Back to Journals » Journal of Inflammation Research » Volume 16
Potential Mechanism of Fatigue Induction and Its Management by JAK Inhibitors in Inflammatory Rheumatic Diseases
Authors Felis-Giemza A , Massalska M , Roszkowski L, Romanowska-Próchnicka K , Ciechomska M
Received 28 March 2023
Accepted for publication 27 July 2023
Published 8 September 2023 Volume 2023:16 Pages 3949—3965
DOI https://doi.org/10.2147/JIR.S414739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Anna Felis-Giemza,1 Magdalena Massalska,2 Leszek Roszkowski,3 Katarzyna Romanowska-Próchnicka,4 Marzena Ciechomska2
1Biologic Therapy Center, National Institute of Geriatrics, Rheumatology, and Rehabilitation (NIGRiR), Warsaw, Poland; 2Department of Pathophysiology and Immunology, National Institute of Geriatrics, Rheumatology, and Rehabilitation (NIGRiR), Warsaw, Poland; 3Department of Outpatient Clinics, National Institute of Geriatrics, Rheumatology, and Rehabilitation (NIGRiR), Warsaw, Poland; 4Department of Biophysics, Physiology and Pathophysiology, Faculty of Health Sciences, Warsaw Medical University, Warsaw, Poland
Correspondence: Magdalena Massalska, Department of Pathophysiology and Immunology, National Institute of Geriatrics, Rheumatology, and Rehabilitation, Spartanska 1, Warsaw, 02-637, Poland, Tel +48226709564, Email [email protected]
Abstract: It is well known that fatigue is a highly disabling symptom commonly observed in inflammatory rheumatic diseases (IRDs). Fatigue is strongly associated with a poor quality of life and seems to be an independent predictor of job loss and disability in patients with different rheumatic diseases. Although the pathogenesis of fatigue remains unclear, indirect data suggest the cooperation of the immune system, the central and autonomic nervous system, and the neuroendocrine system in the induction and sustainment of fatigue in chronic diseases. Fatigue does not correspond with disease activity and its mechanism in IRDs. It is suggested that it may change over time and vary between individuals. Abnormal production of pro-inflammatory cytokines such as interleukin-6 (IL-6), interferons (IFNs), granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF, IL-15, IL-17 play a role in both IRDs and subsequent fatigue development. Some of these cytokines such as IL-6, IFNs, GM-CSF, and common gamma-chain cytokines (IL-15, IL-2, and IL-7) activate the Janus Kinases (JAKs) family of intracellular tyrosine kinases. Therapy blocking JAKs (JAK inhibitors – JAKi) has been recently proven to be an effective approach for IRDs treatment, more efficient in pain reduction than anti-TNF. Therefore, the administration of JAKi to IRDs patients experiencing fatigue may find rational implications as a therapeutic modulator not only of disease inflammatory symptoms but also fatigue with its components like pain and neuropsychiatric features as well. In this review, we demonstrate the latest information on the mechanisms of fatigue in rheumatic diseases and the potential effect of JAKi on fatigue reduction.
Plain Language Summary: JAKi block pro-inflammatory cytokines, decrease activation of IDO, diminish the effects of genetic polymorphism (particularly mediated by IL-6 and IL-1β) as well as sickness behavior also connected with IL-1β expression, which result in fatigue and pain inhibition.In patients treated with JAKi, it was observed that there was significantly greater improvement compared to placebo and MTX in fatigue and pain reduction measured using PROs.Administration of JAKi (BARI+UPA) benefits over TNFi treatment in pain and fatigue reduction assessed by PtGA in RA patients.The effectiveness of fatigue reduction in PROs under recently approved JAKi still needs to be proven in clinical practice, especially in selective and nonselective groups of JAKi.
Keywords: fatigue, JAK inhibitors, patient-reported outcomes, tofacitinib, baricitinib, upadacitinib
Introduction
Based on research data, fatigue appears in most chronic inflammatory diseases as well as cancer and some neurological conditions.1 In chronic diseases, it can be described as pain, depression, sleep disturbances, reduced physical activities, autonomic dysfunction, and hormonal disturbances,2,3 Severe fatigue is observed in patients suffering from inflammatory rheumatic diseases (IRDs) including axial spondyloarthritis (AS), psoriatic arthritis (PsA), rheumatoid arthritis (RA), primary Sjögren syndrome (pSS), systemic lupus erythematosus (SLE), scleroderma, and osteoarthritis.4 IRDs patients describe fatigue as one of their most exhausting problems, and while there is a lack of common tools measuring fatigue across IRDs, there is no established treatment of fatigue and the mechanism leading to its regulation is incompletely understood.1 It is possible that multiple mechanisms, which fluctuate over time and vary between individuals, can participate in fatigue development.5
Fatigue is strongly associated with a much poorer quality of life. It is an independent predictor of job loss and disability in patients with different rheumatic diseases, which creates substantial social and economic costs.4,6,7 Patients experiencing a level of fatigue implicated in reduced quality of life determine 40–80% of all RA patient groups,8 50–75% of all AS, and 45–50% of all PsA.9,10 Interestingly, fatigue and well-being were ranked by patients as the most important issues after pain and independence above joint symptoms. Although the aetiology of IRDs is unknown, it has been proved that the balance between pro- and anti-inflammatory cytokines plays a fundamental role in disease progression and chronic fatigue maintenance. In particular, pro-inflammatory cytokines that signal through Janus Kinases (JAKs) family of intracellular tyrosine kinases are essential in these processes.
In mammals, there are 4 members of the JAK family: JAK1, JAK2, JAK3, and Tyrosine Kinase 2 (TYK2). Activation of JAKs resulted in downstream phosphorylation of various isoforms of Signal Transducers and Activators of Transcription (STAT) proteins and their translocation into the nucleus as STATs dimers, where they alter the expression of cytokine-responsive genes.11 This system is known as the JAK-STAT pathway and often leads to the proliferation and/or differentiation of the immune cells, takes part in processes of immunity development (including cytokines release), cell division, and cell death, as well as tumour formation. JAK inhibitors (JAKi) competitively bind to the ATP-binding site of JAKs and suppress the JAKs activity, thereby inhibiting cytokine signal transduction and cytokine action. Presently, JAKi therapy is one of the most effective treatments of disease symptoms and pain for IRDs patients not responding to other biologic disease-modifying anti-rheumatic drugs (bDMARDs), including anti-TNF therapy.12 Therefore, the administration of JAKi to IRDs patients experiencing fatigue may find rational implications as a therapeutic modulator not only of RA symptoms but also fatigue with its components like pain and neuropsychiatric features. In this review we analyze the data concerning fatigue improvement after JAKi treatment in IRDs. The mechanisms of different aspects of fatigue development and its possible control by JAKi treatment have also been proposed.
Potential Mechanism of Fatigue
The inflammation-induced fatigue has been well proved in animal and human studies in IRDs.6,13,14 However, higher concentrations of pro-inflammatory cytokines in patients with rheumatic diseases did not correspond with worse fatigue in RA.13 There was no relationship between fatigue and validated disease activity scores in RA (like DAS28),15 although it often shadows disease flare.12 Interestingly, the time needed to improve fatigue was the same in the early and established disease phase. However, if fatigue becomes chronic, the chances for significant improvement are less likely.12 Medical interventions for RA aiming at fatigue as a primary or secondary outcome have shown that bDMARDs, including anti-TNF agents, rituximab, tocilizumab, canakinumab, abatacept, and anti-IFN-γ, reduced fatigue in a similar proportion in patients suffering from active RA as compared with placebo.16,17 However, it remains elusive if the fatigue improvement results from reduced inflammation and disease activity or is mediated by other mechanisms.8 Inflammation may play a pivotal role in initiating fatigue response, but there is no evidence that it takes part in the maintenance of fatigue in the chronic phase of the disease. Psychosocial factors seem to play a certain role in the mechanism of fatigue development, but increasing data support the genetic and molecular basis of that process.1 There are models explaining fatigue as evolutionary, complex, and automated behaviour increasing the probability of survival during “danger” like infection or injury.18 The “sickness behaviour” investigated in animals is characterized by inactivity, demotivation, and withdrawal. It is signalled by IL-1β in the brain and resembles chronic fatigue in humans.1 JAKi, at least baricitinib (BARI), was shown to decrease IL-1β and TNF (indirectly), thus intervening in the mechanism of fatigue.19
Oxidative Stress
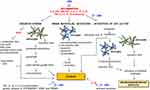
Inflammation might result in metabolic disturbances, including oxidative and nitrosative stress (Figure 1). Oxidative stress takes part when reactive oxygen species (ROS), produced by innate immune cells during the inflammation, dominate over antioxidant defenses. Oxidative and nitrosative stress, mitochondrial dysfunctions together with activated immune-inflammatory pathways and brain metabolic disorders are involved in the pathophysiology of severe fatigue.20 Data presented by Surowiec et al have shown that the metabolic profile of plasma carries information about the severity of fatigue and seems to be independent of inflammation (measured as C-reactive protein; CRP), disease activity (measured as Disease Activity Score; DAS28), and presence of other disease-related factors like anti-cyclic citrullinated peptide (anti-CCP) or rheumatoid factor (RF).1 The metabolomic study of fatigue has shown its strong associations with biochemical patterns connected with oxidative stress as increased fatigue correlated with a metabolic pattern characterized by the down-regulation of metabolites from the urea cycle, fatty acids, tocopherols, aromatic amino acids, and hypoxanthine.1 What’s more, oxidative stress has been linked with initiation or progression of several neurodegenerative disorders like Parkinson’s disease, which are accompanied by fatigue too.21
In the nervous system, it was shown that oxidative stress may stimulate astrocytes to inhibit glutamate transporters.4 Consequently, the accumulation of glutamate may decrease brain-derived growth factor concentrations, resulting in decreased neuroplasticity involved in motivational and cognitive fatigue. Increased production of free radicals and reactive intermediates of oxygen and nitrogen is measured by f2-isoprostanes. Although levels of f2-isoprostanes in urine and plasma independently predicted fatigue scale in SLE patients, the precise mechanism connecting oxidative stress with fatigue is still unclear.22
Brain Microglial Activation
Central nervous system disturbances and strong bi-directional signalling between the immune system and the brain are shown to participate in the development of strong fatigue.4 Cognitive impairment and lack of motivation are declared in RA patients suffering from fatigue.23 There are several mechanisms by which inflammation might alter the neurochemistry of the brain and thus contribute to fatigue (Figure 1). One of them is the presence of pro-inflammatory cytokines that activate microglia in the brain by transferring the signal directly across the blood–brain barrier or indirectly via activated vascular endothelial cells or the vague nerve.24,25 It was shown almost 10 years ago that inflammation originating from joints can be transmitted by sensory afferent neurons through spinal pathways to the central nervous system.26
A few brain areas with different neurotransmitters (including glutamate, acetylcholine, noradrenaline, serotonin, and dopamine) are involved in fatigue development.4 The presence of several pro-inflammatory cytokines (like IL-1, IL-6, TNF, IL-23, and IL-17) in rheumatic disorders causes the reduced level of monoamines (like serotonin, dopamine, and noradrenaline) important for mood and motivation in the synaptic cleft by increasing monoamine transporter. Additionally, the secretion of noradrenaline, a neurotransmitter responsible for increasing arousal, alertness, and attention, is inhibited by pro-inflammatory cytokines and nitric oxide.4
Activation of IDO and TDO
Finally, activation of tryptophan 2.3-dioxygenase (TDO) and indoleamine 2.3-dioxygenase (IDO-1) induced by pro-inflammatory cytokines results in tryptophan conversion to the kynurenine metabolic pathway, further inducing local inflammation in the brain. Tryptophan, degraded into kynurenine cannot serve for serotonin production anymore and this lack further supports motivational fatigue development. Kynurenine is later metabolized into 3-hydroksykynurenine and quinolinic acid in microglia or kynurenic acid in astrocytes.27,28 Both compounds activate oxidative pathways, causing mitochondrial dysfunctions and neurodegenerative effects.29,30 In RA and SLE patients elevated levels of kynurenine correlated with fatigue, but not with depression (in SLE patients).31,32 RA fibroblast-like synoviocytes (FLS), the main effector cells in joint destruction and perpetuation of inflammation in RA, were able to produce IDO as a result of IFN-γ stimulation.33,34 The recent data showed that JAKi (tofacitinib (TOFA), BARI, and upadacitinib (UPA)) significantly suppressed the expression of IDO by FLS and decreased IDO-mediated suppressive effects of FLS on T cell proliferation.35 Observed effects resulted probably from suppression of IFN-γ secretion as well as IFN-γ signalling by JAKi. From the three tested JAKi, UPA decreased IDO-1 expression in FLS the most efficiently and also most strongly reduced IFN-γ production by T cells co-cultured with FLS.35 However, none of the tested JAKi (at the concentration of 0.1 μM, which correspond with plasma levels detected in blood with approved JAKi doses) was able to inhibit IDO-1 expression.35–38
Proteomics
Proteomic analysis of cerebrospinal fluid from pSS patients has revealed 15 proteins discriminating patients with fatigue from those without, but none of the proteins holds the known pro-inflammatory function, which stays in line with oxidative stress-derived fatigue aetiology described above.39 Inflammation stimulates the onset of fatigue, which can further persist despite successful treatment and inhibition of inflammation in rheumatic disorders.40 These observations might result from long-term changes in the brain morphology due to inflammation-induced decrease in nerve growth factors, like brain-derived neurotrophic factor (BDNF), or changes in the striatal microstructure of the human brain predicting fatigue.41
Pain
Musculoskeletal pain often accompanies many inflammatory rheumatic diseases and is a strong predictor of fatigue.8,42,43 It was shown that the treatment of rheumatic patients had a much stronger effect on pain than fatigue,44,45 which suggests different brain mechanisms engaged in pain and fatigue.4 In RA, the causal link between pain and fatigue has not been proved, although pain reduction achieved with DMARDs treatment is followed by improvement in fatigue.8
Genetics
The observation that patients with similar disease activity experienced extremely different fatigue supports the genetic background of the process.18 The last data showed that genetic variation can influence fatigue severity and that specific genes contribute to the regulation of fatigue and pain. Polymorphisms in genes encoding TNF, IL-1β, IL-4, and IL-6 have been already associated with fatigue, while IL-6 and IL-1β influence also pain feeling.46,47 Data published by Norheim et al pointed genetic variants at the RTP4/MASP1 locus associated with fatigue in Scandinavian patients suffering from Sjögren syndrome.18 RTP4, encoded by RTP4 gene, is a Golgi chaperon involved in the organization of γ-δ opioid pain receptors. As JAKi treatment aims to IL-6 (directly) and IL-1β (indirectly), this can explain why this treatment was shown to have a good effect on pain, even more pronounced than bDMARDs.48
There are also other genes like ERN1 and PRDM1 involved in fatigue regulation. ERN1 codes enzyme IRE1a important for sensing cellular stress signals, while PRDM1 encodes a transcription factor expressed by T cells and B cells and is involved in downregulation of immune responses and repression of IFN-γ expression.18 Genetic variants of PRDM1 support the notion that mechanisms downregulating the inflammation may be associated with fatigue.18
Hormones
There is observed dysfunction of two important hormonal axes (hypothalamic-pituitary-gonadal axis and hypothalamic-pituitary-thyroid axis) in patients with pSS, SLE, and RA, but their links with fatigue were not investigated.49 Considering the role of neuroendocrine disturbances in fatigue development, investigation of SLE patients showed that stress, depression, and pain, but not disease activity, independently correlated with fatigue.43 Gonadal hormones may have a role not only in the higher prevalence of autoimmunity in women but also in higher disease activity and stronger fatigue.50
At present, treatment by JAKi seems to modulate the mechanism of fatigue in four possible points as shown in Figure 1. JAKi stop or decrease inflammation mediated by pro-inflammatory cytokines, decrease activation of IDO, diminish the effects of genetic polymorphism (particularly mediated by IL-6 and IL-1β) as well as sickness behaviour also connected with IL-1β expression. Other pro-inflammatory cytokines like TNF-α, IL-1β, and IL-17 signal through different pathways than JAK-STAT.
Fatigue in Selected Rheumatic Diseases
IRDs patients experience progressive and significant restrictions in daily living reporting pain, fatigue, sleep disturbances, and functional impairments in work and leisure-time physical activities.51 Patient-reported symptoms significantly influence patients` quality of life as are often present early in the disease. To assess disease activity in clinical trials, the American College of Rheumatology (ACR) established a core set of measures that includes several patient-reported outcomes (PROs) associated with RA.52 PROs have been recognized by the ACR and the European League Against Rheumatism (EULAR) as important factors in the assessment of patient disease activity, and their evaluation has been recommended as a daily clinical practice when considering a response to therapy53,54 and additional measures of effectiveness in clinical trials of RA.55
Among the most important and often used in clinical practice PROs are as follows: Patient Global Assessment of Disease Activity (PtGA), quality of life assessed by Health Assessment-Questionnaire (HAQ), disability index (DI) (HAQ-DI), fatigue assessed by the Functional Assessment of Chronic Illness Therapy (FACIT-F), assessment of well-being assessed by Short Form Health Survey (SF-36) including physical and mental component summary (PCS and MCS), impact on work assessed by Work Instability Scale for RA (RA-WIS) and pain-intensity assessment on the Visual Analogue Scale (VAS).
Rheumatoid Arthritis
RA is a chronic inflammatory joint disorder characterized by joint stiffness, swelling, and pain, which can have a profound impact on a patient’s health-related quality of life.56,57 The goals of RA treatment are not only symptom relief, reduction in disease activity, and contraction of the rate of joint damage but also improvement in physical functioning and well-being from the patient’s perspective.58,59 So, in addition to effectively treating joint inflammation and reducing the rate of joint deterioration, the treatment aims to improve quality of life as well.60
Fatigue and pain often coexist in rheumatoid arthritis (RA) patients and can be challenging to separate due to various reasons. Both symptoms share common underlying mechanisms, such as chronic inflammation and altered pain processing. Additionally, fatigue and pain are subjective experiences, influenced by psychological and emotional factors, making it difficult to differentiate them based solely on patient reports. EULAR, ACR, and Outcomes Measures in Rheumatology (OMERACT) have outlined the importance of PROs in addition to physician-assessed outcomes for the complete assessment of the progression of the disease and the evaluation of the effectiveness of RA treatment.52 PROs used for the assessment of treatments in RA clinical trials typically include VAS, PtGA, HAQ-DI, and SF-36.61,62 Patients who are intolerant or show an inadequate response (IR) to conventional DMARDs (cDMARDs) are often treated with a biological agent. For DMARD-IR patients, biologics are usually combined with cDMARDs, primarily methotrexate (MTX), but some biologics are approved and are efficacious as monotherapy as well.63,64
Treatments targeting inflammation and pain in RA may simultaneously affect both fatigue and pain symptoms, making it challenging to distinguish between the two solely based on treatment response.
Fatigue in patients with early RA, treated according to modern treatment strategies, is still not well understood. Holten et al explored associations between disease activity and fatigue in patients with early RA during the initial 2 months of modern treat-to-target therapy and predictors of fatigue after 24 months of follow-up.65 The prevalence of fatigue declined from 69% at baseline to 38% at 24 months, with a rapid and sustained reduction during treatment. Fewer swollen joints, lower power Doppler ultrasound score, and higher PtGA increased the risk of clinically relevant fatigue at 24 months. Not achieving remission at 6 months was associated with a higher risk of reporting fatigue at 24 months. Low-objective disease activity and high PtGA at baseline were predictors of clinically relevant fatigue at 24 months.65
Axial Spondyloarthritis
AS is a progressive disease that has a significant impact on the patient’s daily functioning and quality of life. The most frequently reported symptoms in patients with AS are pain, stiffness, and fatigue,66 and back pain is associated with fatigue and work impairment.67 Therefore, the goal of treating AS is not only to relieve symptoms, reduce disease activity, and reduce joint and spine damage but also to improve physical fitness and well-being from the patient’s perspective.68 Inflammatory disease activity and functional capacity are assessed in AS with Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)69 and Bath Ankylosing Spondylitis Functional Index (BASFI), respectively.70 Interestingly, in the first question of the BASDAI questionnaire, there is a question about fatigue, which shows its importance in this disease. A widely used PRO instrument in assessing health-related quality of life in AS is SF-3671 and Fatigue Severity Scale (FSS).72 Ankylosing spondylitis quality of life (ASQoL),73 HAQ-DI,74 and FACIT-F scale are also often used.75
Psoriatic Arthritis
PsA is a combination of dermatological and joint disorders. Little is known about the factors underlying PsA-associated fatigue. In two Canadian studies involving approximately 400 patients with PsA from a single center,9,76 54% of the variability in fatigue can be explained mainly by physical disability, pain, and psychological stress.77 A recent study of 246 patients with PsA from 13 countries demonstrated that high fatigue was mainly explained by disease-related factors (skin psoriasis, number of tender joints, and enthesitis), but also by patient-related characteristics (level of education and female gender), indicating that fatigue in PsA has a multifactorial nature.78
However, another study of fatigue in 101 patients with PsA found that it may also be related to the emotional and social aspects of the disease, rather than to arthritis. Fatigue is often cited by patients in qualitative research, and it has complex meanings covering both the physical and mental aspects.76 In a qualitative study preceding the development of the updated PsA Core GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis)/OMERACT, fatigue was considered a critical component of the impact on the life of PsA patients.79 In a study by Gossec et al which involved 474 patients with PsA, the participants ranked fatigue as the second most important domain after pain.80
Thus, fatigue in RA, AS, and PsA occurs on most days and varies in intensity and frequency, ranging from heaviness and weariness to exhaustion. Individuals distinguish between systemic fatigue, related to their arthritis, and general tiredness. Despite the use of newer and more selective drugs for inflammation (bDMARDs), the percentage of patients complaining of fatigue is still significant.17 In recent years, JAK inhibitors have become a hope for patients with IRDs, which not only block inflammation but also affect other mechanisms of fatigue.11
JAK Inhibitors
Oral JAK inhibitors have been developed for the treatment of RA, PsA, and AS. Currently, there are four JAK inhibitors: tofacitinib (TOFA), baricitinib (BARI), upadacitinib (UPA), and filgotinib (FILGO) licensed for the treatment of RA, two (TOFA and UPA) licensed for the treatment of PsA and one JAKi (UPA) approved for the AS treatment.81–83 They are categorized as targeted synthetic DMARDs (tsDMARDs) to differentiate them from bDMARDs and cDMARDs.
Recommendations for RA treatment underline the importance of early diagnosis and introduction of intensive treatment strategies, with the target of remission or the lowest possible disease activity.84 As suggested by the ACR and EULAR, the treatment regimen for RA includes initial cDMARDs, especially MTX; if not effective, combining cDMARDs with bDMARDs, or oral tsDMARDs that target the intracellular JAK-pathways.53 This early intensive disease management has been shown not only to mitigate joint damage and inflammation but also to improve patient health-related quality of life.85–87 It has also been postulated that the treatment of RA should be a shared decision-making process between the patient and the physician, when together they should address questions of significance to the patients, including the potential improvement of PROs.84 A relevant question to both patients and physicians is whether bDMARDs or tsDMARDs require co-administration of MTX, which itself is associated with potential adverse effects that may affect patient function (fatigue, nausea, etc.).88 Unfortunately, in clinical trials, when registering drugs for rheumatic diseases, the feeling of fatigue is not taken into account either as a primary or secondary endpoint, it is combined with pain and other PROs.
Tofacitinib (TOFA)
TOFA, a pan-JAK-inhibitor blocking JAK1/2/3 and to a lesser extent TYK2, is the first approved JAKi by the FDA in 2012 for the treatment of RA.
Numerous studies have demonstrated that TOFA administration leads to a decrease in the level of pro-inflammatory cytokines that signal through JAK-STAT and a reduction of fatigue sensation in rheumatic patients. For instance, it has been demonstrated that RA patients following 3 months of TOFA treatment had reduced levels of IL-2, IL-4, IL-15, IL-21 (common γ-chain cytokines), IFN-γ, and decreased phospho-STAT and STAT expression levels.89 Another cytokine associated with neuronal symptoms in IRDs is IL-6. It has been demonstrated that a high level of IL-6 correlates not only with disease activity and inflammation but also with fatigue and mood disorders, including depression.90 Moreover, RA patients treated with TOFA for 6 months had decreased sera levels of IL-6, IFN-γ, and other JAK-STAT-independent cytokines like IL-17 and TNF-α and simultaneously reduced VAS and HAQ-DI scores.91
TOFA is currently approved for use in five diseases: RA, PsA, AS, polyarticular juvenile idiopathic arthritis (JIA) or juvenile PsA, and ulcerative colitis.82 During clinical trials, this drug has been proven to be effective and safe in all the above-mentioned indications, and numerous studies have shown its positive effect on fatigue, physical fitness, quality of life, and sleep quality.
Rheumatoid Arthritis
In RA, there was a sustained improvement in PROs in randomized controlled trials, including PtGA, pain, HAQ-DI, and FACIT-F, as well as HRQoL (measured with SF-36), reported in Phase II,92 Phase III,93–97 and Phase IIIb/IV randomized controlled trials of TOFA. These clinical trials included patients with insufficiency responses to MTX,94 cDMARDs,96 or bDMARDs,97 as well as in patients with early arthritis95 and TOFA used as monotherapy.93 In RA, TOFA administration alone or in combination therapy resulted in statistically significant improvement from baseline in all five fatigue-related scores (PtGA, pain, HAQ-DI, FACIT-F, and SF-36) as compared to placebo92–98 or MTX that was maintained throughout the duration of TOFA treatment.95 Furthermore, significantly more patients reported improvement in their physical functioning, which is directly connected with better fatigue control, after treatment with TOFA (10 mg twice a day) vs those treated with ADA.98
Psoriatic Arthritis
Another disease in which fatigue is one of the important accompanying symptoms is PsA. Post hoc analysis results from two Phase III studies of TOFA in patients with active PsA suggest that there is an approximately linear relationship between the Psoriatic Arthritis Disease Activity Score (PASDAS) and SF-36, Patient’s Global Joint and Skin Assessment (PGJS), HAQ-DI, and FACIT-F.99,100 The results in patients with active PsA treated with TOFA confirmed that patients achieving PASDAS-defined remission or low disease activity had greater improvements in health-related quality of life, fatigue, and physical function than patients with high disease activity.101 This confirms the importance of fatigue symptoms in PsA patients and is consistent with other studies that indicate improvement in fatigue as a key result indicating improvement in their condition.79,102 In these studies, both median time and time to initial HAQ-DI and FACIT-F response and disease activity (as determined by minimal disease activity and PASDAS) were generally similar in patients with active PsA treated with TOFA or ADA.103,104
Axial Spondyloarthritis
Patients receiving TOFA experienced greater improvements compared to placebo in the summary of the physical component of SF-36, body pain, FACIT-F, ASQoL, and work impairment in the Phase II study.105 Confirmed in the phase III study, the efficacy endpoints showed significant improvement with TOFA compared to placebo in clinical measurements and PROs for disease activity, mobility, function, and health-related quality of life (also measured by change from baseline at ASQoL, SF-36, and FACIT-F). A recent study confirms that patients with AS treated with TOFA 5 mg twice daily showed significant improvements in fatigue, HRQoL, pain, and work performance compared to placebo at week 16, which was maintained through week 48.106
Juvenile Idiopathic Arthritis
TOFA was the first oral JAK inhibitor to be evaluated in JIA patients. The studies concluded that their primary endpoint was met: the rate of JIA exacerbations was significantly lower with TOFA than with a placebo. Improvements were also seen in secondary endpoints including JIA/ACR response rates and JIA/ACR inactivity, Juvenile Arthritis Disease Activity Score (JADAS), and HAQ-DI change. Treatment with TOFA also showed a statistically significant decrease in VAS, assessing the patient’s well-being, as well as a significant reduction in pain.107
Baricitinib (BARI)
This small molecule along with TOFA belongs to the first generation of JAKi. The pharmacokinetic study demonstrated that BARI suppressed IL-2, IL-4, IL-15, and IL-21 release by peripheral blood mononuclear cells (PBMC) from healthy control (HC), but to a lesser extent than TOFA and UPA.108 In addition, BARI inhibited IL-6, MCP-1, and IP-10 production in oncostatin M-stimulated RA synovial fibroblasts.109
Rheumatoid Arthritis
BARI is an oral selective inhibitor of JAK1 and JAK2,110 molecules that enable intracellular signaling of multiple inflammatory cytokine pathways associated with inflammation in RA. BARI has been approved as a treatment for adults with moderate to severe disease in more than 50 countries, including Europe, the USA, and Japan.81 The results of multi-center, randomized, double-blind, Phase III clinical trials have confirmed the efficacy and safety of the drug.
In a phase III study (RA-BEGIN), therapeutic effect of BARI was investigated in patients with active RA who were naive to cDMARDs (no or limited exposure to MTX) or bDMARDs.51 The results of that study have shown that BARI alone or in combination with MTX demonstrated superior clinical efficacy with acceptable safety compared to MTX as the initial therapy for patients with active RA.
Analysis of RA-BEGIN study in the context of PROs measures reveals that BARI alone or in combination with MTX, when used as initial therapy, resulted in significant improvement compared to MTX in the majority of the pre-specified PRO measures.111 Moreover, patients treated with BARI reported significantly greater and more rapid pain relief, more weeks with limited to no pain, and clinically meaningful improvements in physical health than patients treated with MTX alone over 1 year.112
Fatigue reduction was associated with improved daily activity and work productivity for RA patients treated with ADA or BARI with MTX background investigated in RA-BEAM study.113 At the lowest levels of remaining pain (≤10 mm) at weeks 12 and 24, however, fatigue did not appear to impact work productivity.
Finally, compared with placebo and ADA, BARI showed statistically significant improvements (p≤0.05) in many different PROs, measured by HAQ-DI, PtGA, pain, FACIT-F, SF-36 physical component score, EQ-5D index scores, and WPAI-RA (Work Productivity and Activity Impairment Questionnaire) daily activity at week 12. Improvements were maintained for measures assessed up to week 52.114 Moderate correlations were observed between improvements in disease activity and fatigue and between improvements in pain and fatigue in both MTX-IR and bDMARD-IR patients.115,116
It is worth noting that, mediation analysis showed that the large majority of the improvement in FACIT-F was explained by changes in pain and disease activity for both BARI and ADA in MTX-IR patients, and these factors explained almost all of the improvements in FACIT-F in the bDMARD experienced population.117
In summary, addressing fatigue in RA patients undergoing baricitinib treatment is crucial for optimizing treatment outcomes, monitoring disease activity, improving quality of life, and promoting patient adherence. By recognizing and managing fatigue, health-care providers can support patients in achieving better symptom control and overall disease management.
These studies indicate that baricitinib treatment is associated with improvements in fatigue levels in patients with RA. Fatigue reduction was consistently observed across different patient populations, including those who had an inadequate response to csDMARDs or TNFis. The specific mechanisms underlying baricitinib’s effects on fatigue in RA are not fully elucidated, but it is believed to be related to its ability to suppress inflammation, improve disease activity, and enhance overall well-being. However, it is important to note that individual responses may vary, and fatigue can be influenced by various factors beyond medication alone.
Upadacitinib (UPA)
UPA is another JAKi, which belongs to the next generation of JAKi having high selectivity in blocking JAK1 over JAK2, JAK3, and TYK2. It has been demonstrated that UPA inhibited STAT3 and STAT5 phosphorylation in IL-6 and IL-7 stimulated PBMCs from HC.118 Similarly, UPA along with TOFA and BARI was able to attenuate IL-6 and MMP-1 production in co-cultured T and B cells with RA or OA fibroblasts.35 Interestingly, in animal models, both adjuvant-induced arthritis (AIA) and collagen-induced arthritis (CIA), oral administration of UPA was more efficient in improved synovial hypertrophy, inflammation, cartilage damage, and bone erosion than TOFA.118 UPA is used in patients with active RA, PsA, and AS, as it has been approved for these indications.83
Rheumatoid Arthritis
Strand et al analyzed self-assessed symptoms in a 12-week study in RA patients with an inadequate response to cDMARDs (as part of the randomized SELECT-NEXT study).119 PROs included PtGA, VAS, HAQ-DI, FACIT-F, duration and severity of morning stiffness, depression (SF-36), and RA-WIS, compared to placebo, patients treated with UPA reported statistically significant improvement (p<0.05) in all scales, regardless of the UPA dose (15 and 30 mg/day were used).
The SELECT-COMPARE study compared the UPA directly with an active comparator, the ADA.120 The clinical efficacy of UPA against ADA in combination with MTX was assessed in terms of the following endpoints: ACR response; disease activity, quality of life, pain intensity, morning stiffness, and radiological progression. The group treated with UPA as compared to ADA achieved a statistically significant improvement of fatigue in the FACIT-F scale, and general health assessment using the LSM method: SF-36 and in 6/8 other domains. After 12 weeks of UPA treatment, the improvement in the physical component (PCS) of quality of life (7.89) was significantly higher compared to the placebo group [3.56 (p<0.001)] and ADA-treated patients [6.27 (p=0.002)].
Fatigue significantly impacts a patient`s quality of life. UPA`s effectiveness is managing RA symptoms, including fatigue, can improve physical function and psychosocial well-being, thereby reducing fatigue levels.
While these mechanisms offer insights into UPA`s impact on fatigue, further research is needed to fully understand the precise pathways involved. Additionally, individual responses may vary, and non-pharmacological interventions and consideration of psychological factors may complement the management of fatigue in RA patients.
Psoriatic Arthritis
UPA reduces the number of swollen and painful joints but also affects symptoms typically reported by patients, such as morning stiffness and pain. The efficacy of UPA treatment was similar in patients with PsA who showed no adequate improvement after methotrexate, as well as in patients who failed biological therapy. The results of SELECT-PsA 1 demonstrated that a significantly higher percentage of patients treated with UPA 30 mg/day compared to the group of patients treated with ADA 40 mg every 2 weeks achieved improved pain assessment (PtGA) from week 4 and in BASDAI/SF-36 endpoints at weeks 12 (except BASDAI Q2) and 24 (p<0.05). Improvements in symptoms in PsA patients treated with UPA at a dose of 15 mg/day were also observed in week 24.121
The reduction in fatigue observed in PsA patients treated with UPA can be attributed to its potent anti-inflammatory effects. UPA selectively inhibits JAK1, which plays a crucial role in the signaling of pro-inflammatory cytokines involved in the pathogenesis of PsA. By reducing inflammation and disease activity, UPA can alleviate fatigue symptoms.
Axial Spondyloarthritis
The SELECT-AXIS 1 study demonstrated a UPA-mediated improvement in disease activity and patient perception of the fatigue.122 After 1 year of treatment, there was a continuous and persistent improvement in the reduction of Ankylosing Spondylitis Disease Activity Score (ASDAS) disease activity and pain sensation, including back pain and nocturnal back pain. Improvement was seen in patients treated with UPA from the beginning as well as patients in the placebo group who were started on UPA at week 14 of the study. A similar improvement over time was observed in the assessment of the quality of life (ASQoL and ASAS Health Index) and decreased PtGA. It should be added that regardless of the timing of UPA inclusion, the improvement was similarly fast and of equivalent magnitude. In addition, clinically meaningful improvements in fatigue, as measured by FACIT-F among patients receiving continuous UPA, were maintained from week 14 through weeks 52 and 104.123
These findings suggest that UPA has a positive impact on fatigue perception in AS patients. By effectively reducing disease activity and pain, UPA contributes to an overall improvement in fatigue levels. Managing fatigue is crucial in enhancing the quality of life for AS patients, and UPA offers a potential therapeutic option to address this aspect of the disease.
Filgotinib (FILGO)
Filgotinib (FILGO) is also a JAK1 selective inhibitor which is currently approved in Europe and Japan to treat severe to moderate RA.124,125 FILGO was more efficient in blocking JAK1-dependent IL-6/pSTAT1 by monocytes from HC than other JAKi including TOFA, BARI, and UPA.126 In contrast, IFN-γ-mediated JAK2 inhibition was less suppressed by FILGO than by the other JAKi. A similar observation was previously seen by Dowty et al, demonstrating that FILGO had relatively low clinical suppression towards JAK2-dependent IL-12, IL-23, and G-CSF, GM-CSF.127 FINCH3 Phase III clinical trial also revealed that FILGO improved PROs including HAQ-DI and FACIT-F in patients with active RA.128
Other Current JAK Inhibitors
Ruxolitinib (JAK1/2 inhibitor) significantly reduced IL-6, IL-12, and IFN-γ in a dose-dependent manner, but did not show a significant reduction of IL-4 and IL-5 in animal models mimicking hyper inflammation syndrome.129 This may indicate that ruxolitinib treatment ameliorates inflammation without initiation of broad immunosuppression. A case report study showed that 4 years old patient suffering from refractory systemic idiopathic juvenile arthritis-like associated with interstitial had decreased chest abnormalities, increased oxygen saturation, and reduced frequencies of IFN-γ producing Th1 cells after 12 months of ruxolitinib administration.130 A completed clinical trial (NCT00550043) exploring the safety, tolerability, and efficacy of ruxolitinib demonstrated that 83% of patients achieved ACR20 Improvement. Patients with chronic lymphocytic leukaemia also noted an improvement in the average fatigue score (BFI) 3 months following ruxolitinib treatment.131 This suggests that ruxolitinib may be another promising JAKi in rheumatic diseases, reducing fatigue and affecting ACR20. Recent animal studies demonstrated the promising role of izencitinib (TD-1473) in targeting inflammation locally.132 Izencitinib is a gut-selective pan-JAKi that minimizes systemic exposure following oral administration. Although differences in coagulation parameters and fertility ratio before and after chronic izencitinib administration were not observed in rats and dogs, the phase IIb of the clinical trial failed to meet the primary endpoint to treat ulcerative colitis (NCT03758443).
Overview of the Group of JAK Inhibitors
Recently published meta-analysis gives a comprehensive overview of JAK inhibitors and provides evidence for their superiority in improving PROs and disease activity indices in RA.133 Fatigue and depression measured using different PROs showed significantly greater improvement in the group of RA patients treated with JAKi compared to placebo (SF-36 (PCS, MCS), FACIT-F, and EuroQol 5 dimensions questionnaire—UK and US scoring algorithm, FACIT-F) and versus patients treated with MTX (SF-36 -PCS, FACIT-F, HAQ-DI). In comparison to bDMARDs, JAKi yielded better results in PROs, and significantly better results in 2 of the questionnaires regarding fatigue and general health status (SF-36, FACIT-F). It is, however, important to note that the different JAKi were pooled into one group and probably this is the reason why the significant differences between JAK inhibitors versus bDMARDs were not observed in other questionnaires.133
There are also papers, claiming that the impact of JAKi on pain and fatigue is not significantly different than bDMARDs, at least during the short and middle term corresponding to the duration of clinical trials.134
In summary, randomized clinical trials (RCT) data in RA patients showed a faster onset of JAKi benefits than comparator ADA in the context of reduction of fatigue and pain in PtGA assessment.120 Interestingly, treatment of UPA and BARI was superior to ADA in fatigue reduction in PROs, while other JAKi (TOFA and FILGO) were non-inferior to ADA in fatigue reduction in RA patients who use combined therapy with MTX.120,135–138 Also, in RCT with PsA it was shown that the median time and time to baseline HAQ-DI and FACIT-F response and disease activity were substantially similar in patients with active PsA treated with JAKi or ADA.103,104,121 In addition, RCT data confirm that AS patients treated with JAKi showed significant improvements in pain, fatigue, HRQoL, and work performance.106,122
It should be emphasized that there are new recommendations announced by European Medicines Agency (EMA) concerning treatment by JAKi, generally JAKi should be used with great caution.139 EMA has found that, compared with TNF-alpha inhibitors, JAKi used to treat chronic inflammatory are linked to a higher risk of major adverse cardiovascular events (MACE), venous thromboembolism (VTE), malignancy, serious infections, and all-cause mortality. JAKi (TOFA, BARI, UPA, and FILGO) should be used only in patients, in whom no suitable treatment alternatives are available: those aged 65 years or above, current or past long-time smokers, those with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, or those with other malignancy risk factors. Cautious usage is also recommended in patients with known risk factors for VTE other than those listed above.139
Summary
In this review, we have focused on fatigue – its mechanism and results of JAK-targeted therapies exerted on fatigue. The importance of fatigue for patients with IRDs has been confirmed and described in many studies. However, standardized fatigue assessments and comprehensive research into a range of rheumatic diseases are still needed, as it is a subjective clinical manifestation that can only be assessed with PROs.
To inhibit the development and intensification of fatigue in rheumatic diseases, it is necessary to better understand the mechanism of its pathogenesis. JAKi seem to modulate the mechanism of fatigue in a few possible ways: to limit the release of the pro-inflammatory cytokines, decrease IDO activation, and ameliorate the sickness behaviour connected with IL-1β expression. Importantly, the administration of JAKi to IRDs patients experiencing fatigue may have rational implications also as a therapeutic modulator of neuropsychiatric features. However, in the context of EMA`s reaffirmed efforts to minimize the risk of serious side effects with the use of JAKi in chronic inflammatory diseases, despite the good effect on reducing inflammation, pain, and fatigue, caution is advised in choosing this group of drugs.
It is important to underline the need for a multidisciplinary approach to understanding fatigue and the complexity underlying its various causes and confounding factors. It is suggested that metabolic pathways are more important in fatigue modulation than inflammatory processes. However, unique metabolic-dependent molecule that could be used for the diagnosis of fatigue is still undefined.1. Further studies investigating the long-term effects of JAK inhibitors in comparison to other medications (especially bDMARDs) as well as head-to-head trials comparing different JAKi (selective vs non-selective) would be important to further evaluate their effectiveness not only in the context of their anti-inflammatory effects but also patient-related outcomes including fatigue. Importantly, there are various instruments used to assess fatigue in patients with rheumatic diseases that are correlated with each other, but there is no commonly agreed questionnaire/tool for use in a particular disease. Therefore, optimal management of fatigue certainly requires an individualized and holistic approach to the patient and his disease.
Abbreviations
anti-CCP, anti-cyclic citrullinated peptide; AS, axial spondyloarthritis; ASDAS, Ankylosing Spondylitis Disease Activity Score; ASQoL, ankylosing spondylitis quality of life; BARI, baricitinib; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biologic disease-modifying anti-rheumatic drugs; BDNF, brain-derived neurotrophic factor; cDMARDs, conventional DMARDs; CRP, C-reactive protein; DAS28, Disease Activity Score; DMARDs, disease-modifying anti-rheumatic drugs; FACIT-F, the Functional Assessment of Chronic Illness Therapy; FILGO, filgotinib; FLS, Fibroblast-like synoviocytes; FSS, Fatigue severity scale; GM-CSF, granulocyte-macrophage colony-stimulating factor; HAQ, Health Assessment-Questionnaire; HC, healthy control; IFNs, interferons; IL-6, interleukin-6; IRDs, inflammatory rheumatic diseases; IDO-1, indoleamine 2.3-dioxygenase; JADAS, Juvenile Arthritis Disease Activity Score; JAKi, JAK inhibitors; JAKs, Janus Kinases; MACE, major adverse cardiovascular events; MTX, methotrexate; PASDAS, Psoriatic Arthritis Disease Activity Score; PBMC, peripheral blood mononuclear cells; PGJS, Patient’s Global Joint and Skin Assessment; PROs, patient-reported outcomes; PsA, psoriatic arthritis; pSS, primary Sjögren syndrome; PtGA, Patient Global Assessment of Disease Activity; RA, rheumatoid arthritis; RA-WIS, Work Instability Scale for RA; RCT, randomized clinical trials; RF, rheumatoid factor; ROS, reactive oxygen species; SLE, systemic lupus erythematosus; STAT, signal transducers and activators of transcription; TDO, tryptophan 2.3-dioxygenase; TYK2, Tyrosine Kinase 2; TOFA, tofacitinib; tsDMARDs, targeted synthetic DMARDs; UPA upadacitinib; VAS, Visual Analogue Scale; VTE, venous thromboembolism; WPAI, Work Productivity and Activity Impairment Questionnaire.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by core grant to the National Institute of Geriatrics, Rheumatology, and Rehabilitation from the Polish Ministry of Science and Higher Education (S99).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Surowiec I, Gjesdal CG, Jonsson G, et al. Metabolomics study of fatigue in patients with rheumatoid arthritis naive to biological treatment. Rheumatol Int. 2016;36(5):703–711. doi:10.1007/s00296-016-3426-2
2. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature reviews. Clin Oncol. 2014;11(10):597–609.
3. Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology. 2011;50(6):1009–1018.
4. Korte SM, Straub RH. Fatigue in inflammatory rheumatic disorders: pathophysiological mechanisms. Rheumatology. 2019;58(Suppl 5):v35–v50.
5. Versteeg GA, Ten Klooster PM, van de Laar M. Fatigue is associated with disease activity in some, but not all, patients living with rheumatoid arthritis: disentangling “between-person” and “within-person” associations. BMC Rheumatol. 2022;6(1):3.
6. Druce KL, Basu N. Predictors of fatigue in rheumatoid arthritis. Rheumatology. 2019;58(Suppl 5):v29–v34.
7. Westhoff G, Dorner T, Zink A. Fatigue and depression predict physician visits and work disability in women with primary Sjogren’s syndrome: results from a cohort study. Rheumatology. 2012;51(2):262–269.
8. Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology. 2006;45(7):885–889.
9. Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum. 2001;45(2):151–158.
10. Dagfinrud H, Vollestad NK, Loge JH, Kvien TK, Mengshoel AM. Fatigue in patients with ankylosing spondylitis: a comparison with the general population and associations with clinical and self-reported measures. Arthritis Rheum. 2005;53(1):5–11. doi:10.1002/art.20910
11. Massalska M, Maslinski W, Ciechomska M. Small molecule inhibitors in the treatment of rheumatoid arthritis and beyond: latest updates and potential strategy for fighting COVID-19. Cells. 2020;9(8):1876. doi:10.3390/cells9081876
12. Pope JE. Management of Fatigue in Rheumatoid Arthritis. RMD Open. 2020;6(1):e001084. doi:10.1136/rmdopen-2019-001084
13. Howard Tripp N, Tarn J, Natasari A, et al. Fatigue in primary Sjogren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. 2016;2(2):e000282. doi:10.1136/rmdopen-2016-000282
14. Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol. 2018;14(4):214–228. doi:10.1038/nrrheum.2018.31
15. Druce KL, Bhattacharya Y, Jones GT, Macfarlane GJ, Basu N. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: results from the British society for rheumatology biologics register for rheumatoid arthritis. Rheumatology. 2016;55(10):1786–1790. doi:10.1093/rheumatology/kew241
16. Almeida C, Choy EH, Hewlett S, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2016;2016(6):CD008334. doi:10.1002/14651858.CD008334.pub2
17. Bonek K, Roszkowski L, Massalska M, Maslinski W, Ciechomska M. Biologic drugs for rheumatoid arthritis in the context of Biosimilars, Genetics, Epigenetics and COVID-19 Treatment. Cells. 2021;10(2):323. doi:10.3390/cells10020323
18. Norheim KB, Imgenberg-Kreuz J, Alexsson A, et al. Genetic variants at the RTP4/MASP1 locus are associated with fatigue in Scandinavian patients with primary Sjögren’s syndrome. RMD Open. 2021;7(3):e001832. doi:10.1136/rmdopen-2021-001832
19. Deepak HB, Prince SE, Deshpande P. Effect of baricitinib in regulating programmed death 1 and ligand programmed cell death ligand 1 through JAK/STAT pathway in psoriasis. Indian J Pharmacol. 2022;54(3):183–193. doi:10.4103/ijp.ijp_1089_20
20. Morris G, Berk M, Galecki P, Walder K, Maes M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol. 2016;53(2):1195–1219.
21. Teleanu DM, Niculescu AG, Lungu II, et al. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int J Mol Sci. 2022;23:11.
22. Avalos I, Chung CP, Oeser A, et al. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16(3):195–200.
23. Sharpe M, Wilks D. Fatigue. BMJ. 2002;325(7362):480–483.
24. Davies K, Dures E, Ng WF. Fatigue in inflammatory rheumatic diseases: current knowledge and areas for future research. Nat Rev Rheumatol. 2021;17(11):651–664.
25. McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216(Pt 1):84–98.
26. Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16(5):470.
27. Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54(1):15–36.
28. Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303(1):1–10.
29. Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):702–721.
30. Sas K, Robotka H, Toldi J, Vecsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257(1–2):221–239.
31. Akesson K, Pettersson S, Stahl S, et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci Med. 2018;5(1):e000254.
32. Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364(1–2):82–90.
33. Tykocinski LO, Lauffer AM, Bohnen A, et al. Synovial Fibroblasts selectively suppress Th1 Cell Responses through IDO1-mediated tryptophan catabolism. J Immunol. 2017;198(8):3109–3117.
34. Massalska M, Kuca-Warnawin E, Janicka I, et al. Survival of lymphocytes is not restricted by IDO-expressing fibroblast from rheumatoid arthritis patients. Immunopharmacol Immunotoxicol. 2019;41(2):214–223.
35. Yao N, Tretter T, Kvacskay P, et al. Targeting of janus kinases limits pro-inflammatory but also immunosuppressive circuits in the crosstalk between synovial fibroblasts and lymphocytes. Biomedicines. 2021;9:10.
36. Lamba M, Wang R, Fletcher T, Alvey C, Kushner J, Stock TC. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J Clin Pharmacol. 2016;56(11):1362–1371.
37. Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54(12):1354–1361.
38. Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin Pharmacokinet. 2016;55(12):1547–1558.
39. Larssen E, Brede C, Hjelle A, et al. Fatigue in primary Sjogren’s syndrome: a proteomic pilot study of cerebrospinal fluid. SAGE Open Medicine. 2019;7:2050312119850390.
40. Holdren M, Schieir O, Bartlett SJ, et al. Improvements in fatigue lag behind disease remission in early rheumatoid arthritis: results from the Canadian early arthritis cohort. Arthrit Rheumatol. 2021;73(1):53–60.
41. Dowell NG, Bouyagoub S, Tibble J, Voon V, Cercignani M, Harrison NA. Interferon-alpha-induced changes in NODDI predispose to the development of fatigue. Neuroscience. 2019;403:111–117.
42. Davies K, Mirza K, Tarn J, et al. Fatigue in primary Sjogren’s syndrome (pSS) is associated with lower levels of proinflammatory cytokines: a validation study. Rheumatol Int. 2019;39(11):1867–1873.
43. Azizoddin DR, Gandhi N, Weinberg S, Sengupta M, Nicassio PM, Jolly M. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus. 2019;28(2):163–173.
44. Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain. 2015;156(2):297–304.
45. Reygaerts T, Mitrovic S, Fautrel B, Gossec L. Effect of biologics on fatigue in psoriatic arthritis: a systematic literature review with meta-analysis. Joint Bone Spine. 2018;85(4):405–410.
46. Atzeni F, Nucera V, Masala IF, Sarzi-Puttini P, Bonitta G. Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. Pharmacol Res. 2019;149:104402.
47. Wu P, Wu X, Zhou G, et al. P2X7 receptor-induced bone cancer pain by regulating microglial activity via NLRP3/IL-1beta Signaling. Pain Physician. 2022;25(8):E1199–E1210.
48. Alciati A, Di Carlo M, Siragusano C, Palumbo A, Masala IF, Atzeni F. Effect of biological DMARDs and JAK inhibitors in pain of chronic inflammatory arthritis. Expert Opin Biol Ther. 2022;22(10):1311–1322.
49. Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279.
50. Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11(1):R7.
51. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthrit Rheumatol. 2017;69(3):506–517.
52. Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The committee on outcome measures in rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–740.
53. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthrit Care Res. 2016;68(1):1–25.
54. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977.
55. Food and Drug Administration. Guidance for industry rheumatoid arthritis: developing drug products for treatment https://www.fda.gov/regulatory-information/search-fda-guidance-documents/rheumatoid-arthritis-developing-drug-products-treatment.
56. Whalley D, McKenna SP, de Jong Z, van der Heijde D. Quality of life in rheumatoid arthritis. Br J Rheumatol. 1997;36(8):884–888.
57. Cole JC, Motivala SJ, Khanna D, Lee JY, Paulus HE, Irwin MR. Validation of single-factor structure and scoring protocol for the Health Assessment Questionnaire-Disability Index. Arthritis Rheum. 2005;53(4):536–542.
58. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–784.
59. Luqmani R, Hennell S, Estrach C, et al. British society for rheumatology and British health professionals in rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology. 2009;48(4):436–439.
60. Jansen JP, Buckley F, Dejonckheere F, Ogale S. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs--a systematic review and network meta-analysis. Health Qual Life Outcomes. 2014;12:102.
61. Ramey DR, Raynauld JP, Fries JF. The health assessment questionnaire 1992: status and review. Arthritis Care Res. 1992;5(3):119–129.
62. Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14(4):234–254.
63. Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22(1):128–139.
64. van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of Adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63(5):508–516.
65. Holten K, Paulshus Sundlisater N, Lillegraven S, et al. Fatigue in patients with early rheumatoid arthritis undergoing treat-to-target therapy: predictors and response to treatment. Ann Rheum Dis. 2022;81(3):344–350.
66. Calin A, Edmunds L, Kennedy LG. Fatigue in ankylosing spondylitis--why is it ignored? J Rheumatol. 1993;20(6):991–995.
67. Hammoudeh M, Zack DJ, Li W, Stewart VM, Koenig AS. Associations between inflammation, nocturnal back pain and fatigue in ankylosing spondylitis and improvements with etanercept therapy. J Int Med Res. 2013;41(4):1150–1159.
68. Kotsis K, Voulgari PV, Drosos AA, Carvalho AF, Hyphantis T. Health-related quality of life in patients with ankylosing spondylitis: a comprehensive review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):857–872.
69. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21(12):2286–2291.
70. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol. 1994;21(12):2281–2285.
71. Boonen A, van der Heijde D, Landewe R, et al. How do the EQ-5D, SF-6D and the well-being rating scale compare in patients with ankylosing spondylitis? Ann Rheum Dis. 2007;66(6):771–777.
72. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123.
73. Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. 2003;62(1):20–26.
74. Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25.
75. Singh H, Arya S, Talapatra P, et al. Assessment of fatigue in rheumatoid arthritis (by functional assessment of chronic illness therapy-fatigue score) and its relation to disease activity and anemia. J Clin Rheumatol. 2014;20(2):87–90.
76. Carneiro C, Chaves M, Verardino G, et al. Evaluation of fatigue and its correlation with quality of life index, anxiety symptoms, depression and activity of disease in patients with psoriatic arthritis. Clin Cosmet Investig Dermatol. 2017;10:155–163.
77. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(5):405–417.
78. Gudu T, Etcheto A, de Wit M, et al. Fatigue in psoriatic arthritis - a cross-sectional study of 246 patients from 13 countries. Joint Bone Spine. 2016;83(4):439–443.
79. Orbai AM, de Wit M, Mease P, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. 2017;76(4):673–680.
80. Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–1019.
81. European Union. Olumiant. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant.
82. European Union. Xeljanz. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz.
83. European Union. Rinvoq. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq.
84. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15.
85. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–2386.
86. Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96.
87. Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–382.
88. Nash P, Nicholls D. Perceptions of methotrexate use in rheumatoid arthritis by rheumatologists and their patients: an Australian survey study. Int J Rheum Dis. 2013;16(6):652–661.
89. Palmroth M, Kuuliala K, Peltomaa R, et al. Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associates with treatment response. Front Immunol. 2021;12:738481.
90. Choy EHS, Calabrese LH. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology. 2018;57(11):1885–1895.
91. Li Y, Yuan L, Yang J, et al. Changes in serum cytokines may predict therapeutic efficacy of tofacitinib in rheumatoid arthritis. Mediators Inflamm. 2019;2019:5617431.
92. Wallenstein GV, Kanik KS, Wilkinson B, et al. Effects of the oral Janus kinase inhibitor tofacitinib on patient-reported outcomes in patients with active rheumatoid arthritis: results of two Phase 2 randomised controlled trials. Clin Exp Rheumatol. 2016;34(3):430–442.
93. Strand V, Kremer J, Wallenstein G, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized Phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307.
94. Strand V, van Vollenhoven RF, Lee EB, et al. Tofacitinib or Adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology. 2016;55(6):1031–1041.
95. Strand V, Lee EB, Fleischmann R, et al. Tofacitinib versus methotrexate in rheumatoid arthritis: patient-reported outcomes from the randomised phase III ORAL Start trial. RMD Open. 2016;2(2):e000308.
96. Strand V, Kremer JM, Gruben D, Krishnaswami S, Zwillich SH, Wallenstein GV. Tofacitinib in combination with conventional disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: patient-reported outcomes from a phase iii randomized controlled trial. Arthrit Care Res. 2017;69(4):592–598.
97. Strand V, Burmester GR, Zerbini CA, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthrit Care Res. 2015;67(4):475–483.
98. Strand V, Mysler E, Moots RJ, et al. Patient-reported outcomes for tofacitinib with and without methotrexate, or Adalimumab with methotrexate, in rheumatoid arthritis: a phase IIIB/IV trial. RMD Open. 2019;5(2):e001040.
99. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550.
100. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–1536. doi:10.1056/NEJMoa1615977
101. Coates LC, Bushmakin AG, FitzGerald O, et al. Relationships between psoriatic arthritis composite measures of disease activity with patient-reported outcomes in phase 3 studies of tofacitinib. Arthritis Res Ther. 2021;23(1):94. doi:10.1186/s13075-021-02474-2
102. Overman CL, Kool MB, Da Silva JA, Geenen R. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol. 2016;35(2):409–415. doi:10.1007/s10067-015-3035-6
103. Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Tofacitinib or Adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open. 2019;5(1):e000806. doi:10.1136/rmdopen-2018-000806
104. Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the Phase III, randomised controlled trial: OPAL Beyond. RMD Open. 2019;5(1):e000808. doi:10.1136/rmdopen-2018-000808
105. van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a Phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76(8):1340–1347. doi:10.1136/annrheumdis-2016-210322
106. Navarro-Compan V, Wei JC, Van den Bosch F, et al. Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial. RMD Open. 2022;8(2):e002253. doi:10.1136/rmdopen-2022-002253
107. Ruperto N, Brunner HI, Synoverska O, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet. 2021;398(10315):1984–1996. doi:10.1016/S0140-6736(21)01255-1
108. McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi:10.1186/s13075-019-1964-1
109. Weston S, Macdonald JL, Williams LM, et al. The JAK inhibitor baricitinib inhibits oncostatin M induction of proinflammatory mediators in ex-vivo synovial derived cells. Clin Exp Rheumatol. 2021;40:1620–1628. doi:10.55563/clinexprheumatol/cfsajk
110. Choy EHS, Miceli-Richard C, Gonzalez-Gay MA, et al. The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib. Clin Exp Rheumatol. 2019;37(4):694–704.
111. Schiff M, Takeuchi T, Fleischmann R, et al. Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Res Ther. 2017;19(1):208. doi:10.1186/s13075-017-1410-1
112. Taylor PC, Alten R, Alvaro Gracia JM, et al. Achieving pain control in early rheumatoid arthritis with baricitinib monotherapy or in combination with methotrexate versus methotrexate monotherapy. RMD Open. 2022;8(1):e001994. doi:10.1136/rmdopen-2021-001994
113. Michaud K, Pope JE, Emery P, et al. Relative Impact of pain and fatigue on work productivity in patients with rheumatoid arthritis from the RA-BEAM baricitinib trial. Rheumatol Therap. 2019;6(3):409–419. doi:10.1007/s40744-019-0164-4
114. Keystone EC, Taylor PC, Tanaka Y, et al. Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or Adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis. 2017;76(11):1853–1861. doi:10.1136/annrheumdis-2017-211259
115. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252. doi:10.1056/NEJMoa1507247
116. Smolen JS, Kremer JM, Gaich CL, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis. 2017;76(4):694–700. doi:10.1136/annrheumdis-2016-209821
117. Fautrel B, Wu J, Wang D, Haladyj E, van de Laar M, Takeuchi T. Relative impact of pain and disease activity on improvements in fatigue: results from 2 baricitinib phase 3 clinical trials. J Clin Rheumatol. 2022;2022:1.
118. Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23. doi:10.1186/s41927-018-0031-x
119. Strand V, Pope J, Tundia N, et al. Upadacitinib improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying antirheumatic drugs: results from SELECT-NEXT. Arthritis Res Ther. 2019;21(1):272. doi:10.1186/s13075-019-2037-1
120. Strand V, Tundia N, Bergman M, et al. Upadacitinib improves patient-reported outcomes vs placebo or Adalimumab in patients with rheumatoid arthritis: results from SELECT-COMPARE. Rheumatology. 2021;60(12):5583–5594. doi:10.1093/rheumatology/keab158
121. McInnes IB, Ostor AJK, Mease PJ, et al. Effect of upadacitinib on reducing pain in patients with active psoriatic arthritis or ankylosing spondylitis: post hoc analysis of three randomised clinical trials. RMD Open. 2022;8(1):e002049. doi:10.1136/rmdopen-2021-002049
122. Deodhar A, van der Heijde D, Sieper J, et al. Safety and efficacy of upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthrit Rheumatol. 2022;74(1):70–80. doi:10.1002/art.41911
123. van der Heijde D, Deodhar A, Maksymowych WP, et al. Upadacitinib in active ankylosing spondylitis: results of the 2-year, double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension. RMD Open. 2022;8(2):e002280. doi:10.1136/rmdopen-2022-002280
124. European Union. Jyseleca. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca.
125. Namour F, Anderson K, Nelson C, Tasset C. Filgotinib: a clinical pharmacology review. Clin Pharmacokinet. 2022;61:819–832. doi:10.1007/s40262-022-01129-y
126. Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80(7):865–875. doi:10.1136/annrheumdis-2020-219012
127. Dowty ME, Lin TH, Jesson MI, et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect. 2019;7(6):e00537. doi:10.1002/prp2.537
128. Bingham CO 3rd, Walker D, Nash P, et al. The impact of filgotinib on patient-reported outcomes and health-related quality of life for patients with active rheumatoid arthritis: a post hoc analysis of Phase 3 studies. Arthritis Res Ther. 2022;24(1):11. doi:10.1186/s13075-021-02677-7
129. Huarte E, Peel MT, Verbist K, et al. Ruxolitinib, a JAK1/2 Inhibitor, ameliorates cytokine storm in experimental models of hyperinflammation syndrome. Front Pharmacol. 2021;12:650295. doi:10.3389/fphar.2021.650295
130. Bader-Meunier B, Hadchouel A, Berteloot L, et al. Effectiveness and safety of ruxolitinib for the treatment of refractory systemic idiopathic juvenile arthritis like associated with interstitial lung disease: a case report. Ann Rheum Dis. 2022;81(2):e20. doi:10.1136/annrheumdis-2020-216983
131. Jain P, Keating M, Renner S, et al. Ruxolitinib for symptom control in patients with chronic lymphocytic leukaemia: a single-group, phase 2 trial. Lancet Haematol. 2017;4(2):e67–e74. doi:10.1016/S2352-3026(16)30194-6
132. Hardwick RN, Brassil P, Badagnani I, et al. Gut-selective design of orally administered izencitinib (TD-1473) limits systemic exposure and effects of janus kinase inhibition in nonclinical species. Toxicol Sci. 2022;186(2):323–337. doi:10.1093/toxsci/kfac002
133. Toth L, Juhasz MF, Szabo L, et al. Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: a systematic review and meta-analysis of 24,135 patients. Int J Mol Sci. 2022;23(3):1246. doi:10.3390/ijms23031246
134. Odriozola ICC, Barnetche T, Richez C, Bannwarth B, Schaeverbeke T Is there a specific effect of jak-inhibitors on pain and fatigue in rheumatoid arthritis? Available from: https://acrabstracts.org/abstract/is-there-a-specific-effect-of-jak-inhibitors-on-pain-and-fatigue-in-rheumatoid-arthritis/.
135. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi:10.1056/NEJMoa1608345
136. Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthrit Rheumatol. 2019;71(11):1788–1800. doi:10.1002/art.41032
137. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and Adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–468. doi:10.1016/S0140-6736(17)31618-5
138. Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or Adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80(7):848–858. doi:10.1136/annrheumdis-2020-219214
139. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. Available from: https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.