Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Effect of System Inflammation Response Index for Progression of Chronic Kidney Disease in Non-Dialyzing Patient
Authors Tang L , Deng Y, Lai J, Guo X, Liu P, Li S, Liu X
Received 23 August 2023
Accepted for publication 7 November 2023
Published 15 November 2023 Volume 2023:16 Pages 5273—5285
DOI https://doi.org/10.2147/JIR.S432699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Leile Tang,1,* Ying Deng,2,* Jiahui Lai,2 Xinghua Guo,3 Peijia Liu,4 Shaomin Li,2 Xun Liu2
1Department of Cardiology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Nephrology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China; 3Department of Rheumatology, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 4Department of Nephrology, Guangzhou Eighth People’s Hospital, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xun Liu, Department of Nephrology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, 510630, People’s Republic of China, Tel +8613711692006, Email [email protected]
Purpose: Scant research has been conducted on the interplay between the systemic inflammation response index (SIRI) and chronic kidney disease (CKD). The present study endeavors to meticulously scrutinize the association between SIRI and renal function. Additionally, we aim to assess its efficacy in predicting the progression of CKD in non-dialysis patients.
Patients and Methods: Adult patients with CKD who were not undergoing dialysis were enrolled, and follow-up data were obtained. Data from distinct groups were extracted and meticulously compared. A comprehensive analytical approach was adopted, including logistic regression analysis, Kaplan-Meier analysis, Cox proportional hazards regression analysis, and subgroup analysis.
Results: Our study included 1420 patients, with a mean age of 61 ± 17 years, and 63% were male. 244 (17.2%) patients experienced the progression of CKD. As the level of ln(SIRI) increased, patients tended to be older, with a higher proportion of males, and increased prevalence rates of hypertension, stroke, heart failure, and progression of CKD. Additionally, the levels of baseline creatinine and C-reactive protein were elevated, while the levels of estimated glomerular filtration rate and hemoglobin decreased. Upon adjusting for demographic and biochemical variables, logistic regression analysis indicated that ln(SIRI) was independently associated with advanced CKD in pre-dialysis patients (OR=1.59, 95% CI: 1.29– 1.95, P< 0.001). Moreover, Cox proportional-hazard analysis revealed that ln(SIRI) independently predicted CKD progression (HR: 1.3, 95% CI: 1.07– 1.59, P=0.009). Conducting a subgroup analysis, we observed significant interactions between ln(SIRI) levels and gender (p< 0.001), age (p=0.046), and hypertension (p=0.028) in relation to the progression of CKD.
Conclusion: Our study’s findings demonstrate a significant association between SIRI and fundamental renal function, and independently establish a correlation between SIRI and the progression of CKD in pre-dialysis patients. These observations suggest that SIRI holds promise as a potential predictor for CKD progression.
Keywords: system inflammation response index, progression, pre-dialysis, chronic kidney disease
Introduction
The incidence of CKD is on the rise,1 and numerous studies have indicated that the worsening of CKD is linked to a heightened risk of mortality.2–5 Consequently, CKD has emerged as a prevalent health concern in China, leading to an escalation in the societal burden. It is imperative to gain a deeper comprehension of the clinical predictors associated with the onset and progression of CKD. Addressing this unmet need will provide valuable insights into early prevention strategies and innovative management approaches. Indeed, risk factors related to the progression of CKD have been consistently associated with inflammation.6 The presence of excess adipose tissue, particularly in central obesity, triggers the production of pro-inflammatory adipose hormones while reducing the levels of anti-inflammatory adipose hormones. Consequently, this imbalance leads to an accumulation of macrophages within adipose tissue. These infiltrating macrophages are known to produce elevated levels of tumor necrosis factor-α (TNF-α). Additionally, the visceral adipose tissue contains abundant free fatty acids, which are associated with the production of interleukin-6 (IL-6).
The infiltration of inflammatory cells and the production of pro-inflammatory cytokines, such as TNF-α and IL-6, contribute significantly to the exacerbation of chronic inflammation in patients with CKD. This chronic inflammatory state is linked to the progression and worsening of CKD in affected individuals.7 Following the inflammatory processes, activation of the renin-angiotensin system and endothelial dysfunction play pivotal roles in the deterioration of renal function. These mechanisms further contribute to the progression of chronic kidney disease (CKD) and its associated complications.8–11
In response to inflammation, neutrophils and lymphocytes play crucial roles. A high neutrophil count is primarily indicative of infection, whereas a low lymphocyte count reflects poor general health and physiological stress.12 Monocytes, on the other hand, can undergo differentiation into inflammatory macrophages, monocyte-derived dendritic cells, or reemerge as activated monocytes.13 The neutrophil/lymphocyte ratio (NLR) and monocyte/lymphocyte ratio (MLR) have emerged as important markers of inflammation. High monocyte counts and low lymphocyte counts are used as markers of inflammation based on the hypothesis that the MLR is more effective and robust in detecting inflammation compared to assessing the number of lymphocytes or monocytes separately.
The association between NLR (neutrophil/lymphocyte ratio), MLR (monocyte/lymphocyte ratio), and CKD progression has been widely investigated.
In the study conducted by Kuo et al,14 it was found that a high NLR is associated with a higher risk of CKD in men younger than 60 years of age. Similarly, MLR has been identified as an independent predictor of the risk of new-onset CKD and diabetic kidney injury in other studies.15,16 Moreover, Yoshitomi et al,17 observed that a high NLR is associated with poor renal outcomes, including end-stage renal disease requiring dialysis or death. However, there have been conflicting findings in some investigations, where NLR did not act as an independent risk factor affecting the estimated glomerular filtration rate (eGFR).12
The Systemic Inflammation Response Index (SIRI), which considers three distinct white blood cell subsets (neutrophils, lymphocytes, and monocytes), reflects the intricate interplay between inflammation and immunity. Past research has demonstrated a strong association between SIRI and cardiovascular death as well as all-cause mortality.18 On the other hand, elevated SIRI levels have been linked to an increased risk of stroke and certain stroke subtypes. Additionally, higher SIRI has been associated with an increased incidence of myocardial infarction (MI). These findings indicate that SIRI serves as a valuable marker in predicting adverse cardiovascular outcomes and underscores the importance of monitoring and addressing systemic inflammation in the context of cardiovascular health.19
Given the limited research on the association between Systemic Inflammation Response Index (SIRI) and chronic kidney disease (CKD), we conducted this study to explore and elucidate the predictive capability of SIRI concerning CKD progression. By investigating this relationship, we aim to contribute valuable insights into the potential role of SIRI as a predictor for CKD advancement, which may have important implications for early detection and improved management of CKD patients.
Materials and Methods
Population
This study was a retrospective cohort study which was conducted at the Department of Nephrology in Guangzhou, China, specifically at the Third Affiliated Hospital of Sun Yat-sen University, which covered the Tian-he and Ling-nan districts. The participants included adult individuals who were admitted to the nephrology clinic between July 6, 2015, and December 29, 2018. These individuals were screened for the presence of International Classification of Diseases (ICD) codes related to chronic kidney disease (CKD) (specifically N18, N18.8, N18.9, N19).
Inclusion criteria encompassed pre-dialysis CKD patients who had follow-up data, while certain exclusion criteria were applied. Patients who were undergoing dialysis, those age less than 18 years, individuals with follow-up intervals less than 30 days, patients with acute infectious diseases, autoimmune diseases, malignancy, or incomplete data were excluded from the study. This study was performed in accordance with the 1964 Helsinki Declaration and was approved by the ethics committees of the Third Affiliated Hospital of Sun Yat-sen University, ensuring adherence to ethical guidelines and patient confidentiality ([2019]02-490-01). All patients or their legally authorized representatives provided the written informed consents.
Data Collection
Clinic data for the study were obtained by consulting the electronic medical record system, which allowed researchers to access relevant patient information and medical history. On the other hand, biochemical data were collected using the Hitachi 7600 automatic biochemical analyzer. This advanced equipment enabled the accurate measurement of various biochemical parameters. The test results were then accessed and collected through the test results query system, ensuring a systematic and standardized approach to data retrieval for analysis in the study.
Definition and Diagnosis Criteria
The Systemic Inflammation Response Index (SIRI) was calculated using the following formula: 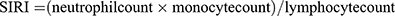 .20 eGFR is calculated by 2009 CKD-EPI creatinine equation:
.20 eGFR is calculated by 2009 CKD-EPI creatinine equation: 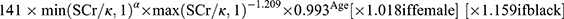 , where SCr is serum creatinine (in mg/dl), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min is the minimum of SCr/κ or 1, and max is the maximum of SCr/κ or 1.21 Chronic Kidney Disease (CKD) is defined as either renal injury or a glomerular filtration rate (GFR) below 60 mL/min per 1.73 m² sustained for a period of three or more months, with profound implications for an individual’s overall health. Indicators of renal impairment encompass various elements, including albuminuria (defined as an albumin excretion rate [AER] equal to or exceeding 30 mg per 24 hours, or an albumin-to-creatinine ratio [ACR] of 30 mg/g [equivalent to 3 mg/mmol]), urinary sediment irregularities, electrolyte imbalances and other manifestations resulting from tubular disorders, pathological findings unveiled through histological examination, structural anomalies detected by imaging techniques, and a prior history of kidney transplantation.22
, where SCr is serum creatinine (in mg/dl), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min is the minimum of SCr/κ or 1, and max is the maximum of SCr/κ or 1.21 Chronic Kidney Disease (CKD) is defined as either renal injury or a glomerular filtration rate (GFR) below 60 mL/min per 1.73 m² sustained for a period of three or more months, with profound implications for an individual’s overall health. Indicators of renal impairment encompass various elements, including albuminuria (defined as an albumin excretion rate [AER] equal to or exceeding 30 mg per 24 hours, or an albumin-to-creatinine ratio [ACR] of 30 mg/g [equivalent to 3 mg/mmol]), urinary sediment irregularities, electrolyte imbalances and other manifestations resulting from tubular disorders, pathological findings unveiled through histological examination, structural anomalies detected by imaging techniques, and a prior history of kidney transplantation.22
Diabetic Kidney Disease (DKD) is a clinical syndrome characterized by persistent albuminuria and a progressive decline in renal function. It typically implies the presence of a specific pattern of glomerular disease. The clinical approach to diagnosing DKD involves the following criteria. 1. Persistent albuminuria or reduced eGFR: A clinical diagnosis of DKD can be established when there is persistent moderate (A2) or severe (A3) albuminuria, or when there’s a consistent decrease in estimated glomerular filtration rate (eGFR) to less than 60 mL/min/1.73 m2. In Type 1 Diabetes (T1DM), this typically occurs at least 5 years after the onset of diabetes, whereas in Type 2 Diabetes (T2DM), it can occur at the onset of diabetes. Persistent abnormalities are defined as a minimum of two elevated Albumin-to-Creatinine Ratio (ACR) levels more than three months apart, or two eGFR values below 60 mL/min/1.73 m2 at least 90 days apart. 2. Association with Diabetic Retinopathy: In T1DM, approximately 95% of patients with diabetic nephropathy (DKD) also have diabetic retinopathy. Thus, the absence of retinopathy may suggest a diagnosis other than diabetic nephropathy. However, in T2DM, the absence of diabetic retinopathy does not provide a strong negative predictive value for the diagnosis of diabetic nephropathy. 3. Exclusion of Alternative Kidney Disease: The diagnosis of DKD is typically based on clinical features. Kidney biopsy is recommended when there are features suggestive of other renal diseases or diagnostic uncertainty.23–25
Hypertensive nephropathy (HTN) is a kind of the kidney injury due to chronic high blood pressure. It was defined as a presumptive diagnosis that was clinically characterized in accordance to the following criteria: 1) development of renal dysfunction only after more than 10 years from the diagnosis of hypertension; 2) negative urinalysis except for 24 h proteinuria that however had to be <1 gr/24h at all determinations (at least 3) in the 12 months before study enrollment; 3) exclusion of patients with a documented diagnosis of a different renal disease.26,27
CKD progression is defined as the last eGFR decreased by 40% from baseline, and the time interval ≥ 30 days.28 Hypertension is defined as systolic blood pressure (SBP) ≥ 140 mm Hg and/or diastolic blood pressure (DBP) ≥ 90 mm Hg, previously diagnosed hypertension, or usage of any antihypertensive medications.
Patients are considered to have diabetes mellitus if they had a fasting glucose ≥ 7.0 mmol/L, an HbA1c ≥ 6.5%, took insulin or other anti-diabetic medications or reported a history of diabetes. Body mass index (BMI) was calculated as initial body weight divided by height squared (kg/m2).
Statistical Analysis
The SIRI was transformed into ln (SIRI) as it was non-normal distribution. There is no established standard for grouping SIRI according to previous reports.
In the study of Lin et al20 they divided SIRI into four groups based on quartiles, and evaluated the association between SIRI and the adverse prognosis of atrial fibrillation-associated ischemic stroke patients. Based on previous studies, according to ln(SIRI) distribution, participants were equally classified into four groups: Q1group [ln(SIRI) ≤-0.39], Q2 group [ln(SIRI):-0.39~0.037], Q3 group [ln(SIRI): 0.037~0.5] and Q4 group [ln(SIRI)>0.5].
In our study, continuous variables were subjected to comparison through either the independent Student’s t-test or the Mann–Whitney U-test, while categorical variables underwent analysis using the Chi-Square test. The relationship between ln(SIRI) and CKD (3–5) was explored utilizing logistic regression analysis. Survival curves were constructed using the Kaplan–Meier method, and their comparison was conducted through the Log rank test. In order to identify significant independent risk factors for the desired outcomes, a comprehensive multivariable Cox proportional hazard regression analysis was conducted. Lastly, a subgroup analysis was performed to further investigate potential associations. All calculations were performed using the SPSS software package, version 23 (IBM).
Results
The study encompassed a total of 9142 patients diagnosed with CKD in the system. After excluding individuals with malignancy, patients undergoing renal replacement therapy, individuals with acute infectious disease and autoimmune disease, as well as those lacking follow-up data, a final cohort of 1420 adult participants was enrolled for analysis (Figure 1).
 |
Figure 1 Inclusion process. |
The Baseline Characteristics of Quantiles of ln(SIRI) Within the Pre-Dialysis CKD Adult Population Were Examined
In the realm of chronic kidney disease etiology, a notable classification encompasses four distinct categories: hypertensive kidney disease, diabetic kidney disease, chronic glomerular kidney disease, and various other causal factors. Among our observed cases, 35 (2.5%) were attributed to hypertensive kidney disease, 286 (20.1%) to diabetic kidney disease, 241 (17%) to glomerulonephritis, and 858 (60.4%) to other underlying causes. The average age of the adult participants in the study was 61 ± 17 years, with 63% being male. Among them, 435 (48.6%) male and 263 (50.1%) female patients were older than 65 years old, showing no statistical differences in age distribution between genders. The mean BMI was 23.28 ± 3.67 kg/m2, and it was observed that the level of BMI was higher in male participants compared to female participants (23.49 ± 3.67 vs 22.92 ± 3.66, P=0.005). Throughout the median 390 days of follow-up, 244 (17.2%) participants experienced progression of CKD. The patients were categorized into four groups based on quartiles of ln(SIRI) (Q1~Q4). As the ln(SIRI) levels increased, the patients tended to be older, and the proportion of male participants, prevalence rates of hypertension, stroke, heart failure, and progression of CKD also increased. Additionally, higher levels of baseline serum creatinine (Cr) and C-reactive protein (CRP) were observed with increasing ln(SIRI), while the levels of estimated glomerular filtration rate (eGFR) and hemoglobin decreased. Furthermore, statistical differences were observed among the four groups in terms of serum levels of uric acid (UA), phosphorus (P), sodium (Na), and chloride (Cl). (Table 1).
 |
Table 1 Baseline Characteristics of Quantiles of Ln(SIRI) Among the Non-Dialysis CKD Adult Population |
The Relationship of SIRI and CKD Stages 3–5
As demonstrated in Table 2, our study conducted a logistic regression analysis, which was adjusted for demographic and biochemical variables, to examine the independent association of ln(SIRI) with the advance stages of CKD. The results revealed a significant and independent association between ln(SIRI) and the advance stages of CKD (odds ratio [OR] = 1.59, 95% confidence interval [CI]: 1.29–1.95, P < 0.001).
 |
Table 2 Logistic Regression Analysis of CKD 3–5 with Ln(SIRI) |
Additionally, we identified several other factors that showed associations with CKD stages 3–5. Specifically, age, hypertension, uric acid, potassium, and phosphorus were positively associated with CKD stages 3–5. On the other hand, female gender, coronary artery disease, diabetes mellitus, and low-density lipoprotein were found to be negatively associated with CKD stages 3–5.
The Predictive Power of ln(SIRI) for CKD Progression
In accordance with the classification principle described earlier, ln(SIRI) was transformed into categorical variables, specifically divided into four groups denoted as Q1, Q2, Q3, and Q4. The Kaplan–Meier survival analysis was conducted to examine the relationship between ln(SIRI) categories and the likelihood of CKD progression, with the Q1 group serving as the reference. The results revealed that higher ln(SIRI) values (specifically in Q4) exhibited the strongest predictive power for CKD progression when compared to the Q1 group (p < 0.001), as depicted in Figure 2. Furthermore, a Cox proportional-hazard analysis was performed to evaluate the independent predictive capability of ln(SIRI) for CKD progression. The analysis indicated that ln(SIRI) independently predicted CKD progression, with a hazard ratio (HR) of 1.3 and a 95% confidence interval (CI) of 1.07–1.59 (P = 0.009). This suggests that elevated ln(SIRI) levels are associated with an increased risk of CKD progression in the studied population. Moreover, it is noteworthy that other factors, including age, gender, BMI, hypertension, phosphorus (P), and hemoglobin (HGB), were also identified as predictors for CKD progression in the study, as indicated in Table 3.
 |
Table 3 Cox Proportional-Hazard Analysis of Ln(SIRI) and CKD Progression |
 |
Figure 2 Kaplan-Meier survival estimates in patients according to categories of ln(SIRI) are given. |
Subgroup Analysis
Based on the recognized differences in ln(SIRI) levels with respect to demographic characteristics, we conducted a subgroup analysis by stratifying the study population based on age, gender, and history of certain diseases (hypertension, coronary atherosclerotic heart disease, heart failure, diabetes mellitus, and stroke) as well as BMI classification. Our findings from the subgroup analysis suggest that gender (p < 0.001 for interactions), age (p = 0.046 for interactions), and hypertension (p = 0.028 for interactions) were significant modifiers for the association between ln(SIRI) levels and the progression of CKD, as shown in Figure 3. In the analysis stratified by gender, it was observed that male patients were associated with a 1.7-fold higher risk of CKD progression (95% CI, 1.34–2.17). Additionally, participants aged 65 years or older had a 1.46-fold higher risk of CKD progression (95% CI, 1.09–1.97).
In our study, it was observed that patients with hypertension had a 1.26-fold higher risk of CKD progression (95% CI, 1.01–1.59), indicating a potential association between hypertension and the advancement of CKD. On the other hand, we did not find significant interactions between ln(SIRI) and other factors such as BMI (body mass index), CAD (coronary artery disease), HF (heart failure), DM (diabetes mellitus), and stroke for the progression of CKD (p > 0.05 for interaction).
Discussion
In our study, we observed a strong correlation between the level of ln(SIRI) and various CKD clinical characteristics and inflammatory status. As the level of ln(SIRI) increased, several notable trends were observed: Age: The patients tended to be older with higher ln(SIRI) levels. Gender: There was a higher proportion of male participants with elevated ln(SIRI) levels. Cardiovascular diseases: The prevalence rates of cardiovascular diseases, such as coronary artery disease, heart failure, and other positive outcomes, were found to increase with higher ln(SIRI) levels. Renal function: Worsening renal function was associated with elevated ln(SIRI) levels, indicating a potential link between systemic inflammation and declining kidney function. CRP (C-reactive protein): The level of CRP, an inflammatory marker, increased with higher ln(SIRI) levels, suggesting a strong association between systemic inflammation and ln(SIRI) in the context of CKD. These findings suggest that ln(SIRI) may serve as a valuable biomarker for evaluating the inflammatory status and clinical characteristics of CKD patients. The significant associations observed in our study underscore the potential utility of ln(SIRI) in predicting disease progression and guiding therapeutic interventions in CKD management (Table 1).
In the context of Logistic Regression analysis, we observed positive correlations not only between the natural logarithm of SIRI (ln(SIRI)) but also with age, gender, hypertension, potassium, and phosphorus concerning CKD3–5. These significant associations align with the established pathophysiological mechanisms of CKD, thereby augmenting the authenticity and validity of our study (Table 2). Of particular interest, Coronary Artery Disease (CAD) and Diabetes Mellitus (DM) exhibited a negative association with CKD3–5. This unexpected discovery could be attributed to specific characteristics of the study population.
First of all, we had 648 patients with diabetes, representing 45.6% of the total population. There were 283 cases of diabetic nephropathy, accounting for 19.9% of the total population and 43.6% of diabetic patients. The prevalence of AECI/ARB in diabetic patients was higher than that in non-diabetic patients [ARB:405 (62.5%) VS 377 (48.8%), p<0.001; ACEI: 120 (18.5%) VS 104 (13.5%), p=0.009]. In diabetic patients, the proportion of ARB use was higher in patients with diabetic nephropathy than in patients without diabetic nephropathy [213 (75.3%) VS 192 (52.6%), p<0.001], while the proportion of ACEI use was similar between the two groups [57 (20.1%) VS 63 (17.4%), =0.349] (Supplementary Table 1). It can be seen that even though the use of ACR drugs in non-diabetic patients is close to 50%, the use of renal-protective drugs in diabetic patients and diabetic nephropathy patients is more than 60%. Therefore, in this study, especially in diabetic patients, active treatment was taken to protect the kidney.
Otherwise, we observed that the average glucose level in our cohort remained below 7 mmol/l, the glomerular filtration rate may have been artificially elevated due to the increased osmotic pressure resulting from the slight elevation in glucose levels.
Additionally, the occurrence and progression of renal damage induced by diabetes can be categorized into five distinct stages. Importantly, it’s worth noting that the estimated glomerular filtration rate (eGFR) may exhibit an increase during the first three stages. Here’s an overview of previous three stages: Stage 1: This stage marks the early phase of diabetes-related renal damage. A prominent feature is the enhancement of glomerular ultrafiltration. At this point, there is an increase in kidney volume, dilation of glomerular arterioles, elevated renal plasma flow, and a substantial rise in glomerular pressure, all of which contribute to a significant increase in the glomerular filtration rate (GFR). Stage 2: In this stage, thickening of the glomerular capillary basement membrane (GBM) and slight widening of the mesangial matrix become evident. Most cases still maintain a normal urinary albumin excretion rate (UAER), although it may intermittently increase, such as after physical activity or during periods of stress. GFR may also experience a modest increase. Stage 3: In the early phases of diabetic nephropathy, notable thickening of the GBM and widening of the mesangial matrix are observed, along with the appearance of arteriolar wall hyalinization. During this stage, persistent microalbuminuria develops, with UAER consistently falling between 20 and 200 µg/min (normal < 10 µg/min), while GFR remains either above normal or within the normal range.29–31
The diagnosis of CAD typically necessitates procedures such as computed tomography angiography or coronary angiography, both of which involve the administration of iodine-containing contrast media. However, these examinations are not commonly conducted in patients with chronic kidney disease due to the potential risk of renal function impairment associated with these agents. Importantly, patients with normal estimated Glomerular Filtration Rate (eGFR) tend to tolerate contrast media better, so our findings indicate a positive association between CAD and eGFR.32
As anticipated, the natural logarithm of Systemic Immune-Inflammation Index (ln(SIRI)) demonstrated an independent association with the progression of Chronic Kidney Disease (CKD) (see Table 3). This finding can be attributed to several underlying mechanisms. Systemic or intrarenal inflammation plays a crucial role in the dysregulation of the microvascular response to its regulatory factors, which sustains the production of various tubular toxins, including reactive oxygen species (ROS). Consequently, this leads to tubular injury, nephron dropout, and the initiation of CKD.
Circulating proinflammatory cytokines activate intrarenal micro-vessels, particularly endothelial cells and leukocytes, resulting in a localized amplification of proinflammatory factors and ROS. These processes, in turn, affect cell-surface adhesion molecules and disrupt the glycocalyx layer. Consequently, endothelial barrier function, activation of the coagulation system, and receptor-mediated vasoreactivity become compromised. These inflammation-mediated alterations can ultimately induce irreversible tubular injury and failure of nephrons.33
Neutrophils and lymphocytes play crucial roles in responding to inflammation. A high neutrophil count is typically indicative of an ongoing infection, while a low lymphocyte count often reflects poor general health and physiological stress. Monocytes, on the other hand, possess the ability to differentiate into inflammatory macrophages, monocyte-derived dendritic cells, or can circulate as activated monocytes.
The Systemic Immune-Inflammation Index (SIRI), which incorporates the counts of neutrophils, lymphocytes, and monocytes, reflects the intricate interplay between inflammation and immunity. It serves as a pertinent reminder of the interconnectedness of these two vital processes. Furthermore, in our study, we observed a positive correlation between SIRI and C-reactive protein (CRP), implying that SIRI can serve as a reliable indicator of the inflammatory status in patients with CKD.12
In a stratified analysis, it was determined that the interactions between ln(SIRI) and BMI, CAD, heart failure, diabetes mellitus, and stroke were not statistically significant. This outcome implies that these specific conditions or factors did not exert a significant influence on the association between ln(SIRI) and the progression of CKD.
As anticipated, there was a positive correlation between ln(SIRI) and patient age, as evident from Table 1. Notably, when we employed age 65 years as the threshold, a significant interaction was observed while analyzing ln(SIRI) concerning CKD progression. Consequently, age emerged as an independent factor influencing the impact of SIRI levels on the risk of CKD progression.
Age-related changes in the renal system encompass a reduction in both the size and quantity of nephrons, along with tubulointerstitial alterations, thickening of the glomerular basement membrane, and elevated glomerulosclerosis. The aging process is further characterized by the gradual decline of tubular function, diminished sodium reabsorption, decreased potassium excretion, and compromised urine concentrating capacity. These age-associated factors may collectively contribute to an augmented vulnerability to Acute Kidney Injury (AKI).34 On the other hand, aging induces systemic changes throughout the entire body. Mechanistically, age-related elevations in inflammatory levels are referred to as aging-associated inflammatory responses.35 Senescent cells, predominantly fibroblasts, found in the elderly, play a role in generating proinflammatory factors collectively termed as senescence-associated secretory phenotypes.36 As individuals age, the immune system becomes less efficient in degrading misfolded proteins and organelles, leading to the accumulation of senescent cells and subsequently causing systemic inflammation.
In the subgroup analysis based on hypertension, a significant interaction was observed when analyzing ln(SIRI) concerning the progression of CKD. Existing evidence supports the notion that inflammation plays a role in the pathogenesis of hypertension. Notably, immune cells have been detected in the kidneys of hypertensive patients. Moreover, biomarkers of inflammation, including high-sensitive CRP, cytokines, and products of the complement pathway, are found to be elevated in individuals with hypertension. Recent findings indicate that hypertension is closely associated with the activation of complement and the inflammasome,37 along with alterations in the phenotype of circulating immune cells, particularly myeloid cells. These inflammatory processes are interconnected, ultimately leading to the engagement of the adaptive immune system through mechanisms involving oxidative stress, modifications of endogenous proteins, and changes in antigen processing and presentation. As a result, hypertension is often accompanied by additional inflammation, which contributes to the worsening of CKD. The interplay between hypertension and inflammation plays a pivotal role in the progression of chronic kidney disease.
Remarkably, the gender-stratified analysis revealed that male patients exhibited a 1.7-fold higher risk of CKD progression (95% CI, 1.34–2.17), while no significant association was observed among female patients. This suggests that inflammation may manifest more aggressively in males, thereby contributing to the worsening of renal function. However, it should be noted that this finding differs from some previous studies. For instance, in the study conducted by Al-Daghri et al, a significant association was found between elevated levels of IL-6 and female subjects. Additionally, elevated levels of CRP were significantly associated with the risk of hypertension only in female subjects, while in male subjects, a significant association between elevated levels of TNF-α and the risk of developing insulin resistance (IR) was observed. These discrepancies in inflammatory characteristics between the two genders underscore the complexity of the relationship. Nevertheless, it is evident that both sexes are linked to inflammation, albeit with distinct patterns and associations with specific inflammatory markers. Such gender-specific differences may be crucial in understanding the underlying mechanisms of inflammation-related diseases and their impact on various health outcomes.38
In the study by Eytan Cohen et al, the researchers concluded that inflammatory markers are indeed significantly higher in women compared to men.39
The authors put forward several theoretical explanations to account for this observation.
Firstly, it is noted that female patients tend to have a higher proportion of subcutaneous fat when compared to males.40 This disparity in body fat distribution between the sexes may play a role in the differences in inflammation levels observed. Secondly, sex hormones are known to influence the circulating levels of inflammatory proteins. Specifically, postmenopausal hormonal replacement treatment has been associated with increased concentrations of C-reactive protein.41,42 This suggests that hormonal fluctuations or interventions could be contributing factors to the variation in inflammatory marker levels between men and women.
Upon reviewing the results of our study, we observed that out of the total patient population, 435 individuals (48.6%) were male, and 263 individuals (50.1%) were female, both of which were older than 65 years old, without any statistical differences in age distribution. The average Body Mass Index (BMI) was calculated to be 23.28 ± 3.67 kg/m2. Interestingly, male patients exhibited a higher BMI level compared to female patients, with means of 23.49±3.67 kg/m2 and 22.92±3.66 kg/m2, respectively, and this difference was found to be statistically significant (P=0.005). In our study population, although half of the female CKD patients were postmenopausal, it was noted that none of the patients had undergone hormonal replacement treatment, as indicated in the medical records. Moreover, the average BMI level in our population was less than 25 kg/m2, which suggests that the inflammation related to excess body fat might be less pronounced in our patient cohort. This observation could potentially explain why male patients were associated with a higher risk of CKD progression, while no significant association was observed in female patients. The differences in BMI levels and the absence of hormonal replacement treatment may contribute to the variation in inflammation between the two genders and the subsequent impact on CKD progression. It is important to consider these factors when interpreting the study results and their implications for the management and understanding of CKD in different gender populations.
Our study exhibited several limitations. Firstly, it was a retrospective study, which implies that inherent data biases might be present. Secondly, further investigation is required to comprehensively understand the underlying mechanisms revealed in this study. Prospective studies, cellular models, and animal model studies will be necessary to confirm and expand upon our findings. Thirdly, the unavailability of raw pathological data represents another limitation in our study. Addressing these limitations in future research may contribute to a more robust and complete understanding of the subject matter.
Conclusion
Our study’s findings demonstrate a significant association between SIRI and fundamental renal function, and independently establish a correlation between SIRI and the progression of CKD in pre-dialysis patients. These observations suggest that SIRI holds promise as a potential predictor for CKD progression.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was partially supported by: Key Project of Basic and Applied Basic Research Fund of Guangdong Province (No. 2020B1515120037) to Dr Xun Liu; Special Fund for Clinical Medical Research of the Third Affiliated Hospital of Sun Yat-sen University (No. YHJH201806) to Dr Xun Liu. The National Natural Science Foundation of China (Grant No.81873631, 81370866, 81070612) to Dr Xun Liu; The Guangzhou Science and technology planning project (Grant No.202002020047) to Dr Xun Liu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6
2. Yeh HC, Lo YC, Ting IW, et al. 24-hour serum creatinine variation associates with short- and long-term all-cause mortality: a real-world insight into early detection of acute kidney injury. Sci Rep. 2020;10(1):6552. doi:10.1038/s41598-020-63315-x
3. Xs G, Sq C, Cy D, et al. Association of post-procedural early (within 24h) increases in serum creatinine with all-cause mortality after coronary angiography. Clin Chim Acta. 2017;474:96–101. doi:10.1016/j.cca.2017.08.036
4. Losito A, Nunzi E, Pittavini L, et al. Cardiovascular morbidity and long term mortality associated with in hospital small increases of serum creatinine. J Nephrol. 2018;31(1):71–77. doi:10.1007/s40620-017-0413-y
5. Mh B, Ristl R, Neugebauer T, et al. Very early changes in serum creatinine are associated with 30-day mortality after cardiac surgery: a cohort study. Eur J Anaesthesiol. 2020;37(10):898–907.
6. Stuveling EM, Hillege HL, Bakker SJ, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63(2):654–661. doi:10.1046/j.1523-1755.2003.00762.x
7. Chen S, Liu H, Liu X, et al. Central obesity, C-reactive protein and chronic kidney disease: a community-based cross-sectional study in southern China. Kidney Blood Press Res. 2013;37(4–5):392–401. doi:10.1159/000355718
8. Sesso HD, Wang L, Buring JE, et al. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49(2):304–310. doi:10.1161/01.HYP.0000252664.24294.ff
9. Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):152–158. doi:10.1097/01.mnh.0000203189.57513.76
10. Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12(13):1623–1635. doi:10.2174/138161206776843313
11. Di Napoli M, Papa F. Systemic inflammation, blood pressure, and stroke outcome. J Clin Hypertens. 2006;8(3):187–194. doi:10.1111/j.1524-6175.2005.04590.x
12. Sevencan NO, Ozkan AE. Associations between neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, albuminuria and uric acid and the estimated glomerular filtration rate in hypertensive patients with chronic kidney disease stages 1–3. Arch Med Sci. 2019;15(5):1232–1239. doi:10.5114/aoms.2018.76262
13. Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. doi:10.1016/j.immuni.2013.08.007
14. Kuo YT, Wang YY, Lin SY, et al. Age and sex differences in the relationship between neutrophil-to-lymphocyte ratio and chronic kidney disease among an adult population in Taiwan. Clin Chim Acta. 2018;486:98–103. doi:10.1016/j.cca.2018.07.025
15. Zhang M, Wang K, Zheng H, et al. Monocyte lymphocyte ratio predicts the new-onset of chronic kidney disease: a cohort study. Clin Chim Acta. 2020;503:181–189. doi:10.1016/j.cca.2019.11.021
16. Kocak MZ, Aktas G, Duman TT, et al. Monocyte lymphocyte ratio as a predictor of Diabetic Kidney Injury in type 2 diabetes mellitus; the MADKID study. J Diabetes Metab Disord. 2020;19(2):997–1002. doi:10.1007/s40200-020-00595-0
17. Yoshitomi R, Nakayama M, Sakoh T, et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41(1):238–243. doi:10.1080/0886022X.2019.1595645
18. Xia Y, Xia C, Wu L, et al. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. 2023;12(3):1128. doi:10.3390/jcm12031128
19. Jin Z, Wu Q, Chen S, et al. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/JIR.S283835
20. Lin KB, Fan FH, Cai MQ, et al. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res. 2022;27(1):106. doi:10.1186/s40001-022-00733-9
21. Drawz PE, Beddhu S, Bignall ON, et al. KDOQI US Commentary on the 2021 KDIGO Clinical Practice Guideline for the management of blood pressure in CKD. Am J Kidney Dis. 2022;79(3):311–327. doi:10.1053/j.ajkd.2021.09.013
22. de Boer IH, Caramori ML, Chan JC, et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115. doi:10.1016/j.kint.2020.06.019
23. American Diabetes Association.11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S151–S167. doi:10.2337/dc21-S011
24. Tsai IT, Wu CC, Hung WC, et al. FABP1 and FABP2 as markers of diabetic nephropathy. Int J Med Sci. 2020;17(15):2338–2345. doi:10.7150/ijms.49078
25. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15. doi:10.1111/dom.14007
26. Chen Z, Wu H, Wang G, et al. Identification of potential candidate genes for hypertensive nephropathy based on gene expression profile. BMC Nephrol. 2016;17(1):149. doi:10.1186/s12882-016-0366-8
27. Vettoretti S, Caldiroli L, Zanoni F, et al. Patients with hypertensive nephropathy and chronic kidney disease might not benefit from strict blood pressure control. Kidney Blood Press Res. 2018;43(6):1706–1715. doi:10.1159/000495388
28. Levin A, Agarwal R, Herrington WG, et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020;98(4):849–859. doi:10.1016/j.kint.2020.07.013
29. Shen Y, Cai R, Sun J, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. 2017;55(1):66–76. doi:10.1007/s12020-016-1014-6
30. Kim Y, Park CW. Can management of the components of metabolic syndrome modify the course of chronic kidney disease?. Kidney Res Clin Pract. 2020;39(2):118–120. doi:10.23876/j.krcp.20.066
31. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. doi:10.1681/ASN.2016060666
32. Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC State-of-The-Art review. J Am Coll Cardiol. 2019;74(14):1823–1838. doi:10.1016/j.jacc.2019.08.1017
33. Qian Q. Inflammation: a key contributor to the genesis and progression of chronic kidney disease. Contrib Nephrol. 2017;191:72–83.
34. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–420. doi:10.1681/ASN.2015121308
35. Milan-Mattos JC, Anibal FF, Perseguini NM, et al. Effects of natural aging and gender on pro-inflammatory markers. Braz J Med Biol Res. 2019;52(9):e8392. doi:10.1590/1414-431x20198392
36. Guan Y, Zhang C, Lyu G, et al. Senescence-activated enhancer landscape orchestrates the senescence-associated secretory phenotype in murine fibroblasts. Nucleic Acids Res. 2020;48(19):10909–10923. doi:10.1093/nar/gkaa858
37. Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36(5):635–647. doi:10.1016/j.cjca.2020.01.013
38. Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Gender-specific associations between insulin resistance, hypertension, and markers of inflammation among adult Saudis with and without diabetes mellitus type 2. Adv Med Sci. 2010;55(2):179–185. doi:10.2478/v10039-010-0052-1
39. Cohen E, Margalit I, Shochat T, et al. Markers of chronic inflammation in overweight and obese individuals and the role of gender: a cross-sectional study of a large cohort. J Inflamm Res. 2021;14:567–573. doi:10.2147/JIR.S294368
40. Festa A, D’Agostino Jr RJ, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25(10):1407–1415. doi:10.1038/sj.ijo.0801792
41. Ridker PM, Hennekens CH, Rifai N, et al. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100(7):713–716. doi:10.1161/01.CIR.100.7.713
42. Cushman M, Legault C, Barrett-Connor E, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100(7):717–722. doi:10.1161/01.CIR.100.7.717
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

