0.022), video-assisted retroperitoneal debridement intervention (P = 0.015), and longer intensive care unit stay (P = 0.044) were correlated with PEI development. Furthermore, the logistic regression analyses showed that MCTSI during hospitalization is an independent risk factor for PEI development during the AP recovery period.
Conclusion: ASA grade, severity of AP, multiple organ dysfunction syndrome, MCTSI, infected pancreatic necrosis, local complications, higher levels of triacylglycerol, video-assisted retroperitoneal debridement intervention, and longer intensive care unit stay were potentially associated with PEI development during the AP recovery period. High MCTSI was independently associated with the development of PEI during the AP recovery period, which may help alert to the possibility of PEI to help with its early detection and treatment.
Keywords: acute pancreatitis, pancreatic exocrine insufficiency, modified computed tomography severity index, fecal elastase-1
Plain Language Summary
We conducted this study to identify the differences in the clinical characteristics between patients with pancreatic exocrine insufficiency that developed after acute pancreatitis and those without pancreatic exocrine insufficiency.
We also aimed to identify the predictive risk factors for pancreatic exocrine insufficiency after acute pancreatitis.
Pancreatic exocrine insufficiency could lead to indigestion and absorption disorders. Studies on the clinical features and predictive risk factors for pancreatic exocrine insufficiency development are still limited, especially in China.
The findings of our study may benefit patients by providing early indicators for pancreatic exocrine insufficiency that has developed during the acute pancreatitis recovery period, and this can contribute to the early detection of pancreatic exocrine insufficiency and its treatment.
We conducted a prospective cohort study, and all acute pancreatitis patients admitted at our center during October 1st 2019 to July 30th 2021 were included. We applied the fecal elastase-1 assay for the diagnosis of pancreatic exocrine insufficiency.
You are welcome to communicate with our corresponding author on the results and discoveries of our study through email.
Introduction
Pancreatic exocrine insufficiency (PEI) is a maldigestion condition which is induced by inadequate production, insufficient and asynchronous secretion, and inactivation of pancreatic enzymes. PEI could lead to symptoms such as indigestion and absorption disorders. Malnutrition arising from PEI usually leads to decreased levels of essential amino acids, fatty acids, microelements, fat-soluble vitamins, high-density lipoprotein C, and lipoprotein A, resulting in osteoporosis, weakened immunity, and increased risk of cardiovascular events.1–7 All these pathological changes seriously affect the quality of life of patients with PEI.
Acute pancreatitis (AP) is one of the most common inflammatory diseases that causes PEI, due to direct pancreatic acinar injury, obstruction in pancreatic secretion caused by pancreatic duct blockage, or unsynchronized release of pancreatic enzymes.8
PEI can be detected by direct and indirect tests. Direct detection methods including the detection of pancreatotropin, cholecystokinin, or transdermal hormone after medical stimulation, have the advantage of high accuracy, but these methods are invasive and time-consuming.9,10 Among these, the fecal elastase 1(FE-1) test is the most reliable indirect pancreatic function test, with relatively high accuracy, practicability, and lower cost.11,12
Since the quality of life for AP patients with PEI may be seriously affected, early detection of PEI is of great importance to guide proper nutritional management.3,13 The present study was conducted to evaluate the PEI status and characteristics of patients recovering from AP in a single center from China, and this study explores the predictive risk factors for PEI, which may provide early detection and treatment of these patients.
Materials and Methods
Patients and Clinical Records
Patients diagnosed with AP between October 1st 2019 and July 30th 2021 in “Pancreatic Disease Clinical Center”, Department of General Surgery were contacted. The present study was reviewed and approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University (NO.: [2019]057), and all patients signed written informed consent. All patients were followed for six to twelve months with outpatient clinic visits. The definition for the diagnosis of AP followed the Atlanta criteria (2012).14–16 Patients with chronic pancreatitis, pancreatic cancer, cystic fibrosis, neuroendocrine tumors, inflammatory intestinal disease, a history of gastrointestinal surgery, a history of diabetes, or a prior diagnosis of PEI were excluded.
Assessment of Pancreatic Exocrine Function
Pancreatic exocrine function was assessed by an FE-1 testing kit (ScheBo® Biotech, Pancreatic Elastase 1 stool test, Giessen, Germany). The recovery rate ranges from 85.0 to 115.0. The limit of blank is no more than 1.18. The limit detection ranges from 15ug/g to 300ug/g, and the correlation coefficient should be no less than 0.9900. The intra CV is no more than 15.0 under 10 repetition tests. The inter CV is no more than 15.0 among the ELISA kits of 3 different batch Numbers. Fecal samples were collected from the patients who recovered from their AP attack without drainage tubes and pancreatic fistulae, and these samples were taken between six to twelve months after the AP attack. All the samples were immediately stored at −20°C for the FE-1 tests. According to the manufacturer, FE-1 levels < 200 µg/g were defined as PEI.17,18 The FE-1 tests for each sample were performed three times.
Outcomes of Interests
The severity of AP was assessed according to the Atlanta criteria (2012).16,19 The modified CT severity index (MCTSI) was adopted to assess the extent of necrosis and evaluate the severity of AP.20 The presence of infected pancreatic necrosis (IPN) can be confirmed, when extraluminal gas is found in the pancreatic and peripancreatic tissues on CECT or if there is a positive fine-needle aspiration culture of peripancreatic effusion.21
After admission, all patients received intravenous fluid resuscitation, nutritional support, and anti-inflammatory treatment. When the patient’s condition deteriorated irrespective of the antibiotic treatment, interventional approaches including percutaneous catheter drainage (PCD), video-assisted retroperitoneal debridement (VARD), and laparoscopic pancreatic debridement were performed as described previously.22,23
The items of interest include biochemical tests, inpatient therapy, complications, etc. Questionnaires during the follow-up were completed, and were used to investigate the patients’ symptoms, latest biochemical tests, and FE-1 test results. EuroQoL Five-dimensions (EQ-5D) was adopted to assess the daily health status of the patients.
Statistical Analysis
All analyses were performed using SPSS Statistics version 26.0 software (IBM, NY, USA). Only those who completed at least a six month follow-up were analyzed. Continuous variables were compared using t tests or the Mann–Whitney-U test. Categorical variables were compared using chi-square or Fisher’s exact-tests. A univariable analysis was performed between the values of FE-1 and other characteristic items. Then, the items of interest that obtained statistical significance during the univariable analysis were further analyzed in multivariable logistic regression. P value < 0.05 were considered significant.
Results
Baseline Clinical Characteristics, Treatments During Hospitalization, and Outcomes After AP
During the study period, a total of 165 AP patients were admitted to our center. Among them, 22 patients were excluded due to death or loss of contact. Thirty-eight patients could not present to our hospital to complete the clinic visit and FE-1 test, due to the long distance or the COVID-19 prevention and control protocols. Finally, 105 patients participated in the present study and completed the follow-up.
There were 63 males and 42 females, with a mean age of 50.105 ± 13.865 years old. The ASA status, etiologies and severity of AP, MCTSI, local complications, and levels of laboratory tests at admission of the enrolled patients are shown in Table 1. For treatment, 39 patients underwent VARD. The surgical strategy and length of ICU stay are shown in Table 2. The median follow-up time was 9(8–11) months. During the follow-up, the median level of FE-1 was 278.204(160.063, 642.835) ug/g, with 38 patients diagnosed with PEI after AP. The median weight loss for the patients was 3.000(0.000, 9.250) kg, and the median quality of life (QOL) was 85.000(75.000, 88.000).
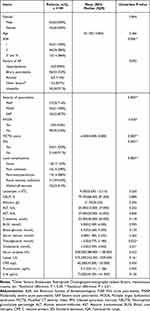 |
Table 1 Baseline Characteristics for Patients and Their Correlation with Pancreatic Exocrine Insufficiency After Acute Pancreatitis |
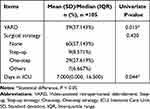 |
Table 2 Treatment During Hospitalization for Acute Pancreatitis Patients and Their Correlation with Pancreatic Exocrine Insufficiency After Acute Pancreatitis |
Baseline Clinical Characteristics and Treatments During Hospitalization Compared Between PEI and Non-PEI Patients Who Were Diagnosed After AP
As shown in Table 3, the patients who developed PEI had higher ASA scores than those who did not develop PEI (P=0.009). The percentage of hyperlipidemia related AP was higher in the patients who developed PEI than in those who did not (P=0.000, Table 3). The incidences of MSAP and SAP were higher in the patients who developed PEI than in those who did not (P=0.000, Table 3). In addition, the incidences of MODS was higher in the patients who developed PEI (P=0.046, Table 3). The MCTSI were 8.000(6.000, 10.000) in the patients who developed PEI, higher than in the non-PEI patients [4.000(4.000, 8.000), P=0.000; Table 3]. Moreover, in the patients who developed PE-1, there was had a higher percentage of patients with IPN than that in the non-PEI patients (P=0.002, Table 3). Regarding local complications, the percentages of the patients with acute necrotic collection and walled-off necrosis were higher in the patients who developed PEI than in those who did not (P=0.001, Table 3). As for gender, age, and etiology, there was no significant difference between the patients with and without PEI (Table 3).
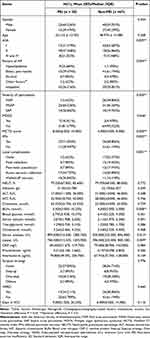 |
Table 3 Baseline Clinical Characteristics and Treatments Between Patients with and without Pancreatic Exocrine Insufficiency That Developed After Acute Pancreatitis |
Regarding the laboratory tests upon admission, the levels of blood urea nitrogen (BUN) were significantly higher in the PEI patients than in the non-PEI patients [5.500(3.000, 10.250) vs 4.000(3.000, 5.000), P = 0.008; Table 3]. Moreover, both the levels of serum amylase and lipase were significantly higher in the patients who developed PEI [899.500(510.500, 1385.750) for amylase and 760.500(312.525, 1243.900) for lipase, respectively] than in the patients who did not develop PEI [509.000(335.000, 896.000) for amylase and 396.500(223.000, 800.100) for lipase, respectively; P = 0.015 and P = 0.049, respectively; Table 3]. There was no significant difference found in the other laboratory tests between the patients who developed PEI and those who did not (Table 3). During hospitalization, there were no significant differences found in surgical strategy and length of ICU stay between the patients who developed PEI and those who did not (Table 3).
Follow-Up Outcomes for PEI and Non-PEI Patients Diagnosed After AP
During the follow-up, 19 PEI patients suffered weight loss, which was higher than the 9 patients who had weight loss in the non-PEI group (P=0.000, Table S1). Fatty diarrhea was found in 9 PEI patients, and this number was significantly higher than in the non-PEI group, which had no patients who had fatty diarrhea (P=0.001, Table S1). During follow-up, 33 patients developed recurrences of AP (r-AP), with 17 patients who did not develop PEI and 16 in those who developed PEI (P=0.076, Table S1). A total of 8 patients developed chronic pancreatitis, with 2 in the patients who did not develop PEI and 6 in those who developed PEI (P=0.046, Table S1). And 29 patients developed diabetes, with 16 in the patients who did not develop PEI and 13 in those who developed PEI (P=0.255, Table S1). During the follow-up, a total of 30 patients with PEI required pancreatic enzyme replacement therapy. The quality of life (QOL) scores were similar between the PEI group and the non-PEI group [85.000(80.000, 88.000) vs 85.000(75.000, 95.000), P = 0.879, Table S1].
Univariate and Multivariate Analyses of Factors Associated with PEI After AP
The univariate correlation analysis showed that higher ASA grade (P = 0.006), more severe acute pancreatitis (P = 0.000), the presence of MODS (P = 0.030), higher MCTSI score (P = 0.000), the development of IPN (P = 0.002), local complications (P = 0.000), higher level of triacylglycerol (P = 0.022), VARD intervention (P=0.015), and longer ICU stay (P = 0.044) were correlated with PEI (Table 1 and Table 2). Further multivariate analysis was conducted based on the univariate analysis. As shown in Table 4, MCTSI was independently associated with PEI after AP (OR = 3.308; 95% CI: 1.537–7.121; P = 0.002; Table 4).
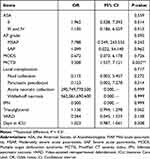 |
Table 4 Multivariate Analysis of Factors Associated with Pancreatic Exocrine Insufficiency That Developed After Acute Pancreatitis |
Discussion
The previously published experiment has confirmed that decreased enzyme, bicarbonate, and pancreatic fluid secretions happened at the early stage after an episode of AP.24 PEI after acute pancreatitis may be referred to as obstruction in the ductal outlet, or the reduction of pancreatic parenchyma injured during AP.25 Injuries of pancreatic parenchyma caused by AP were observed, including acinar cell necrosis, dilatation and degranulation in the rough endoplasmic reticulum (RER), swelling of mitochondria, and retardation of autophagic vacuoles. RER degranulation could induce decreased synthesis of proteins. Mitochondrial damage could induce cellular injury to pancreatic cells. The dysfunctional autophagy process is toxic to pancreatic tissues. These dysfunctions of cellular organelles together with acinar cell necrosis may lead to damage to acinar and ductal cells, accounting for PEI in AP. During the AP attack, surgical interventions and drainage obstruction of secretions in the pancreas could contribute to a varying decrease in exocrine function.26 Moreover, during the AP attack, the responsiveness and sensitivity of pancreatic cells are markedly reduced, which may partly contribute to PEI.27
The manifestations of PEI are related to the decompensation of pancreatic exocrine function. In the present study, the incidence of PEI after AP was approximately 36.190%, which was coincident with the previously reported incidences ranging from 29%-56%.21 Previous studies have reported about 10% of patients with AP will develop chronic pancreatitis.28 The percentage of chronic pancreatitis in the present study was 7.619%. The lower rate may be attributed to the relatively short follow-up period in the present study. The percentage of r-AP in the present study was 31.429%, which is near the range reported by Huang et al.29 The percentage of diabetes is 27.62% in the present study, which is near the result reported by Tu et al.30
Regarding the laboratory tests upon admission, the levels of serum amylase and lipase were significantly higher in patients who developed PEI. These results to a certain extent reflected a relatively more severe AP for the patients who developed PEI than those who did not. In addition, the levels of blood urea nitrogen from patients who developed PEI were significantly higher. The higher level of blood urea nitrogen may indicate hypoperfusion, renal dysfunction, and eating disorder related to AP attack. Moreover, higher levels of blood urea nitrogen in patients upon AP attack were prediction factors for SAP and mortality.31,32 All these results indicated a relatively more severe form of AP upon admission for patients who developed PEI after AP. More severe AP may cause more injuries of pancreatic parenchyma, which may contribute to PEI development.
The present study showed that PEI was associated with more severe acute pancreatitis and the presence of MODS. This result is in line with the study conducted by Garip et al, which showed that the levels of FE-1 in SAP patients were lower than those in non-SAP patients, indicating a more severe PEI in SAP patients.33 The presence of MODS was reported to be correlated with the severity of AP, which may partially explain its association with PEI.34
The present study revealed that the development of IPN and local complications were also potential risk factors for developing PEI after AP, which was coincident with the study conducted by Tu et al.30 In this study, the percentage of patients who developed PEI after AP was higher in the patients with pancreatic necrosis. In the present study, the percentage of acute necrotic collection and walled-off necrosis during hospitalization were higher in the patients who developed PEI after AP. It has been confirmed that IPN could directly cause damage to pancreatic acinar cells, and exacerbate the obstruction to the ductal system, which could increase the incidence of PEI.18,35
The present study showed that PEI was associated with higher levels of triacylglycerol. This result was also closely related to the higher percentage of hyperlipidemia related AP in the patients who developed PEI. A previous systematic review noted that hyperlipidemia pancreatitis was a more severe form of pancreatitis.36 It has been inferred that hyperlipidemia may damage pancreatic acinar cells, induce endoplasmic reticulum stress, and lead to pancreatic microcirculation disorder.37,38 Due to a lack of direct evidence, the relationship between hypertriglyceridemia and PEI is potentially debatable.
The present study showed that PEI was associated with VARD intervention and a longer ICU stay. VARD intervention was the main surgical intervention for pancreatic necrosis. This result was in line with the study conducted by Huang et al,29 which reported that necrosectomy was associated with the development of PEI. Longer ICU stay was also an indicator for the severity of AP, indicating its potential correlation with PEI development.
The present study revealed that MCTSI was independently associated with PEI, indicating that patients with higher MCTSI scores during hospitalization are prone to developing PEI. The MCTSI scoring system emphasizes the crucial role of the clinical systemic score during the early phase and CECT imaging evaluation during the late phase,16,20 which is accurate in assessing the severity of AP. Thus, this result further indicated that the severity of AP was associated with PEI.
The clinical diagnosis of PEI in China is mainly based on the clinical symptoms, but this method has low sensitivity and accuracy. The present study applied the FE-1 test in diagnosing PEI. During follow-up, we found that there were significant differences between the PEI group and the non-PEI group in weight loss and fatty diarrhea. These results confirmed the accuracy of FE-1 testing for PEI diagnosis. The present study identified MCTSI as an independent risk factor for PEI based on a Chinese patient group, and this study may with the early detection of PEI and the initiation of PERT, which can prevent the risk of malnutrition and long-term complications.1,39
There are several limitations in our study. First, it was a single-center, cohort study, and selection bias could not be prevented. Second, the collection of stool samples from the patients was difficult due to the COVID-19 pandemic, with the quarantine and hampered express delivery. Third, due to a lack of relevant studies, the associations between ASA grade, MODS, and triacylglycerol with PEI were not fully discussed. Fourth, this research mainly employed the FE-1 assay in the diagnosis of PEI. Studies showed the sensitivity of FE-1 in the detection of mild PEI was about 0.47, which can lead to false negative results and missing diagnosis of PEI.40 Moreover, the watery stool samples of nonpancreatic origin may be tested as falsely low results.25 When conducting the FE-1 test, the patient should eat a normal diet. However, during the early AP recovery period, it is unlikely that patients are back to eating normal diets, which may contribute to false results.41 More diagnostic tools should be employed in future follow-up studies. Finally, the follow-up period of the present study is only 6–12 months. Studies had reported that the prevalence of PEI during the AP attack could be as high as 60%. The prevalence of PEI decreased along with the time to about 30% at 3 years after AP.29,30 And the prevalence of EPI could continue to change until 5 years after the AP attack. Thus, the relatively short follow-up limited the visit times and recordings. The Study with a longer follow-up time is still needed.
Conclusion
This study investigated the predictive factors within the clinical characteristics, laboratory tests, and treatments for the development of PEI during the recovery period of AP. The present study showed that PEI development after AP was potentially associated with ASA grade, severity of AP, presence of MODS, MCTSI, local complications, level of triacylglycerol, VARD intervention, and length of ICU stay. However, only high MCTSI score was independently associated with the development of PEI after AP. The quantitative assessment MCTSI scoring system may help alert clinicians for the early detection and treatment of PEI following AP.
Abbreviations
ASA, the American Society of Anesthesiologists; MAP, Mild acute pancreatitis; MSAP, Moderately severe acute pancreatitis; SAP, Severe acute pancreatitis; MODS, Multiple organ dysfunction syndrome; MCTSI, Modified CT severity index; IPN, Infected pancreatic necrosis; NEUT%, Neutrophilic granulocyte percentage; ALT, Alanine aminotransferase; AST, Aspartic transaminase; BUN, Blood urea nitrogen; CRP, C reactive protein; Step-up, Step-up strategy; One-step, One-step strategy; VARD, Video-assisted retroperitoneal debridement; ICU, Intensive Care Unit, PEI, pancreatic exocrine insufficiency.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University (NO.: [2019]057) and informed consents were taken from all individual participants. The present study was registered at the Chinese Clinical Trial Registry, and the registration number is ChiCTR1900028634.
Acknowledgments
In the process of preparing our article, we received valuable advice and assistance with study design from Professor Taiping Zhang and Qiang Xu from basic surgery of Beijing Union Medical College due to the Construction Project of Clinical Advanced subjects of Capital Medical University. We would like to say thank you for them to serve as scientific advisors. The authors declare that we have no conflicts of interest to disclose.
Author Contributions
All authors made a significant contribution to the work reported:
Conception and design: Yulin Guo, Feng Cao, Fei Li;
Execution support: Yulin Guo, Shuo Wang, Xiaohui Wang, Ang Li, Feng Cao;
acquisition of data: Yulin Guo, Shuo Wang, Xiaohui Wang, Ang Li;
Data analysis and interpretation: Yulin Guo, Feng Cao, Fei Li;
All authors took part in drafting, revising or critically reviewing the article:
Manuscript drafted and written: Yulin Guo, Shuo Wang;
Manuscript supervision and revised: Xiaohui Wang, Ang Li, Feng Cao, Fei Li;
All authors gave final approval of the version to be published;
All authors have agreed on the journal to which the article has been submitted;
All authors are agree to be accountable for all aspects of the work.
Funding
This study was supported by the Construction Project of Clinical Advanced subjects of Capital Medical University [No. 1192070312 to F.L.]; and the Beijing Municipal Science & Technology Commission [No. Z171100001017077 to F.L., No. Z191100006619038 to F.C.]; and the Capital Health Research and Development of Special [No.2020-1-2012 to F.L.]; and the Beijing Postdoctoral Funding for Scientific Research [No. ZZ2019-18 to Y.G.].
Disclosure
The authors have no competing interests to declare.
References
1. Domínguez-Muñoz J, Iglesias-García J, Iglesias-Rey M, et al. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005;21:993–1000. doi:10.1111/j.1365-2036.2005.02390.x
2. Duggan S, Smyth N, O’Sullivan M, et al. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract. 2014;29(3):348–354. doi:10.1177/0884533614528361
3. de la Iglesia-Garcia D, Vallejo-Senra N, Iglesias-Garcia J, et al. Increased risk of mortality associated with pancreatic exocrine insufficiency in patients with chronic pancreatitis. J Clin Gastroenterol. 2018;52(8):e63–e72. doi:10.1097/MCG.0000000000000917
4. Bresnahan K, Tanumihardjo S. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr. 2014;5(6):702–711. doi:10.3945/an.114.006361
5. Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015;2015:109275. doi:10.1155/2015/109275
6. Barkin BJA, Barkin Jamie S. Chronic pancreatitis and bone disease. J Clin Densitom. 2020;23(2):237–243. doi:10.1016/j.jocd.2019.08.004
7. Shintakuya R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017;17(1):70–75. doi:10.1016/j.pan.2016.10.005
8. Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: systematic review and study level meta-analysis. Pancreatology. 2018;18:253–262. doi:10.1016/j.pan.2018.02.009
9. Dominguez-Muñoz J. Diagnosis and treatment of pancreatic exocrine insufficiency. Curr Opin Gastroenterol. 2018;34(5):349–354. doi:10.1097/MOG.0000000000000459
10. Laterza L, Scaldaferri F, Bruno G, et al. Pancreatic function assessment. Eur Rev Med Pharmacol Sci. 2013;17(Suppl):65–71.
11. Löser C, Brauer C, Aygen S, et al. Comparative clinical evaluation of the 13C-mixed triglyceride breath test as an indirect pancreatic function test. Scand J Gastroenterol. 1998;33:327–334. doi:10.1080/00365529850170946
12. Löser C, Möllgaard A, Fölsch U. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–586. doi:10.1136/gut.39.4.580
13. Sikkens E, Cahen D, Kuipers E, et al. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24(3):337–347. doi:10.1016/j.bpg.2010.03.006
14. Banks P, Freeman M. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400. doi:10.1111/j.1572-0241.2006.00856.x
15. Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;Suppl 3:iii1–iii9.
16. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi:10.1136/gutjnl-2012-302779
17. Leeds J, Oppong K, Sanders D. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011;8:405–415. doi:10.1038/nrgastro.2011.91
18. Partelli S, Frulloni L, Minniti C, et al. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44(11):945–951. doi:10.1016/j.dld.2012.05.017
19. Thoeni R. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3):751–764. doi:10.1148/radiol.11110947
20. Bollen T, Singh V, Maurer R, et al. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol. 2011;197(2):386–392. doi:10.2214/AJR.09.4025
21. Hollemans R, Bakker O, Boermeester M, et al. Superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. 2019;156(4):1016–1026. doi:10.1053/j.gastro.2018.10.045
22. Gao C, Li J, Cao F, et al. Infection recurrence following minimally invasive treatment in patients with infectious pancreatic necrosis. World J Gastroenterol. 2020;26(22):3087–3097. doi:10.3748/wjg.v26.i22.3087
23. Cao F, Duan N, Gao C, et al. One-step verse step-up laparoscopic-assisted necrosectomy for infected pancreatic necrosis. J Dig Surg. 2020;37(3):211–219. doi:10.1159/000501076
24. Jin Y, Bai Y, Li Q, et al. Reduced pancreatic exocrine function and organellar disarray in a canine model of acute pancreatitis. PLoS One. 2016;11(2):e0148458. doi:10.1371/journal.pone.0148458
25. Sand J, Nordback I. Acute pancreatitis: risk of recurrence and late consequences of the disease. Nat Rev Gastroenterol Hepatol. 2009;6(8):470–477. doi:10.1038/nrgastro.2009.106
26. Xu Y, Wu D, Zeng Y, et al. Pancreatic exocrine function and morphology following an episode of acute pancreatitis. Pancreas. 2012;41(6):922–927. doi:10.1097/MPA.0b013e31823d7f2d
27. Czakó L, Yamamoto M, Otsuki M. Exocrine pancreatic function in rats after acute pancreatitis. Pancreas. 1997;15:83–90.
28. Weiss FU, Laemmerhirt F, Lerch MM. Acute pancreatitis: genetic risk and clinical implications. J Clin Med. 2021;10(2):190. doi:10.3390/jcm10020190
29. Huang W, de la Iglesia-García D, Baston-Rey I, et al. Exocrine pancreatic insufficiency following acute pancreatitis: systematic review and meta-analysis. Dig Dis Sci. 2019;64:1985–2005. doi:10.1007/s10620-019-05568-9
30. Tu J, Zhang J, Ke L, et al. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long term follow-up study. BMC Gastroenterol. 2017;17:114. doi:10.1186/s12876-017-0663-0
31. Dai M, Fan Y, Pan P, et al. Blood urea nitrogen as a prognostic marker in severe acute pancreatitis. Dis Markers. 2022;2022:7785497. doi:10.1155/2022/7785497
32. Lin S, Hong W, Basharat Z, et al. Blood urea nitrogen as a predictor of severe acute pancreatitis based on the revised Atlanta criteria: timing of measurement and cutoff points. Can J Gastroenterol Hepatol. 2017;2017:9592831. doi:10.1155/2017/9592831
33. Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol. 2013;19:8065–8070. doi:10.3748/wjg.v19.i44.8065
34. Düzenci D, Yalnız M, Ispiroğlu M. Comparison between prognostic indicators in organ insufficiency with acute pancreatitis. Ulus Travma Acil Cerrahi Derg. 2021;27:410–420. doi:10.14744/tjtes.2020.18552
35. Chandrasekaran P, Gupta R, Shenvi S, et al. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology. 2015;15:478–484. doi:10.1016/j.pan.2015.08.006
36. Adiamah A, Psaltis E, Crook M, et al. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clinic Nutr. 2018;37:1810–1822. doi:10.1016/j.clnu.2017.09.028
37. Bruce J, Elliott A. Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase. Am J Physiol Cell Physiol. 2007;293:C938–C950. doi:10.1152/ajpcell.00582.2006
38. Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689–694. doi:10.1016/j.ejim.2014.08.008
39. Ockenga J. Importance of nutritional management in diseases with exocrine pancreatic insufficiency. HPB. 2009;Suppl 3:11–15. doi:10.1111/j.1477-2574.2009.00134.x
40. Vanga RR, Tansel A, Sidiq S, et al. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(8):1220–1228.e4. doi:10.1016/j.cgh.2018.01.027
41. Phillips AE, Ooka K, Pothoulakis I, et al. Assessment of weight loss and gastrointestinal symptoms suggestive of exocrine pancreatic dysfunction after acute pancreatitis. Clin Transl Gastroenterol. 2020;11(12):e00283. doi:10.14309/ctg.0000000000000283
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
