Back to Journals » Nature and Science of Sleep » Volume 12
Postoperative 24-h Acute Sleep Deprivation Improves Learning and Memory Through Inhibition of Tau Phosphorylation in the Hippocampal Neurons of Splenectomized Rats
Authors Zhang Y, Li X, Tan W , Fang B, Ma H
Received 3 April 2020
Accepted for publication 11 August 2020
Published 24 August 2020 Volume 2020:12 Pages 603—613
DOI https://doi.org/10.2147/NSS.S254449
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Yu-wei Zhang,1,2 Xiao-qian Li,1 Wen-fei Tan,1 Bo Fang,1 Hong Ma1
1Department of Anesthesiology, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of Anesthesiology, The First Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Wen-fei Tan Email [email protected]
Purpose: As tau pathology is involved in impaired postoperative learning and memory in rats, we attempted to identify the possible mechanisms by which tau pathology affects postoperative sleep deprivation.
Methods: Adult male Sprague-Dawley rats were randomly assigned into six groups as follows: the Control group, Anaesthesia group, Surgery group, Sleep deprivation (SD) group: 24-h SD with the modified multiple platform method (MMPM), Anaesthesia and SD (ASD) group, and Surgery and SD (SSD) group. Tau396 and FOXQ1 protein expression levels in the hippocampal neurons of all groups were analysed. Changes following co-culture of hippocampal neurons with IL-6 were detected by flow cytometry.
Results: Twenty-four hours of acute SD decreased the error scores on postoperative day 5 in the ASD and SSD groups compared with the Anaesthesia and Surgery groups. Compared with the tau levels in the Control group, tau levels in the Anaesthesia and Surgery groups were increased, but SD decreased the expression of tau in the ASD and SSD groups. The expression levels of tau and FOXQ1 were inversely regulated. When hippocampal neurons were co-cultured with IL-6, the same changes were observed.
Conclusion: Postoperative 24-h acute SD improves learning and memory through inhibition of tau phosphorylation and increases IL-6-induced expression of FOXQ1 in the hippocampal neurons of splenectomized rats.
Keywords: tau pathology, interleukin-6, postoperative sleep deprivation, FOXQ1, learning, memory
Introduction
Sleep deprivation (SD) is a risk factor for perioperative neurocognitive disorders.1 Insufficient sleep increases the risk of neurodegeneration in Alzheimer’s disease (AD).2 Studies have indicated that restricted sleep increases the formation of neurofibrillary tangles and tau pathology.3,4 Although risk factors, such as kinase dysregulation, phosphatase dysregulation, reactive oxygen species, endoplasmic reticulum damage, and glymphatic system dysfunction, have been identified as the same important factors that mediate the interaction between SD and tau, the exact mechanisms by which the pathology of tau affects postoperative sleep deprivation (PSD) have not yet been fully identified. In our previous research, we found that tau pathology is involved in postoperative learning and memory impairment in rats through different mechanisms.5,6 However, it remains unknown whether PSD influences learning and memory through the same mechanisms as tau pathology.
The role of interleukin-6 (IL-6) in sleep regulation is controversial,7–10 and IL-6 may play a secondary role in addition to its primary role in the acute phase response. Therefore, it is necessary to elucidate the precise changes in both central and peripheral IL-6 levels to clarify the role of IL-6 in PSD. The results of our clinical trials suggest that IL-6 causes postoperative sleep disturbances.11,12
Forkhead box (FOX) family proteins are involved in a wide range of crucial cellular processes, including stress resistance, metabolism, cell cycle arrest, ageing and apoptosis.13 Foxq1 was first found to modulate hair follicle development in mammals,13 and FOXQ1 remarkably inhibits replicative senescence through suppressing IL-6 expression.14 FOXQ1 is the direct target gene of miR-125b, which is involved in neuronal apoptosis and the phosphorylation of tau.15 However, it remains unknown whether IL-6, FOXQ1 and tau pathology are related.
We found that tau pathology is involved in learning and memory impairment in splenectomized rats, and that increased peripheral IL-6 impairs learning and memory by influencing PSD in clinical trials. Based on the cumulative evidence, we hypothesize that postoperative 24-h acute SD increases IL-6 plasma levels in splenectomized rats, and that IL-6 increases tau phosphorylation by mediating FOXQ1 in hippocampal neurons, leading to impaired postoperative learning and memory.
Materials and Methods
Animals
Adult (6-month-old) male Sprague-Dawley rats weighing 350–400 g were housed at 22°C under a 12 h/12 h light/dark cycle with free access to food and water. The whole study was approved by the Ethics Committee of China Medical University. All research followed the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health publication No. 85–23, National Academy Press, Washington, DC).
Y-Maze Testing and Training
Spatial learning and memory were evaluated using a modified Y-maze apparatus with three opaque arms (60 cm × 15 cm × 20 cm),5 and light ON in the Y-maze indicates that testing has started. In total, 20 trials per day were carried out for each animal, and the number of errors was used to evaluate spatial learning and memory. All tests were performed between 20:00 and 24:00. As shown in Figure 1A, 7 days of training, 3 days of testing and another two days of testing on postoperative days 3 and 5 were carried out. Brains and blood plasma were harvested from anaesthetized rats 2 h after Y-maze testing.
Surgical Procedure
Rats were anaesthetized with 10% chloral hydrate (0.4 mL/kg), and their body temperatures were maintained between 36.0°C and 37.0°C using a heating pad during the whole operation. Electrocardiograms were continuously recorded using three subcutaneous needle electrodes. For splenectomy, the spleen was mobilized, isolated and removed through a small incision made in the abdomen. The wound was infiltrated with 0.25% bupivacaine just before being sutured closed.
Sleep Deprivation
The SD procedure was performed using a modified multiple platform method (MMPM) at 24 h after anaesthesia or surgery as described in previous studies.16 Briefly, SD continued for 24 h (08:00 to 08:00 on the next day), after which the rats were placed inside a tiled water tank (120 cm L × 44 cm W × 44 cm H) containing 15 platforms (5 cm in diameter) spaced 7 cm apart and water up to 2 cm beneath the platform surface. The animals were group-housed. In the SD procedure, the rat falls into the water when it enters sleep and then wakes up.
Experimental Protocol
After 7 days of Y-maze training,5 the rats were randomly assigned to one of the six groups (n=36 for each group) shown in Figure 1A as follows: (1) Control group: only Y-maze testing, (2) Anaesthesia group: anaesthesia with 0.4 mL/kg 10% chloral hydrate plus surgical incision, (3) Surgery group: anaesthesia with 10% chloral hydrate at 0.4 mL/kg and splenectomy, (4) SD group: 24 h SD with MMPM, (5) Anaesthesia and SD (ASD) group: anaesthesia and next day 24 h SD, and (6) Surgery and SD (SSD) group: splenectomy with anaesthesia and next day 24 h SD. Three days of Y-maze testing were recorded as a learning and memory baseline after group assignment.
Double Immunofluorescence Analysis
Hippocampal tissue was cut into 5 μm thick pieces using a vibrating microtome (VT1000 S; Leica, Baden-Baden, Germany). The hippocampal slices were blocked with 10% bovine serum albumin (BSA) for 1 h at room temperature (RT) to avoid non-specific staining. The sections were washed with phosphate-buffered saline (PBS), blocked in 5% milk in PBS with 0.1% Triton X-100, and incubated overnight with either tau (phosphor-tau, Ser396) antibody (BM4496; 1:100, Boster, Wuhan, China) or FOXQ1 antibody (sc-166265, 1:100; Santa Cruz, CA, USA). After washing, the antibodies were visualized with fluorescein-conjugated goat anti-rabbit or rhodamine-conjugated goat anti-mouse antibodies (Proteintech, Chicago, USA) diluted in blocking buffer for 2h at RT.
Similarly, hippocampal neurons were permeabilized with 0.1% Triton X-100 for 10 min and blocked with 1% BSA at RT for 2 hours. The cells were then incubated with primary antibody against phospho-tau (Ser396) or FOXQ1 overnight at 4°C, followed by incubation with secondary antibodies at RT for 1 hour. Additionally, cell nuclei were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI; Beyotime Biotechnology, Shanghai, China) for 10 min at room temperature.
For all steps, the major cell types in the hippocampal images (lamina IX grey matter) were recorded at 400× magnification using a Leica TCS SP2 fluorescence microscope (Leica Microsystems, Buffalo Grove, IL, USA). The number of immunoreactive cells in a similar area of each section (6 sections per rat) was determined with a LI-COR Odyssey Infrared Imager (LI-COR Biosciences, NE, USA).
Blood–Brain Barrier (BBB) Permeability Assessment
BBB permeability was assessed by Evans blue (EB) extravasation as described before.2 EB (20 mg/kg, Sigma, MO, USA) was injected through the tail vein 60 min before sacrifice. Tissues were cut into 10 μm sections and visualized using a BX-60 fluorescence microscope (Olympus, NY, USA) with a green filter.
Transmission Electron Microscopy
To carry out glutaraldehyde-osmium tetroxide fixation, rat hippocampal tissues were fixed with 2.5% glutaraldehyde, and 1% osmic acid was then added for fixation.17 Afterwards, the tissues were dehydrated with an acetone gradient, embedded in Epon 812 epoxy resin, and sliced into ultra-thin sections by an ultratome followed by staining with lead citrate. The hippocampal neurons were observed under a JEM-1400 Plus electron microscope.
Measurement of IL-6 in the Hippocampus Using ELISA
IL-6 concentrations were determined with ELISA kits (DKW12-3060-048, Dakewe Bioengineering Co. Ltd., Beijing, China) according to the manufacturer’s instructions. Each sample concentration was calculated based on a standard curve.
Quantification of IL-6 mRNA Levels with Real-Time PCR
Plasma IL-6 mRNA expression was measured in triplicate with an Applied Biosystems 7500 Real-Time PCR System (Foster City, CA, USA). Total RNA from different groups was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). IL-6 was quantified using a TaqMan MicroRNA Assay Kit (Applied Biosystems).18
The following primers were used:
IL-6 (forward, 5′-ATCTGCTCTGGTCTTCTGG-3′ and reverse, 5′- TCTGGCTTTGTCTTTCTTGT-3′), and the expression levels were normalized to GAPDH (forward, 5′-GGTTGTCTCCTGCGACTTCA-3′ and reverse, 5′-GGTGGTCCAGGGTTTCTTACT-3′). The results were analysed with the 2−ΔΔCt method.
Western Blotting
Proteins were extracted from hippocampal tissue or neurons and purified using a protein extraction kit (Kangchen, Shanghai, China).5,18 Briefly, proteins were extracted from tissue homogenates in lysis buffer on ice and then centrifuged at 12,000 rpm for 15 min at 4°C. The protein concentrations were assessed using a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China), and the total protein concentration was adjusted to a final value of 5 μg/μL. Samples containing 50 μg of protein were loaded onto 12% SDS-PAGE gels for electrophoresis and then transferred to PVDF membranes. The membranes were then treated with 5% skim milk for 1 h, and the proteins were then incubated with the following primary antibodies overnight at 4°C: anti-tau (phospho-tau, Ser396) or anti-FOXQ1. After three washes with PBS-T (Beyotime, Shanghai, China), the membranes were treated with horseradish peroxidase-conjugated secondary antibodies (1:1000, Sigma, MA, USA). Immunoreactive bands were assessed with an ECL kit (Biyuntian, Beijing, China). The intensities of the protein bands were normalised to those of the β-actin or GAPDH band and quantified by Quantity One software (Bio-Rad Laboratories, Milan, Italy).
Hippocampal Neuron Culture and Co-Culture with IL-6
Hippocampal neurons obtained from Wuhan Biofavor Biotech (CP-R107, Wuhan, China) were initially plated in minimum essential medium (MEM)-10 and maintained at 37ºC in a humid atmosphere of 5% CO2/95% air. One hour after plating, the MEM-10 was removed and replaced with serum-free neurobasal medium supplemented with N2. Neurons were seeded onto 35-mm plastic Petri dishes coated with 0.1% poly-lysine (106 cell/35-mm culture dish) for biochemical experiments. IL-6 (10 ng/mL) was added to hippocampal neurons, which were then cultured for 24 h in vitro.19,20 Parallel cultures that did not receive IL-6 treatment were used as controls.
Detection of Neuronal Apoptosis by Flow Cytometry
Annexin V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) double staining (BD Bioscience, MA, USA) and flow cytometry were used to assess the effects of IL-6 on the induction of neuronal apoptosis. Neurons (1×106) from each group co-culture with IL-6 were harvested and washed twice with PBS. According to the manufacturer’s instructions, the cells were first incubated with 10 μL of Annexin V-FITC at 37°C for 15 min and subsequently counterstained with 5 μL of PI for 30 min in the dark. FITC/PI double staining was assessed by a BD FACSCalibur flow cytometer (BD Bioscience, MA, USA). Early apoptotic cells were defined as Annexin V-positive and PI-negative cells (quadrant A4), while late apoptotic or necrotic cells were defined as both Annexin V- and PI-positive cells (quadrant A2). Each sample was prepared in triplicate. Only Annexin V-positive cells were counted for statistical purposes.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 22 for Windows; IBM, Armonk, NY, USA). Data are presented as the means ± SEMs. Normality was assessed using the one-sample Kolmogorov–Smirnov test. Quantitative comparisons between two groups were performed by t-tests, and those between three or more groups were performed by one-way or repeated-measures ANOVA followed by the post hoc Dunnett test. A value of P < 0.05 indicated statistical significance.
Results
Changes in Learning and Memory After Different Interventions
The effects of different interventions on learning and memory, as measured by the Y-maze test, are shown in Figure 1B. Rats in the same group and in different groups that exhibited the ability to learn and remember on all test days are indicated by 20 stable trial error scores after 7 days of training (Figure 1B). The numbers of total errors made by rats in each group compared to that of the controls in the 20 trials were compared and noted postoperative 3 and 5 day, revealing a significant difference between total errors by day and by group (Figure 1B). Both the Anaesthesia and Surgery groups had higher error scores than the Control group. Compared with the error scores of rats that underwent only anaesthesia and surgery, postoperative 24 h acute SD decreased the error scores in the ASD and SSD groups on postoperative 5 day.
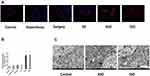
Changes in the BBB and Hippocampal Tissue Ultrastructure After Different Interventions
As shown in Figure 2A, almost no EB extravasation in the form of red fluorescence was observed in the first four groups. ASD and SSD induced a robust increase in red fluorescence in the hippocampus18 and exacerbated BBB leakage. Quantification of the fluorescence intensity due to EB extravasation confirmed the above results (Figure 2B, P < 0.05). There were no detectable differences in red fluorescence among the other four groups (P > 0.05). Hippocampal neurons were observed under a transmission electron microscope, which revealed mitochondrial swelling, vacuolation and endoplasmic reticulum dilatation in the hippocampal tissues of rats in the ASD and SSD groups (Figure 2C; white arrow).
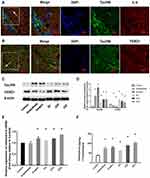
Changes in Tau and FOXQ1 After Different Interventions
The effects of IL-6 and FOXQ1 on the regulation of tau396 expression were assessed. The colocalization of phosphorylated tau immunoreactivity was confirmed by double immunostaining with IL-6 or FOXQ1 antibodies in the hippocampus (Figure 3A and B). Compared with tau levels in the Control group, the tau levels in the Anaesthesia (1.8-fold) and Surgery (3.2-fold) groups were increased, but SD (0.68-fold) decreased the increased tau levels in the ASD (1.2-fold) and SSD (1.2-fold) groups (Figure 3C and D). Compared with the FOXQ1 expression in the Control group, the FOXQ1 expression in the Anaesthesia and Surgery groups decreased, and FOXQ1 expression increased progressively in the SD, ASD and SSD groups. Tau and FOXQ1 expression levels were inversely regulated.
Changes in IL-6 Expression After Different Interventions
Compared with plasma IL-6 mRNA levels at baseline and in the Control group, all groups except for the Anaesthesia group showed significant increases in plasma IL-6 mRNA by RT-PCR (Figure 3E, P < 0.05). Compared with the Control group, all groups exhibited higher IL-6 levels by ELISA (Figure 3F, P < 0.05). The IL-6 level was highest in the SSD group and lowest in the SD group (Figure 3F, P < 0.05).
Changes in Hippocampal Neurons After IL-6 Intervention
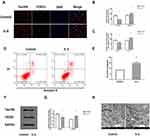
We found that IL-6 regulates tau phosphorylation through assessing FOXQ1 by Western blotting, flow cytometry, double immunofluorescence labelling, and transmission electron microscopy.
As shown, double immunofluorescence labelling revealed the colocalization of tau396 and FOXQ1 (Fig. 4A). Quantitative analysis further showed that co-culture with IL-6 simultaneously increased the level of FOXQ1 and decreased that of tau396 (Fig. 4B, C; P < 0.05).
Neuronal apoptosis was further assessed by flow cytometry in vitro. Compared with the Control group, the IL-6 group contained a significantly higher percentage of apoptotic neurons (Fig. 4D, E; P < 0.05).
Western blotting showed that co-culture with IL-6 significantly inhibited tau396 phosphorylation compared with that in the Control group, whereas co-culture with IL-6 increased the levels of FOXQ1 (Fig. 4F, G; P < 0.05).
The ultrastructure of hippocampal tissues was observed by transmission electron microscopy. In the Control group, the following cell morphological features were observed: normal hippocampal neuron ultrastructure, a complete cell structure, nucleoli in the middle of nuclei, and evenly distributed chromatin. In contrast, the hippocampal neurons in the IL-6 groups exhibited chromatin assembly, dissolution, mitochondrial swelling, vacuolation and endoplasmic reticulum dilation (Fig. 4H).
Discussion
In our present research, we found that anaesthesia and surgery increased total error scores during the Y-maze test compared with the Control group, and that postoperative 24-h acute SD may alleviate such increases even though the total error scores in the ASD and SSD groups were still higher than those in the Control group. Compared with IL-6 in the Control group, hippocampal and plasma IL-6 was more pronounced in the SSD group. ASD and SSD exacerbated BBB leakage, as demonstrated by EB fluorescence, and the hippocampal neurons of these two groups exhibited mitochondrial swelling, vacuolation and endoplasmic reticulum dilatation when viewed by transmission electron microscopy. These results showed that ASD and SSD may increase peripheral and central IL-6 levels and influence BBB leakage and hippocampal neurons. Further evidence has shown colocalization of phosphorylated tau and IL-6 or FOXQ1 and that anaesthesia and surgery may induce the expression of phosphorylated tau, but SD may alleviate this induction. Tau and FOXQ1 expression levels were inversely regulated. Finally, we tested this trend using hippocampal neurons co-cultured with IL-6. As expected, all analyses revealed that IL-6 simultaneously increases the expression of FOXQ1 and decreases tau396 expression. Postoperative 24-h acute SD improved learning and memory through inhibition of tau phosphorylation and increased IL-6-induced expression of FOXQ1 in the hippocampal neurons of splenectomized rats.
SD is a common complaint in modern life, and postoperative SD has not been adequately examined.21 Insufficient sleep increases the risk of neurodegenerative diseases, such as Alzheimer’s disease. Several studies have indicated that restricted sleep increases β-amyloid deposition and the formation of neurofibrillary tangles, the major microstructural hallmarks of Alzheimer’s disease in the brain.2–4 In our previous research, we found that tau pathology is involved in postoperative impaired learning and memory in rats induced through different mechanisms.5,6 Other research has suggested that sevoflurane induces tau phosphorylation and elevates IL-6 expression in the hippocampi of young mice and cognitive impairment in mice.22 These findings suggest that postoperative 24 h acute SD increases the plasma levels of IL-6 in splenectomized rats and that an increase in IL-6 increases tau phosphorylation, which influences postoperative learning and memory.
Interestingly, even though SD and anaesthesia or surgery increased peripheral IL-6, tau phosphorylation at Ser396 was alleviated by 24 h of acute SD and was accompanied by a decrease in total error scores, suggesting that the rats exhibited improved learning and memory due to SD on postoperative day 5. Although most research on SD shows that it impairs learning and memory, several paradox results on the influence of SD on learning and memory have been reported. Although sleep fragmentation (SF) and surgery independently produce significant memory impairment, perioperative SF significantly increases hippocampal inflammation without further cognitive impairment. The dissociation between neuroinflammation and cognitive decline may be related to the use of a memory paradigm alone that does not encapsulate other aspects of cognition, especially learning.23 Another study has reported that a brief 4h SD period is sufficient to induce delayed neurochemical changes indicative of improved avoidance learning and spatial memory.24 Sleep-deprived participants recognize negative emotional memories at rates similar to those of participants who have a full night of sleep.25 All these findings suggest that the exact mechanism of PSD is unclear. Different surgeries, time points and testing tools may produce different results.
Sleep disturbance and a long sleep duration, but not a short sleep duration, are associated with increases in IL-6,26 and peripheral IL-6 trans-signalling contributes profoundly to sleep regulation.27 The sleep-wake cycle regulates tau, and SD increases tau and spreads tau pathology.28 There are some mechanisms by which increases in peripheral IL-6 and the inhibition of tau phosphorylation are regulated. Based on the evidence from our research, increased peripheral IL-6 increases BBB permeability and FOXQ1 expression in the hippocampi of rats subjected to SSD in vivo and hippocampal neurons co-cultured with IL-6 in vitro. Although we did not test changes in tau396 with a FOXQ1 inhibitor or agonist, our results showed that tau396 and FOXQ1 expression levels are inversely regulated. FOXQ1 remarkably inhibits explicative senescence through suppressing IL-6 expression via modulation of the SIRT1-NF-κB pathway.14 Furthermore, FOXQ1 is the direct target gene of miR-125b and is involved in the effects of miR-125b on neuronal apoptosis and tau phosphorylation.15 Although we did not test the pathway or miRNA involved in the regulation of FOXQ1 and tau396, our data suggested that an increase in IL-6 may decrease tau phosphorylation by increasing FOXQ1 in hippocampal neurons, thereby improving learning and memory on postoperative day 5.
There are several limitations to this research. Previous findings have suggested that sleep-deprived young women are at particular risk for overestimating their working memory performance.29 We used only male rats to obtain our results, which may have introduced sex-based bias. Furthermore, the splenectomized rat model itself influences the level of IL-6.
Conclusion
In conclusion, postoperative 24 h acute SD improves learning and memory through inhibiting tau phosphorylation and increasing the IL-6-induced expression of FOXQ1 in the hippocampal neurons of splenectomized rats.
Abbreviations
SD, sleep deprivation; ASD, anaesthesia and sleep deprivation; SSD, surgery and sleep deprivation; MMPM, modified multiple platform method; IL-6, interleukin-6; FOX, Forkhead box; BBB, blood–brain barrier; EB, Evans blue; ANOVA, one-way analysis of variance.
Data Sharing Statement
Datasets are available on request.
Author Contributions
All authors substantially contributed to the conception and design of the study, data acquisition, or analysis and interpretation of the data, participated in drafting the article or revising it critically for important intellectual content, provided final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Rasmussen LS, Steinmetz J. Ambulatory anaesthesia and cognitive dysfunction. Curr Opin Anaesthesiol. 2015;28(6):631–635. doi:10.1097/ACO.0000000000000247
2. Ahmadian N, Hejazi S, Mahmoudi J, Talebi M. Tau pathology of alzheimer disease: possible role of sleep deprivation. Basic Clin Neurosci. 2018;9(5):307–316. doi:10.32598/bcn.9.5.307
3. Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200–208. doi:10.1016/j.brainres.2013.07.010
4. Di Meco A, Joshi YB, Pratico D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 2014;35(8):1813–1820. doi:10.1016/j.neurobiolaging.2014.02.011
5. Tan W, Cao X, Wang J, Lv H, Wu B, Ma H. Tau hyperphosphorylation is associated with memory impairment after exposure to 1.5% isoflurane without temperature maintenance in rats. Eur J Anaesthesiol. 2010;27(9):835–841. doi:10.1097/EJA.0b013e32833a6561
6. Tan WF, Cao XZ, Wang JK, Lv HW, Wu BY, Ma H. Protective effects of lithium treatment for spatial memory deficits induced by tau hyperphosphorylation in splenectomized rats. Clin Exp Pharmacol Physiol. 2010;37(10):1010–1015. doi:10.1111/j.1440-1681.2010.05433.x
7. Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85(10):3597–3603. doi:10.1210/jcem.85.10.6871
8. Spath-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83(5):1573–1579. doi:10.1210/jcem.83.5.4795
9. Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51(7):887–892. doi:10.1053/meta.2002.33357
10. Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi:10.1093/sleep/30.9.1145
11. Tan WF, Guo B, Ma H, Li XQ, Fang B, Lv HW. Changes in postoperative night bispectral index of patients undergoing thoracic surgery with different types of anaesthesia management: a randomized controlled trial. Clin Exp Pharmacol Physiol. 2016;43(3):304–311. doi:10.1111/1440-1681.12530
12. Jin F, Li Z, Tan WF, Ma H, Li XQ, Lu HW. Preoperative versus postoperative ultrasound-guided rectus sheath block for improving pain, sleep quality and cytokine levels in patients with open midline incisions undergoing transabdominal gynecological surgery: a randomized-controlled trial. BMC Anesthesiol. 2018;18(1):19. doi:10.1186/s12871-018-0485-9
13. Zhu H. Forkhead box transcription factors in embryonic heart development and congenital heart disease. Life Sci. 2016;144:194–201. doi:10.1016/j.lfs.2015.12.001
14. Wang P, Lv C, Zhang T, et al. FOXQ1 regulates senescence-associated inflammation via activation of SIRT1 expression. Cell Death Dis. 2017;8(7):e2946. doi:10.1038/cddis.2017.340
15. Ma X, Liu L, Meng J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett. 2017;661:57–62. doi:10.1016/j.neulet.2017.09.043
16. Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004(1–2):45–51. doi:10.1016/j.brainres.2004.01.019
17. Zheng P, Bin H, Chen W. Inhibition of microRNA-103a inhibits the activation of astrocytes in hippocampus tissues and improves the pathological injury of neurons of epilepsy rats by regulating BDNF. Cancer Cell Int. 2019;19:109. doi:10.1186/s12935-019-0821-2
18. Li XQ, Chen FS, Tan WF, Fang B, Zhang ZL, Ma H. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation. 2017;14(1):205. doi:10.1186/s12974-017-0977-4
19. Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB. Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotox Res. 2005;8(3–4):295–304. doi:10.1007/BF03033983
20. Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004;295(1):245–257. doi:10.1016/j.yexcr.2004.01.002
21. Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18(3):273–282. doi:10.1016/j.smrv.2013.07.002
22. Tao G, Zhang J, Zhang L, et al. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology. 2014;121(3):510–527. doi:10.1097/ALN.0000000000000278
23. Vacas S, Degos V, Maze M. Fragmented sleep enhances postoperative neuroinflammation but not cognitive dysfunction. Anesth Analg. 2017;124(1):270–276. doi:10.1213/ANE.0000000000001675
24. Azogu I, de la Tremblaye PB, Dunbar M, Lebreton M, LeMarec N, Plamondon H. Acute sleep deprivation enhances avoidance learning and spatial memory and induces delayed alterations in neurochemical expression of GR, TH, DRD1, pCREB and Ki67 in rats. Behav Brain Res. 2015;279:177–190. doi:10.1016/j.bbr.2014.11.015
25. Vargas I, Payne JD, Muench A, Kuhlman KR, Lopez-Duran NL. Acute sleep deprivation and the selective consolidation of emotional memories. Learn Mem. 2019;26(6):176–181. doi:10.1101/lm.049312.119
26. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi:10.1016/j.biopsych.2015.05.014
27. Oyanedel CN, Kelemen E, Scheller J, Born J, Rose-John S. Peripheral and central blockade of interleukin-6 trans-signaling differentially affects sleep architecture. Brain Behav Immun. 2015;50:178–185. doi:10.1016/j.bbi.2015.07.001
28. Holth JK, Fritschi SK, Wang C, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363(6429):880–884. doi:10.1126/science.aav2546
29. Rangtell FH, Karamchedu S, Andersson P, et al. A single night of sleep loss impairs objective but not subjective working memory performance in a sex-dependent manner. J Sleep Res. 2019;28(1):e12651. doi:10.1111/jsr.12651
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.