Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Prediction of Non-Transplantable Recurrence After Liver Resection for Solitary Hepatocellular Carcinoma
Authors Zhang C, Tao Y, Yang R, Wang Y, Yu Y , Zhou Y
Received 7 November 2023
Accepted for publication 29 December 2023
Published 26 January 2024 Volume 2024:11 Pages 229—240
DOI https://doi.org/10.2147/JHC.S412933
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Chunhui Zhang,1,* Yuqing Tao,1,* Rui Yang,1 Yueqi Wang,1 Yanyan Yu,2 Yang Zhou2
1Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, 150010, People’s Republic of China; 2Department of Radiology, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, 150010, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Zhou, Department of Radiology, Harbin Medical University Cancer Hospital, 150 Haping Road, Harbin, Heilongjiang, 150010, People’s Republic of China, Email [email protected]
Purpose: Using a combination model of preoperative imaging and clinical factors to predict non-transplantable recurrence (NTR) after liver resection and assist solitary hepatocellular carcinoma (HCC) patients in the selection of early treatment options.
Patients and Methods: A retrospective analysis was conducted on 253 solitary HCC patients who underwent radical resection and had preoperative MRI. NTR patients were defined as those exceeding the University of California, San Francisco (UCSF) criteria at the time of recurrence. Cox regression analysis was employed to identify preoperative factors associated with NTR based on clinical and tumor imaging characteristics. A risk scoring model (NTRScore) was developed and validated.
Results: Among the 253 patients, 86 (33.9%) experienced recurrence, and among those with recurrence, 34 patients (39.5%) developed NTR. In multivariate analysis, factors associated with NTR included alpha-fetoprotein (AFP) [> 10 ng/mL] [HR: 3.42, 95% confidence interval (CI): 1.54– 7.63, P: 0.003], arterial phase hyperenhancement (APHE) [HR: 2.23, 95% CI: 1.03– 4.81, P: 0.041], washout[HR: 0.35, 95% CI: 0.15– 0.84, P: 0.019], and capsule [HR: 0.44, 95% CI: 0.22– 0.88, P: 0.021]. The β-coefficients of these variables were utilized to develop the weighted NTRScore(c-index 0.72, 95% CI: 0.65– 0.79). The NTR occurrence increased across the three categories (low: 5.6%, medium: 13.6%, high: 35.1%, p < 0.001), and the Kaplan-Meier curves of recurrence-free survival(RFS) and overall survival(OS) show significant differences (p = 0.004 and p< 0.001). Furthermore, the higher NTR categories may be associated with an increased risk of extrahepatic recurrence.
Conclusion: The NTRScore demonstrated strong discriminatory ability and may serve as a clinically useful tool to assist in risk stratification and potential to guide treatment and optimal surveillance for patients of solitary hepatocellular carcinoma within UCSF criteria.
Keywords: hepatocellular carcinoma, salvage liver transplantation, non-transplantable recurrence, treatment, prognosis
Introduction
Hepatocellular carcinoma (HCC) is a major health concern and ranks as the third leading cause of cancer-related deaths worldwide.1 Liver transplantation is considered the optimal curative treatment option for early-stage HCC, particularly Milan and UCSF criteria.2,3 To address the potential disease progression and dropout while waiting for donor organs, in 2000, Majno et al4 proposed a “salvage” transplantation method that allowed for liver resection in patients with solitary hepatocellular carcinoma of ≤5cm prior to transplantation. This approach has alleviated the pressure on liver transplantation and is increasingly employed.5 However, this strategy does not apply to patients with recurrent disease beyond the salvage liver transplantation criteria.6 Given salvage liver transplantation may be feasible and whether more aggressive early intervention should be taken for those experiencing recurrences beyond transplantable criteria, the preoperative prediction of Non-Transplantable Recurrence (NTR) can better stratify HCC patients and improve long-term outcomes.
The Liver Imaging Reporting and Data System (LI-RADS) has been widely recognized as the mainstream diagnostic criteria for HCC.7,8 The LI-RADS includes five main features, namely arterial phase hyperenhancement (APHE), tumor size, capsule, washout, and threshold growth, as well as additional characteristics such as peritumoral enhancement and mosaic architecture.7,9 A number of previous studies have demonstrated that LI-RADS imaging features can be used to assess the recurrence of HCC.10,11 Meanwhile, AFP has long been used to assess HCC invasiveness.12–14 However, currently, no one has combined these features to predict the occurrence of NTR.
Therefore, the aim of this study is to describe and analyze the recurrence patterns following initial hepatic resection within UCSF criteria in solitary HCC patients, and to develop and validate a straightforward preoperative NTR risk model to optimize early treatment strategies for HCC.
Materials and Methods
Study Population
This retrospective study was approved by the institutional review board and informed consent was waived. A database of patients who had hepatic resections for HCC at our institution from January 2015 to May 2021 was obtained from our institution’s medical database. A total of 958 diagnosed HCC cases underwent abdominal MRI examination within two weeks before curative resection. Patients meeting the following criteria were included: untreated, solitary HCC, and had undergone an acceptable contrast-enhanced MRI examination within two weeks before surgery.
Exclusion criteria were as follows: (1) Multiple tumors. (n = 548); (2) tumor size greater than 65mm before surgery (n = 103); (3) incomplete clinical imaging data (n = 46); (4) severe image artifacts (n = 8). Based on these criteria, a total of 253 patients were included in the study (Figure 1). The characteristics of the patients included sex, age, hepatitis status, liver cirrhosis, alpha-fetoprotein (AFP) levels, gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), Child-Pugh classification, ALBI (Albumin-Bilirubin) and morphological type.
 |
Figure 1 Flowchart of inclusion and exclusion criteria for the study. |
MRI Data Acquisition
All patients underwent liver multiparametric MRI examinations using a 3.0 Tesla MRI system and a 32-channel phased-array coil. Patients were positioned supine, and image acquisition commenced at the end of expiration. The scan range extended from the diaphragm to the inferior border of the liver.
The liver multiparametric MRI protocol included the following sequences:
- Axial dual-echo (in-phase and out-of-phase) T1-weighted imaging.
- Fat-suppressed T2-weighted imaging.
- Dynamic contrast-enhanced imaging. This involved the administration of a liver-specific contrast agent, gadobenate dimeglumine (Primovist), at a rate of 1 mL/s through an intravenous line in the antecubital vein (dose: 0.1 mL/kg) or gadobutrol (dose: 0.2 mL/kg) at a rate of 2 mL/s, followed by a 20 mL saline flush at 2 mL/s. The dynamic contrast-enhanced sequences included the pre-contrast phase, arterial phase (at 20 seconds), portal venous phase (at 55 seconds), equilibrium phase (at 90 seconds), and delayed phase (at 180 seconds). An additional hepatobiliary phase was obtained 20 minutes after injection.
- Post-processing involved subtraction of the pre-contrast images from the post-contrast images to enhance the visualization of tumor enhancement.
Image Analysis
The preoperative abdominal MRI scans were reviewed independently by two radiologists with 3 and 5 years of abdominal MRI experience. The radiologists knew the diagnosis of HCC, but were blinded to all clinical, pathological, and follow-up results. The final consensus reading was reached after a third radiologist with 12 years of abdominal imaging experience resolved discrepancies.
For each HCC lesion, the radiologists assessed the following imaging features as defined in LI-RADS Version 2018 (Specific feature definitions are shown in Table S1):
- Major features: Tumor size, APHE, Washout, Capsule.
- Ancillary features: Satellite nodules, Mosaic architecture, Peritumoral enhancement, Fat in mass, Intratumoral hypoperfusion, and Intratumoral hemorrhage.
Patient Follow-Up
All patients were regularly followed up for the detection of recurrence. The follow-up assessments included a review of medical history, AFP, and ultrasound or contrast-enhanced CT/MRI scans. The first postoperative follow-up occurred at 4–6 weeks after surgery. Subsequent follow-ups were scheduled every 2–3 months within the first 6 months after surgery, followed by once every 6 months thereafter. The primary outcome was NTR. NTR was recognized as recurrence exceeding the following criteria: a solitary tumor ≤ 6.5 cm, or ≤ 3 nodules with the largest lesion ≤ 4.5 cm and total tumor diameter ≤ 8 cm, macrovascular invasion, or extrahepatic recurrence. The dates of the first HCC recurrence, end of follow-up, and death were recorded. The study’s follow-up end date was June 1st, 2023. Secondary endpoints included overall survival (OS) and recurrence-free survival (RFS). RFS is defined as the time from date of surgery to the date of the first recurrence of HCC, death, or last follow-up, whereas OS is defined as the time from date of surgery to date of death or last follow-up.
Data Analysis
The categorical variables were presented as percentages (%) and numbers (n), and chi-square tests or Fisher’s exact tests were used to compare them. The the optimal cut-off point for AFP was obtained using X-tile15 software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA). Univariate and multivariate Cox regression analyses were performed to determine the independent predictors of NTR, hazard ratios (HR), and their 95% confidence intervals (CI). In the multivariable model, variables with p<0.10 in the univariate model were included, and the β-coefficients of the significant variables were used to construct the weighted formula for the NTRScore. NTRScores were categorized into high, medium, and low by X-tile. The performance of the NTRScore was assessed using Harrell’s c-index and was internally validated by bootstrapping with 2000 repetitions. OS and RFS were calculated using the Kaplan-Meier method generated by the Log-rank test for comparison. SPSS version 26.0 (IBM Corporation, Armonk, NY) and R version 4.3.0 (R Project for Statistical Computing, Vienna, Austria) were used for all statistical analyses. Statistical significance was determined using p<0.05.
Results
Patient Characteristics
Table 1 summarizes patient characteristics. The study included 253 patients (201 males and 52 females) with a mean age of 54 years (Interquartile Range 48–61 years). Liver cirrhosis was present in 186 (74%) patients, and 236 (93.3%) patients had viral hepatitis, with 220 (87%) of them having hepatitis B. Most patients had well-compensated liver function, and 97.0% (n=246) classified as Child-Pugh class A. The median AFP level was 13.49 ng/mL (IQR 3.55–188.95 ng/mL), and 49 patients (19%) had high AFP levels (> 400 ng/mL) (Table 1). The median ALBI score was −2.50 (IQR −2.28 to −2.74). Out of the 253 patients, 86 (33.5%) experienced tumor recurrence, with 34 patients (39.5%) developing NTR, accounting for 13.3% of all patients. Table 2 summarizes the results of the correlation analysis between imaging features based on LI-RADS classification and the presence of NTR and non-NTR.
 |
Table 1 Baseline Characteristics |
 |
Table 2 Imaging Features with NTR |
Postoperative Outcomes
The median follow-up of our study was 62.3 months, and a total of 58 patients (22.9%) died. Tumor recurrence occurred in 86 patients (33.5%), with a median time to recurrence of 12.79 months. Among the patients with recurrence, the majority (n=63, 73.2%) experienced tumor recurrence within 24 months after surgery, while 23 patients (26.8%) had recurrence beyond 24 months after liver resection. Regarding the pattern of recurrence, most recurrences (n = 68, 79%) occurred within the liver only, and approximately one-fifth (n = 22, 25.5%) of patients had multiple recurrent tumors. During the study period, 34 patients experienced NTR, resulting in a recurrence rate of 39.5% and a median NTR time of 15.67 months. Figure 2A is a streamplot to show pre - and post-operative HCC status.The overall recurrence rate in all patients included in the cohort was 13.3%, with 73.5% (n = 25) of NTR patients belonging to the BCLC-C stage of recurrence, and over half (n = 18, 53%) of NTR patients experiencing extrahepatic recurrence. The detail information on recurrence is shown in Table S2.
Development of NTRScore
In the multivariate analysis, AFP [>10ng/mL] [HR: 3.42, 95% CI: 1.54–7.63, p: 0.003], APHE [HR: 2.23, 95% CI: 1.03–4.81, p: 0.041], washout[HR: 0.35, 95% CI: 0.15–0.84, p: 0.019], and capsule [HR: 0.44, 95% CI: 0.22–0.88, p: 0.021] (Table 3) were independent prognostic factors for NTR. The β-coefficients of these variables were utilized to develop the weighted NTRScore. The NTRScore ranged from −1.87 to 1.23 in the present cohort. Patients were categorized into low (NTRScore < −0.64; n = 106, 41.9%), medium (NTRScore ≥ −0.64 to < 0.19; n = 110, 43.48%), and high (NTRScore ≥ 0.19; n = 37, 14.62%). And Figure 2B shows the recurrence stratification by NTRScore.
 |
Table 3 Univariate and Multivariate Cox Analyses for NTR of HCC Patients |
Performance of NTRScore
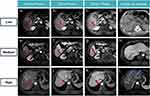
In this cohort, the occurrence rates of NTR were significantly different between the three groups (low: 5.6%, medium: 13.6%, high: 35.1%, p < 0.001). The RFS and OS curves are shown in Figures 2C and D and Kaplan-Meier curves of them show significant differences (p = 0.004 and p<0.001). Patients in the low NTRScore had significantly higher 5-year RFS rates compared to the medium and high (76.4% versus 61.8% versus 54.1%, p = 0.014). Furthermore, postoperative 5-year overall survival rates were significantly better for patients in the low NTRScore compared to the medium and high-risk groups, with rates of 89.6%, 77.3%, and 64.9% (p = 0.002). Figure 3A–L shows three typical cases. Among the 86 recurrent patients, 51 cases experienced BCLC-0/A stage recurrence, while 35 were at BCLC-B stage or higher. Except for one case of transplantable recurrence, all others were classified as NTR (n = 34, 97%). The model demonstrated good discriminative ability in internal bootstrap validation (n = 2000) with a c-index of 0.72 and a 95% CI ranging from 0.649 to 0.793.
Discussion
We have established a preoperative model to predict the development of NTR. Previous research has attempted to predict NTR either preoperatively or postoperatively, but it did not take into account significant preoperative imaging features that could be used to guide treatment strategy selection and postoperative monitoring plans. Considering the possibility of salvage liver transplantation and perioperative treatment, based on our scoring system, patients in the low categories may undergo surgical treatment first, with a lower probability of recurrence and still a significant chance of salvage liver transplantation in case of recurrence. For patients in higher categories of NTRScore, there is a higher risk of recurrence and a greater probability of NTR occurrence upon recurrence, and it is not recommended to undergo liver resection or liver transplantation as the initial treatment.
Based on our study, we recommend perioperative treatment for higher categories patients. In recurrent patients, the proportion of NTR occurrence concerning recurrent patients was 22.2%, 35.7%, and 76.5% for the low, medium, and high categories, respectively (p <0.001). Patients in the higher categories are more prone to recurrence, and show worse outcomes upon recurrence, losing the opportunity for curative treatment. Notably, 13.3% of recurrences are NTR, similar to previous studies.6,16 All NTR patients were at BCLC stage B or higher, and over half of the patients experienced extrahepatic recurrence (n = 18, 53%), both them indicate poor prognosis. The IMbrave05017 study, which has attracted much attention, achieved its predefined primary endpoint during the mid-term analysis, breaking the dilemma of adjuvant systemic therapy for postoperative liver cancer. Inspired by the results of IMbrave050, we speculate that intermediate to high-risk patients in this study may benefit from exploratory and more aggressive perioperative treatment.
Considering that extrahepatic recurrence after liver transplantation is also a key factor for poor overall survival prognosis. Elena Fernandez-Sevilla et al18 revealed that 71.9% of recurrences after liver transplantation are extrahepatic, with 70% of these recurrences diagnosed within two years after liver transplantation. Early recurrence may be due to undetected extrahepatic metastasis during or shortly after liver transplantation, or circulating HCC clones implanting in distant organs.19 Given that post-liver transplantation, extrahepatic recurrence may be a consequence of the primary tumor and that patients prone to extrahepatic metastasis postoperatively may not be suitable for pre-transplant treatment, our results show that as the risk stratification increases, the probability of extrahepatic recurrence gradually increases (4.7% vs 7.3% vs 13.5%, p = 0.217). While no statistically significant difference was observed, a trend is evident, possibly due to the limited sample size. We plan to further expand the sample size for future research. Therefore, our criteria not only predict the occurrence of unresectable recurrence after liver resection but also suggest that patients with high NTRScore are more likely to experience extrahepatic recurrence, emphasizing the potential benefit of early systemic treatment to improve survival.
Our study suggests that arterial-phase enhancement characteristics are independent risk factors. Specifically, rim enhancement and poorly enhanced lesions during the arterial phase had a higher risk of developing NTR compared to non-rim arterial-phase hyperenhancement (26% vs 11%, p=0.027). Previous studies have indicated that rim-enhanced HCC exhibits faster progression and worse prognosis following curative resection, radiofrequency ablation, or transarterial chemoembolization.10,20,21 Furthermore, studies have found a close association between this condition and poor tumor differentiation, more invasive vascular distribution. Poor tumor differentiation implies that cancer cells have lost the characteristics and functions of normal cells, leading to decreased treatment response and worse prognosis. The more invasive vascular distribution indicates vigorous tumor growth and increases the risk of distant metastasis.20,22–24Additionally, our retrospective analysis of lesions without arterial-phase enhancement, which were not enhanced in any phase, is hypothesized to be related to ischemia, potentially leading to worse outcomes.Furthermore, capsule appearance and washout were included as protective factors in our model. Pathological capsule has always been a favorable factor after liver resection, It usually represents slow-growing tumors and well-differentiated histology of the tumor. In this case, the tumor cells exhibit lower malignant features and a slower growth rate, leading to a better survival rate.25–28 While it may not fully represent pathological capsule, the sensitivity and accuracy of this feature are high, reaching 94.0% and 83.0%,29 respectively. Its presence suggests a better prognosis.Washout is not a commonly reported imaging feature related to prognosis, but our study suggests relatively favorable outcomes associated with it. The precise mechanisms behind these observations warrant further exploration. Moreover, AFP has widespread applications as a prognostic biomarker following curative resection for HCC.30–32 It serves as both a static and dynamic variable, providing a more accurate reflection of tumor biology. In our model, patients with AFP levels greater than 10 ng/mL exhibited a worse prognosis, our research is consistent with the findings of the previous study conducted by Tsilimigras et al.33
In summary, Previous studies have largely focused on the clinical factors influencing NTR, without considering the role of radiological factors. Our NTRScore combines preoperatively assessable tumor imaging features and tumor biological behavior, which are key determinants of HCC prognosis. It can help us identify patients with more severe recurrence and hope to provide useful information for perioperative treatment in clinical practice. Therefore, the results of this study provide some information to assist with the complex decisions related to liver cancer resection, transplantation, and perioperative treatment. They provide specific information and guidance for the optimal post-resection monitoring to reduce NTR occurrences.
The results of the current study should be interpreted with several limitations. Firstly, due to the retrospective study design, there may be selection bias. The second limitation is the lack of external validation, which restricts the generalizability of our data. Furthermore, these data rely on patients undergoing LR treatment for HCC, making direct comparisons between LR and other perioperative treatment strategies unfeasible. Future research will delve deeper into comparative studies based on this premise.
Conclusion
NTRScore can help predict NTR and provide precise stratification of recurrence, offering potential to improve patient treatment selection and monitor strategies, ultimately wishing to promot long-term prognosis.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Review Board of Harbin Medical University(protocol code:KY2023-59).The requirement of informed consent from the patients was waived because of the retrospective design of this study, and patients’ information was protected. And the study was performed in accordance with the Declaration of Helsinki.
Consent for Publication
Consent to publish has been received from all participants.
Author Contributions
Chunhui Zhang and Yuqing Tao contributed equally to this work and should be considered co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by: Harbin Medical University Cancer Hospital Haiyan Funds (No. JJZD2022-12). Natural science foundation of Heilongjiang Provincial Government (LH2022H067).
Disclosure
The authors declare that they have no competing interests.
References
1. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi:10.1016/j.jhep.2022.08.021
2. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi:10.1056/NEJM199603143341104
3. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi:10.1053/jhep.2001.24563
4. Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31(4):899–906. doi:10.1053/he.2000.5763
5. Yong CC, Elsarawy AM, Wang SH, et al. The surgical challenges of salvage living donor liver transplantation for Hepatocellular carcinoma; The cumulative experience of 100 cases - A retrospective cohort study and a propensity score analysis. Int J Surg. 2018;54(Pt A):187–192. doi:10.1016/j.ijsu.2018.04.041
6. Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55(1):132–140. doi:10.1002/hep.24680
7. Tang A, Bashir MR, Corwin MT, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: a Systematic Review. Radiology. 2018;286(1):29–48. doi:10.1148/radiol.2017170554
8. Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289(3):816–830. doi:10.1148/radiol.2018181494
9. American College of Radiology. Liver Imaging Reporting and Data System. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS.
10. An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single Hepatocellular Carcinoma: preoperative MR Imaging to Predict Early Recurrence after Curative Resection. Radiology. 2015;276(2):433–443. doi:10.1148/radiol.15142394
11. Wang L, Feng B, Li D, et al. Risk stratification of solitary hepatocellular carcinoma ≤ 5 cm without microvascular invasion: prognostic values of MR imaging features based on LI-RADS and clinical parameters. Eur Radiol. 2023;33(5):3592–3603. doi:10.1007/s00330-023-09484-5
12. Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–994.e983; quiz e914–985. doi:10.1053/j.gastro.2012.05.052
13. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi:10.1016/S0168-8278(02)00360-4
14. Zhang Z, Jiang H, Chen J, et al. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19(1):22. doi:10.1186/s40644-019-0209-5
15. Camp RL, Dolled-Filhart M. -tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
16. Zhang XF, Xue F, Bagante F, et al. Non-transplantable Recurrence After Resection for Transplantable Hepatocellular Carcinoma: implication for Upfront Treatment Choice. J Gastrointest Surg. 2022;26(5):1021–1029. doi:10.1007/s11605-021-05206-8
17. Chow P, Chen M, Cheng A-L, et al. Abstract CT003: iMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Cancer Res. 2023;83(8_Supplement):CT003–CT003. doi:10.1158/1538-7445.AM2023-CT003
18. Fernandez-Sevilla E, Allard MA, Selten J, et al. Recurrence of hepatocellular carcinoma after liver transplantation: is there a place for resection? Liver Transpl. 2017;23(4):440–447. doi:10.1002/lt.24742
19. Sposito C, Citterio D, Virdis M, et al. Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma. World J Gastroenterol. 2022;28(34):4929–4942. doi:10.3748/wjg.v28.i34.4929
20. Rhee H, An C, Kim HY, Yoo JE, Park YN, Kim MJ. Hepatocellular Carcinoma with Irregular Rim-Like Arterial Phase Hyperenhancement: more Aggressive Pathologic Features. Liver Cancer. 2019;8(1):24–40. doi:10.1159/000488540
21. Kang TW, Rhim H, Lee J, et al. Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur Radiol. 2016;26(10):3437–3446. doi:10.1007/s00330-015-4190-5
22. Kierans AS, Leonardou P, Hayashi P, et al. MRI findings of rapidly progressive hepatocellular carcinoma. Magnetic Resonance Imaging. 2010;28(6):790–796. doi:10.1016/j.mri.2010.03.005
23. Kawamura Y, Ikeda K, Hirakawa M, et al. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatology Res. 2010;40(10):1006–1014. doi:10.1111/j.1872-034X.2010.00703.x
24. Kang HJ, Kim H, Lee DH, et al. Gadoxetate-enhanced MRI Features of Proliferative Hepatocellular Carcinoma Are Prognostic after Surgery. Radiology. 2021;300(3):572–582. doi:10.1148/radiol.2021204352
25. Okuda K, Musha H, Nakajima Y, et al. Clinicopathologic features of encapsulated hepatocellular carcinoma: a study of 26 cases. Cancer. 1977;40(3):1240–1245. doi:10.1002/1097-0142(197709)40:3<1240::AID-CNCR2820400339>3.0.CO;2-Y
26. Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205(1):33–40. doi:10.1097/00000658-198701000-00006
27. Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237(4):536–543. doi:10.1097/01.SLA.0000059988.22416.F2
28. Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70(1):45–49. doi:10.1002/1097-0142(19920701)70:1<45::AID-CNCR2820700108>3.0.CO;2-7
29. Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009;250(2):435–443. doi:10.1148/radiol.2501071702
30. Wang MD, Sun LY, Qian GJ, et al. Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: a retrospective cohort study. Int J Surg. 2022;105:106843. doi:10.1016/j.ijsu.2022.106843
31. Hu X, Chen R, Wei Q, Xu X. The Landscape Of Alpha Fetoprotein In Hepatocellular Carcinoma: where Are We? Int J Bio Sci. 2022;18(2):536–551. doi:10.7150/ijbs.64537
32. Trevisani F, Garuti F, Neri A. Alpha-fetoprotein for Diagnosis, Prognosis, and Transplant Selection. Semin Liver Disease. 2019;39(2):163–177. doi:10.1055/s-0039-1677768
33. Tsilimigras DI, Bagante F, Moris D, et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: a multi-institutional analysis of 1010 patients. Surgery. 2019;166(6):967–974. doi:10.1016/j.surg.2019.08.010
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.