Back to Journals » Journal of Inflammation Research » Volume 17
Pre-Implant Immune Status is Associated with Infection Risk After Left Ventricular Assist Device Implantation
Authors Dieterlen MT, Messer EK , Klaeske K, Sieg F, Eifert S, Haunschild J, Jawad K, Saeed D, Dashkevich A, Borger MA
Received 7 June 2023
Accepted for publication 10 November 2023
Published 1 February 2024 Volume 2024:17 Pages 581—589
DOI https://doi.org/10.2147/JIR.S424879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Maja-Theresa Dieterlen,* Eva Katharina Messer,* Kristin Klaeske, Franz Sieg, Sandra Eifert, Josephina Haunschild, Khalil Jawad, Diyar Saeed, Alexey Dashkevich,* Michael Andrew Borger*
University Clinic of Cardiac Surgery, Leipzig Heart Center, HELIOS Clinic, Leipzig, Germany
*These authors contributed equally to this work
Correspondence: Maja-Theresa Dieterlen, University Department for Cardiac Surgery, Heart Center Leipzig, University Hospital, HELIOS Clinic, Strümpellstraße 39, Leipzig, 04289, Germany, Tel +49 – 341 865 1651, Fax +49 – 341 865 1452, Email [email protected]
Purpose: Infection is the most common complication after left ventricular assist device (LVAD) implantation. The immune status of LVAD patients is relevant for the incidence and severity of infection, but it is unknown if there is a predisposing immune status prior to LVAD implantation that contributes to an increased risk for infection in the post-implant period. We analyzed the pre-LVAD immune status in patients with infection within 3 months after LVAD implantation in comparison to infection-free patients.
Patients and Methods: Fifty-four consecutive LVAD patients were included in this study. According to their infectious history in the first 3 months after LVAD implantation, these patients were grouped into an infection (n=23) and an infection-free group (n=31). Pre-LVAD blood samples were obtained for flow cytometric analysis of immunological parameters including B cells, subsets of T, dendritic and natural killer cells. Patient-specific, clinical and laboratory data were recorded.
Results: Blood count analysis prior to LVAD implantation showed comparable counts of erythrocytes (p=0.19), platelets (p=0.33) and leukocytes (p=0.50) between patients with infection and infection-free patients in the post-implant period. Patients with infection in the first 3 months after LVAD implantation had lower concentrations of lymphocytes (p=0.02). Forty percent of the patients with infection showed more often pre-LVAD neutrophil-to-lymphocyte ratios (NLR) > 7 than patients without infection in the first 3 months after LVAD implantation (14%, p=0.05). Patients with infection already had lower percentages of CD3+ T cells (p=0.03), CD19+ B cells (p< 0.01), BDCA2+ pDCs (p=0.03) and BDCA4+ plasmacytoid DCs (pDCs) (p=0.05) prior to LVAD implantation than infection-free patients.
Conclusion: Our results demonstrated that patients with infection in the early post-implant period showed lower concentrations of lymphocytes, especially of CD3+ T cells and CD19+ B cells, decreased percentages of BDCA2+ and BDCA4+ pDCs, and had more often NLRs > 7 indicating moderate-to-severe inflammation. Thus, we identified specific immunological changes pre-LVAD that could help to identify patients at risk for infection in the early post-implant period.
Keywords: LVAD, immune system, T cells, B cells, plasmacytoid dendritic cells, neutrophil–lymphocyte ratio
Introduction
Left ventricular assist device (LVAD) support is an established treatment option for patients with advanced heart failure but comprises the risk for infection following implantation. Infection is the most common complication after LVAD implantation, leading to major morbidity and mortality.1 Between 19% and 39% of the patients with implanted continuous-flow LVAD suffer from infection, and >10% result in LVAD-related death.1 The 1-year mortality is 5.6 times greater in patients with infection compared to infection-free patients.2 An association between infection and cerebrovascular events in LVAD patients is assumed.3,4 Predisposing factors for infection are an elevated body mass index (BMI), young age, female sex, prior cardiac surgery or intra-aortic balloon pump, trauma at the driveline site and duration of LVAD support.1,5 Further, the host’s immune status is relevant for the incidence and severity of infection, and it has been reported that LVAD implantation alters the immunobiology, thereby affecting the response to infection.6,7
However, it has not been investigated so far, if there is a predisposing immune status prior to LVAD implantation that contributes to an increased risk for infection in the post-implant period. Thus, the present study analyzed the pre-LVAD immune status in patients that suffer from infection early (3 months) after LVAD implantation in comparison to infection-free LVAD patients.
Materials and Methods
Study Groups and Clinical Characteristics
This study was approved by the Ethics Committee of the Medical Faculty of the University of Leipzig, Germany (ID: 225/17-ek) and was performed according to the guidelines of the Declaration of Helsinki (2013). Written informed consent was obtained from all patients before study initiation.
The study included 54 patients who underwent LVAD implantation between September 2018 and January 2021. Citrated blood and serum were obtained prior to LVAD implantation. Immunological parameters, including cytokines, B cells, and subsets of T cells, dendritic cells (DCs) and natural killer (NK) cells were quantified. Patient-specific, clinical and laboratory data were recorded. The postoperative course of the first 3 months after LVAD implantation, including the occurrence and type of infection, was documented. Infection was defined according to the definition of the International Society of Heart and Lung Transplantation (ISHLT) and divided into 3 types: LVAD-specific, LVAD-related and non-LVAD infection.8 LVAD-specific infections are related to the device and do not occur in non-LVAD patients such as pump, cannula, pocket or driveline infections. LVAD-related infections can be associated with the implanted device and include for example infective endocarditis, LVAD-related bloodstream infection, mediastinitis or wound infection. Non-LVAD infection comprises infections that are not affected by the LVAD such as respiratory tract infection, urinary tract infection, and Clostridium difficile infection.8
Inclusion criteria were (i) age ≥18 years, (ii) indication for LVAD implantation and (iii) informed consent. Exclusion criteria were (i) pregnancy, (ii) known immunodeficiency, (iii) malignant disease and (iv) infection within 6 weeks prior to LVAD implantation.
Blood Sampling
After peripheral blood withdrawal, citrated blood samples were analyzed using flow cytometry. Sera were centrifuged at 2000 *g at room temperature (RT) for 10 min, aliquoted, and frozen at −20°C until analysis.
Flow Cytometry
Citrated blood samples were used to determine the proportion of the following immunological cell populations: total CD3+ T cells and their proportion of CD4+ and CD8+ T cells as well as their degree of terminal differentiation/senescence (CD57) and activation (CD25); total CD16+CD56+ NK cells and the proportion of their subpopulations (CD56bright, CD56dim and CD56neg NK cells) and the degree of terminal differentiation (CD57); total regulatory T cells (Tregs) defined as CD3+/CD4+/CD25high/CD127low; total CD19+ B cells; total dendritic cells (DCs) and their subsets expressing blood dendritic cell antigen (BDCA) 1, 2, 3 or 4. BDCA 1 and 3 indicate subsets of myeloid DCs (mDCs), whereas BDCA 2 and 4 indicate subsets of plasmacytoid DCs (pDCs). In brief, samples were incubated with different antibody panels for 20 min at RT: panel A: CD57-APC, CD56-FITC, CD16-APC, CD3-PerCP/Cy5.5, CD19-PE; panel B: CD57-APC, CD8-FITC, CD25-PE-Cy7, CD4-APC-H7, CD3-PerCP/Cy5.5; panel C: lineage cocktail 1-FITC, HLA-DR-PerCP, CD304-APC, CD303-PE; panel D: lineage cocktail 1-FITC, HLA-DR-PerCP, CD1c-PE, CD141-APC; panel E: CD127-Alexa Fluor 647, CD25-PE-Cy7, CD4-APC-Cy7, CD3-PerCP-Cy5.5. The antibodies were purchased from Becton Dickinson (BD, Franklin Lakes, NJ, USA) and BioLegend (San Diego, CA, USA). Following antibody incubation, erythrocytes were lysed with 2 mL FACS lysing solution (BD Biosciences) for 10 min. After centrifugation at 300 *g for 5 min at RT, the cells were washed with 4 mL phosphate-buffered saline (PBS), followed by additional centrifugation. The cells were fixed by adding 500 µL of 1% formalin-PBS. Flow cytometric analysis was performed using a BD LSR II cytometer with FACS-Diva 2.0 software version 6.1.3 (BD Biosciences). Standardization of the instrument was performed by weekly measurements of Cytometer Setup and Tracking Beads (BD Biosciences). In general, 100,000 events were recorded in each panel.
Quantification of Cytokines
The cytokines interleukin (IL)-2, IL-4, IL-10 and interferon (IFN)-γ were quantified in the serum samples using the Bio-Plex Pro Human Screening Panel 5plx EXP (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. For multiplex assay analysis, a Luminex® 200 device and Luminex XPonent® software version 3.1 (Luminex, Austin, TX, USA) were used. The cytokines tumor necrosis factor (TNF)-α, IL-6 and IL-1β were quantified using ELISA MAX™ Deluxe Sets (BioLegend) according to the recommended protocols of the manufacturer and the Tecan reader Infinite PRO 200 and the i-control™ software (both Tecan Group AG, Männedorf, Switzerland).
Statistics
Data were collected and evaluated using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). Statistical analyses were performed using SPSS version 28 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± standard deviation of the mean for continuous variables and as the number (percent) for categorical variables. A comparison of the means was performed with Student’s t-test in case of normal distribution or with Mann–Whitney U-test in case of non-normal distribution. Group comparisons of ordinal data were performed using the χ²-test for frequencies greater than 5 or using Fisher’s exact test for frequencies lower or equal to 5. Logistic regression analysis was used to identify confounding factors for early infection after LVAD implantation. P values ≤0.05 were considered significant.
Results
Twenty-three patients (43%) suffered from infection in the first 3 months after LVAD implantation. A comparison of demographic and clinical data between patients with and without infection in the first 3 months after LVAD implantation showed that both groups were comparable (Table 1). No confounding factors for early infection after LVAD implantation could have been detected in logistic regression analysis comprising demographic data (age, gender, BMI), clinical data (eg, indication, etiology, NYHA class, INTERMACS), comorbidities as well as drug, nicotine and alcohol consumption (p > 0.05 for all tested variables).
 |
Table 1 Demographic and Clinical Characteristics of Patients Prior to LVAD Implantation |
Patients of the infection group suffered from either LVAD-specific, LVAD-related or non-LVAD infections, while the major type of infection was a percutaneous driveline infection that occurred in 70% of the patients in the infection group (Table 2).
 |
Table 2 Type of Infection Occurring Within 3 Months Following LVAD Implantation |
Blood count analysis prior to LVAD implantation showed comparable counts of erythrocytes (p = 0.19), platelets (p = 0.33) and leukocytes (p = 0.50) between patients with infection and infection-free patients in the post-implant period (Supplemental Table 1). Patients with infection in the first 3 months after LVAD implantation had lower concentrations of lymphocytes (p = 0.02) (Supplemental Table 1). Lymphopenia, defined as <1×109 lymphocytes/L, occurred in 41% of the patients with infection and in 24% of infection-free patients (p = 0.33). Patients with infection showed more often pre-LVAD neutrophil-to-lymphocyte ratios (NLR) >7, which indicates moderate to severe/critical inflammation, than patients without infection in the first 3 months after LVAD implantation (patients with infection: 40% with NLR > 7, patients without infection: 14% with NLR > 7, p = 0.05).
A detailed flow cytometric analysis revealed remarkable differences between both groups: Patients with infection in the first 3 months after LVAD implantation already had lower percentages of CD3+ T cells (p = 0.03), CD19+ B cells (p < 0.01), BDCA2+ pDCs (p = 0.03) and BDCA4+ pDCs (p = 0.05) prior to LVAD implantation than patients who were free from infection in the first 3 months (Table 3).
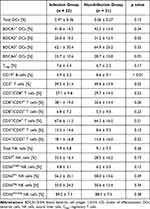 |
Table 3 Flow Cytometric Parameters Prior to LVAD Implantation in Patients Suffering from Infection Within the First 3 Months Following LVAD Implantation and Infection-Free Patients |
The percentages of CD4+ T cells (p = 0.21), CD8+ T cells (p = 0.23), total DCs (p = 0.15), BDCA1+ (p = 0.34) and BDCA3+ DCs (p = 0.33), Tregs (p = 0.17), total NKs (p = 0.36) and subsets of NK cells (CD56bright NK cells: p = 0.13; CD56dim NK cells: p = 0.39; CD56neg NK cells: p = 0.34; CD56dim/neg NK cells: p = 0.38) were comparable between the groups. The CD57 expression of CD4+ T cells, CD8+ T cells and NK cells as well as the activation status of CD4+ and CD8+ T cells measured by CD25 expression did not differ between patients with infection and infection-free patients (Table 3). Serum cytokine concentrations of the proinflammatory cytokines IL-1β (p = 0.44), IL-2 (p = 0.46), IL-6 (p = 0.37), IL-17A (p = 0.14), IFN-γ (p = 0.16) and TNF-α (p = 0.16) as well as the anti-inflammatory cytokines IL-4 (p = 0.20) and IL-10 (p = 0.19) were comparable between both groups in the pre-LVAD period (Table 4).
Discussion
Our study investigated if the pre-LVAD immune status differed between patients that suffer from infection in the first 3 months after LVAD implantation in comparison to infection-free LVAD patients. Our results demonstrated that patients with infection in the early post-implant period showed lower concentrations of lymphocytes, especially of CD3+ T cells and CD19+ B cells, decreased percentages of BDCA2+ and BDCA4+ pDCs, and had more often NLRs >7 indicating moderate-to-severe inflammation. Thus, we identified specific immunological changes pre-LVAD that could help to identify patients at risk for infection in the early post-implant period who could benefit from increased vigilance and/or more liberal antibiotic therapy.
Lymphocytes comprise cell subsets such as innate T helper cells and NK cells, but also antigen-specific T and B cells. These cell subsets exhibit various effector function with the aim to reduce the expansion of pathogens such as viruses, bacteria and parasites.9 Lymphopenia is associated with increased infection risk and an increased risk of infection-related death.10 Although both study groups were comparable for the incidence of lymphopenia prior to LVAD implantation, patients suffering from infection in the early post-implant period had significantly lower concentrations of lymphocytes than infection-free patients. This indicates that even low concentrations of lymphocytes that range within the reference could have an effect on infection risk. Low circulating B and T cell numbers have been shown to predispose patients for infectious diseases.11,12
T cell activation and senescence were analyzed by expression of CD57 and CD25. Comparable values of activated and senescent T cells were documented in patients suffering from infection and infection-free patients. The reduced percentages of T (and B) cells seem to contradict this finding; however, the absolute or relative counts of T and B cells do not allow conclusions on the state of activation or senescence. Therefore, the reduced percentages of T (and B) cells do not contradict the finding of comparable T cell activation and senescence or comparable serum cytokine concentrations in the study groups.
DCs are antigen-presenting cells that are involved in the activation and maturation of T cells, thereby adjusting the immunological reaction to antigens.13 Furthermore, DCs mutually regulate B and NK cells.14 BDCA2+ and BDCA4+ DCs are subsets of pDCs that can be activated by bacteria to produce IFN-α and proinflammatory cytokines as well as prime naïve CD4+ T cells.15 Furthermore, pDCs induce the maturation, activation and cytokine production of NK cells that are a relevant component of viral defense.14 A reduction of pDCs, as it has been shown in our study, might be a relevant factor for a reduced immunological reaction in defending pathogens and seems to lead to a higher infection rate in LVAD patients in the early post-implant period. The infection-reducing properties of pDCs have been described in several studies16–19 and comprise cytoprotective effects and T cell activation as well as fine-tuning of adaptive immune responses through enhanced T cell differentiation.20
We found that patients with infection in the first 3 months after LVAD implantation showed more often moderate to severe/critical inflammation according to NLR in the pre-LVAD period than patients without infection. NLR is a cheap and easy to calculate parameter from blood count analysis that reflects the inflammation status and stress, and indicates the balance between innate and adaptive immune responses.21 NLR reflects the dynamic relationship between the innate immune system, represented by neutrophils, and the adaptive cellular immune system, represented by lymphocytes. It has been used as a rapid and valid marker for acute, subacute and chronic inflammation in association with infectious diseases, and allows a differentiation in mild, moderate, severe and critical inflammation.21 Healthy individuals have mean NLR values around 1.7.22 In LVAD patients, NLR is increased, and studies reported median pre-LVAD NLR of 4.3 and post-implant NLR at 4–6 months after implantation of 4.4.23 Higher pre-LVAD NLR is associated with mortality and right ventricular failure.24,25 According to our study results, pre-LVAD NLR > 7 could be an indicator for patients at risk for infection in the early post-implant period. However, only 40% of the patients with infection in our study had NLR values greater than 7, which indicates that NLR alone is not a valuable marker for post-implant infection and should be combined with additional parameters.
According to our data, it could be hypothesized that a pre-LVAD immune monitoring including the analysis of T cells, B cells, DCs and NLR is helpful to decrease the post-implant infection risk when combined with an increased vigilance or adapted antibiotic therapy. The ISHLT and American Heart Association (AHA) Guidelines recommend a secondary antibiotic prophylaxis for the prevention of infectious events during routine procedures or dental work.26,27 Further, subsets of monocytes and macrophages and their activation status should be investigated to increase the knowledge of the effects on the innate immune system. A prospective study should investigate if patients at risk for post-implant infections would benefit from a post-implant prophylactic antibiotic regimen. Furthermore, it is conceivable to investigate if the application of immunoglobulins or adoptive T cell therapy is helpful to reduce the risk for post-implant infection in predisposed patients.
Conclusion
We identified specific immunological changes pre-LVAD involving lower concentrations of T and B cells, decreased percentages of pDCs, and a higher incidence of NLRs >7 in patients with infection in the early post-implant period compared to infection-free patients. A pre-LVAD immune monitoring could help to identify patients at risk for infection in the early post-implant period. These patients could benefit from increased vigilance and more liberal antibiotic therapy in the early post-implant period.
Abbreviations
AHA, American Heart Association; BDCA, blood dendritic cell antigen; BMI, body mass index; CD, cluster of differentiation; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy; CVA, cerebrovascular accident; DCs, dendritic cells; DCM, dilatative cardiomyopathy; HTx, heart transplantation; ICD, implantable cardioverter-defibrillator; ICM, ischaemic cardiomyopathy; IFN-γ, interferon-γ; IL-2/4/6/10, interleukin-2/4/6/10; ISHLT, International Society for Heart and Lung Transplantation; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; mDCs, myeloid dendritic cells; NK, natural killer; NLR, neutrophil-to-lymphocyte ratio; NYHA, New York Heart Association; pDCs, plasmacytoid dendritic cells; RT, room temperature; TNF-α, tumor necrosis factor α; Tregs, regulatory T cells.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Funding
Eva K. Messer received a Kaltenbach doctoral fellowship of the German Heart Foundation.
Disclosure
Maja-Theresa Dieterlen and Eva Katharina Messer shared first authorship; both authors contributed equally. Alexey Dashkevich and Michael Andrew Borger shared senior authorship; both authors contributed equally. The authors report no conflicts of interest in this work.
References
1. Zinoviev R, Lippincott CK, Keller SC, Gilotra NA. In full flow: left ventricular assist device infections in the modern era. Open Forum Infect Dis. 2020;7(5):ofaa124. doi:10.1093/ofid/ofaa124
2. O’Horo JC, Abu Saleh OM, Stulak JM, Wilhelm MP, Baddour LM, Rizwan Sohail M. Left Ventricular Assist Device Infections: a Systematic Review. ASAIO J. 2018;64(3):287–294. doi:10.1097/MAT.0000000000000684
3. Aggarwal A, Gupta A, Kumar S, et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J. 2012;58(5):509–513. doi:10.1097/MAT.0b013e318260c6a6
4. Kato TS, Schulze PC, Yang JH, et al. Pre-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012;31(1):1–8. doi:10.1016/j.healun.2011.08.014
5. Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: a comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39(8):774–781. doi:10.1016/j.healun.2020.03.002
6. Mondal NK, Sobieski MA, Pham SM, et al. Infection, oxidative stress, and changes in circulating regulatory t cells of heart failure patients supported by continuous-flow ventricular assist devices. ASAIO J. 2017;63(2):128–133. doi:10.1097/MAT.0000000000000487
7. Kimball PM, Flattery M, McDougan F, Kasirajan V. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg. 2008;85(5):1656–1661. doi:10.1016/j.athoracsur.2008.01.050
8. Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30(4):375–384. doi:10.1016/j.healun.2011.01.717
9. Koyasu S, Moro K. Role of innate lymphocytes in infection and inflammation. Front Immunol. 2012;3:101. doi:10.3389/fimmu.2012.00101
10. Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE, Altfeld M. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018;15(11):e1002685. doi:10.1371/journal.pmed.1002685
11. Grammatikos A, Donati M, Johnston SL, Gompels MM. Peripheral B cell deficiency and predisposition to viral infections: the paradigm of immune deficiencies. Front Immunol. 2021;12:731643. doi:10.3389/fimmu.2021.731643
12. Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95(9):2860–2868. doi:10.1182/blood.V95.9.2860.009k35_2860_2868
13. Solano-Gálvez SG, Tovar-Torres SM, Tron-Gómez MS, et al. Human dendritic cells: ontogeny and their subsets in health and disease. Med Sci. 2018;6:88.
14. Thomas R, Yang X. NK-DC crosstalk in immunity to microbial infection. J Immunol Res. 2016;2016:6374379. doi:10.1155/2016/6374379
15. Michea P, Vargas P, Donnadieu MH, et al. Epithelial control of the human pDC response to extracellular bacteria. Eur J Immunol. 2013;43(5):1264–1273. doi:10.1002/eji.201242990
16. Cormier SA, Shrestha B, Saravia J, et al. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol. 2014;88(16):9350–9360. doi:10.1128/JVI.00818-14
17. Lynch JP, Werder RB, Loh Z, et al. Plasmacytoid dendritic cells protect from viral bronchiolitis and asthma through semaphorin 4a-mediated T reg expansion. J Exp Med. 2018;215(2):537–557. doi:10.1084/jem.20170298
18. Rahman T, Brown AS, Hartland EL, van Driel IR, Fung KY. Plasmacytoid dendritic cells provide protection against bacterial-induced colitis. Front Immunol. 2019;10:608. doi:10.3389/fimmu.2019.00608
19. Cervantes-Barragan L, Lewis KL, Firner S, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109(8):3012–3017. doi:10.1073/pnas.1117359109
20. Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity. 2019;50(1):37–45. doi:10.1016/j.immuni.2018.12.027
21. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–488. doi:10.4149/BLL_2021_078
22. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine. 2018;97(26):e11138. doi:10.1097/MD.0000000000011138
23. Ferrera C, Gonzalez Fernandez O, Bouzas N, et al. Neutrophil to lymphocyte ratio is related to thrombotic complications and survival in continuous flow left ventricular assist devices. ASAIO J. 2020;66(2):199–204. doi:10.1097/MAT.0000000000000971
24. Sundararajan S, Kiernan MS, Couper GS, Upshaw JN, DeNofrio D, Vest AR. The neutrophil-lymphocyte ratio and survival during left ventricular assist device support. J Card Fail. 2019;25(3):188–194. doi:10.1016/j.cardfail.2019.01.005
25. Yost GL, Joseph CR, Tatooles AJ, Bhat G. Neutrophil-to-lymphocyte ratio predicts outcomes in patients implanted with left ventricular assist devices. ASAIO J. 2015;61(6):664–669. doi:10.1097/MAT.0000000000000267
26. Wilson WR, Gewitz M, Lockhart PB, et al. Prevention of Viridans Group Streptococcal infective endocarditis: a scientific statement from the American Heart Association. Circulation. 2021;143(20):e963–e978. doi:10.1161/CIR.0000000000000969
27. Potapov EV, Antonides C, Crespo-Leiro MG, et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg. 2019;56(2):230–270. doi:10.1093/ejcts/ezz098
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

