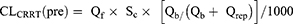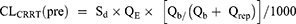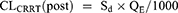Back to Journals » Drug Design, Development and Therapy » Volume 16
Population Pharmacokinetics and Dosing Optimization of Gentamicin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy
Received 8 October 2021
Accepted for publication 23 December 2021
Published 6 January 2022 Volume 2022:16 Pages 13—22
DOI https://doi.org/10.2147/DDDT.S343385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Sha He, Zeneng Cheng, Feifan Xie
Division of Biopharmaceutics and Pharmacokinetics, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, 410013, People’s Republic of China
Correspondence: Zeneng Cheng; Feifan Xie
Division of Biopharmaceutics and Pharmacokinetics, Xiangya School of Pharmaceutical Sciences, Central South University, Tongzipo Road 172, Changsha, 410013, People’s Republic of China
Tel/Fax +86 731 8265 0446
Email [email protected]; [email protected]
Purpose: Appropriate gentamicin dosing in continuous renal replacement therapy (CRRT) patients remains undefined. This study aimed to develop a population pharmacokinetic (PK) model of gentamicin in CRRT patients and to infer the optimal dosing regimen for gentamicin.
Methods: Fourteen CRRT patients dosed with gentamicin were included to establish a population PK model to characterize the variabilities and influential covariates of gentamicin. The pharmacokinetic/pharmacodynamic (PK/PD) target attainment and risk of toxicity for different combinations of gentamicin regimens (3– 7 mg/kg q24h) and CRRT effluent doses (30– 50 mL/h/kg) were evaluated by Monte Carlo simulation. The probability of target attainment (PTA) was determined for the PK/PD indices of the ratio of drug peak concentration/minimum inhibitory concentration (Cmax/MIC > 10) and the ratio of area under the drug concentration–time curve/MIC over 24 h (AUC0-24h/MIC > 100), and the risk of toxicity was estimated by drug trough concentration thresholds (1 and 2 mg/L).
Results: A one-compartment model adequately described the PK characteristics of gentamicin. Covariates including body weight, age, gender, and CRRT modality did not influence the PK parameters of gentamicin based on our dataset. All studied gentamicin regimens failed to achieve satisfactory PTAs for pathogens with an MIC ≥ 2 mg/L. A good balance of PK/PD target attainment and risk of toxicity (> 2 mg/L) was achieved under 7 mg/kg gentamicin q24h and 40 mL/kg/h CRRT dose for an MIC ≤ 1 mg/L. CRRT dose intensity had a significant impact on the target attainment of AUC0-24h/MIC > 100 and risk of toxicity.
Conclusion: A combination of 7 mg/kg gentamicin q24h and 40 mL/kg/h CRRT dose might be considered as a starting treatment option for CRRT patients, and drug monitoring is required to manage toxicity.
Keywords: gentamicin, CRRT, population pharmacokinetics, critically ill
Introduction
Sepsis is the most common cause of patient admission to an intensive care unit (ICU), and the morbidity and mortality of sepsis patients are consistently high.1,2 Early and rational antimicrobial treatment is a key for the successful treatment of sepsis patients,3–5 and daily evaluation of the administered antibiotics in this critically ill patient population is recommended to ensure efficacy, reduce adverse drug reactions and minimize pathogen resistance.6,7
Gentamicin, a member of aminoglycosides, is widely used in the treatment of severe infections caused by gram-negative bacteria in sepsis patients.8 Gentamicin is mainly eliminated by renal excretion through glomerular filtration.9 In healthy subjects, the clearance of gentamicin is about 80 mL/min, and the apparent volume of distribution is 0.25 L/kg, resulting in an elimination half-life of 2–3 h.9–11 As a concentration-dependent bacterial killing drug, the traditionally used pharmacokinetic/pharmacodynamic (PK/PD) index of gentamicin is the ratio of drug peak concentration/minimum inhibitory concentration (Cmax/MIC > 10),12,13 while recent data has suggested that the ratio of area under the drug concentration-time curve/MIC over 24 h (AUC0-24h/MIC > 100) is the preferred PK/PD index.14 The main concern of gentamicin usage is toxicities, such as nephrotoxicity and ototoxicity.15 The predictor for the risk of toxicity of gentamicin is drug trough concentration (Cmin), and the concentration threshold for a safe treatment is Cmin < 1–2 mg/L.12,13,16–18
Critical illness like the sepsis and septic shock is accompanied by vasodilatation and increased vascular permeability, leading to capillary leak syndrome.6 The capillary leak is a driving force for the fluid shift from the intravascular space to the interstitial compartment, resulting in edema formation. This phenomenon often increased the volume of distribution for hydrophilic drugs, such as the aminoglycosides.19 Increased volume of distribution could lower the drug concentrations and consequently impact the PK/PD target attainment for concentration-dependent antibiotics.9 Another common damage of sepsis is acute kidney injury (AKI),20 and this could significantly decreased the drug’s renal clearance. For AKI patients, the continuous renal replacement therapy (CRRT) is often delivered for blood purification. The main modalities of CRRT include continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF).21 As a small-molecular-weight solute removal technique, CRRT removes both of the endogenous toxins and exogenous antibiotics, and this brings extracorporeal drug clearance and leads to decreased drug concentrations.18,22 Therefore, in critically ill patients with CRRT, the combined effects of critical illness and CRRT could make the PK of antibiotics quite complicated, leading to a challengeable antibiotic dosing. Our previous work has studied the dosing of gram-positive antibiotics in CRRT patients,23 while the dosing of gram-negative antibiotics like gentamicin in this patient population deserves attention as well.
To date, the PK data of gentamicin in CRRT patients are limited, and the available dosing recommendations are under debate. Chuk et al reported the PK of 5–7 mg/kg q24h aminoglycosides in 9 critically ill patients with a mean CVVH dose of 30 mL/kg/h, but only one patient dosed with gentamicin was included.15 Petejova et al investigated the PK of 2.4–3.3 mg/kg q24h gentamicin in 7 septic AKI patients under a mean CVVH dose of 45 mL/kg/h, and only three patients achieved the Cmax/MIC target after the first dose in this condition.11 D’Arcy et al studied the PK of 5 mg/kg q24h gentamicin in 7 septic AKI patients under a mean CVVHDF dose of 42.1 mL/kg/h, and they recommended a loading dose of 5–7 mg/kg gentamicin in this patient population.24 In these aforementioned studies, only the traditional compartmental or non-compartmental analysis was performed, and the more preferred AUC/MIC index is not evaluated during the PD analysis. Hence, the available PK/PD analysis failed to provide reliable dosing regimen of gentamicin in this patient population.
The aims of this study are to establish a population PK model of gentamicin in CRRT patients and to explore the optimal dosing regimens of gentamicin in this patient population.
Materials and Methods
Study Data
A search of the literature data was made through the PubMed (until 14 May, 2021) using the search terms: (gentamicin[Title/Abstract]) AND (renal replacement therapy[Title/Abstract] OR continuous venovenous hemofiltration[Title/Abstract] OR continuous venovenous hemodialysis[Title/Abstract] OR continuous venovenous hemodiafiltration[Title/Abstract] OR CVVH[Title/Abstract] OR CVVHD[Title/Abstract] OR CVVHDF[Title/Abstract]) AND (english[Filter]). A total of 25 publications were found, and four of them were identified as the gentamicin PK studies in CRRT patients.11,15,24,25 The PK studies with individual concentration data, complete gentamicin dosing information (eg, drug dose and infusion duration), specific CRRT settings (eg, CRRT effluent dose), and sampling schemes were selected for further analysis. Applying these criteria, one gentamicin CVVH study and one gentamicin CVVHDF study were included.11,24 In these two studies, there were 14 patients and 151 gentamicin concentration measurements. The gentamicin dose ranges from 2.4 to 5 mg/kg with a fixed infusion time of 30 min, and the CRRT effluent dose is between 36.6 and 67.7 mL/h/kg. An overview of the characteristics of the included patients is presented in Table 1, and the scatter plot of plasma concentration-time after dose (TAD) is shown in Figure 1.
 |
Table 1 Demographics and Clinical Characteristics of the Study Patients Receiving Continuous Renal Replacement Therapy (CRRT) |
 |
Figure 1 Scatter plot of gentamicin concentrations for the included 14 critically ill CRRT patients with different gentamicin dosing regimens. |
Calculation of CRRT Clearance and Exploratory Data Analysis
The total clearance (CLtotal) of gentamicin in patients under CRRT consists of endogenous clearance (CLbody) and extracorporeal clearance (CLCRRT) (as shown in equation 1). The CRRT clearance provides information about the relative contribution of CRRT clearance to the total clearance. The clearance capacity of CRRT is indicated by the CRRT effluent dose, which is equal to the body weight-based effluent flow rate (QE, usually expressed in the unit of mL/h/kg). The calculation of CLCRRT (L/h) of a drug depends on the specific CRRT modality, and the formulas of CLCRRT for CVVH and CVVHDF modes are summarized in equations 2–8.11,23
Where CLCRRT (pre) is the clearance from CRRT using the pre-filter hemodilution, and ClCRRT (post) is the clearance from CRRT using the post-filter hemodilution. Qb is the blood flow rate, and Qrep is the replacement fluid rate. Qf is the ultrafiltration rate (mL/h), and Qd is the dialysate flow rate (mL/h). Sc is the sieving coefficient for CVVH mode, and Sd is saturation coefficient for CVVHDF mode.
A non-compartmental model-based exploratory analysis was performed to evaluate the contribution of residual CLbody to the CLtotal (CLratio=1-CLCRRT/CLtotal). As shown in Table 1, the CLratio for four of the patients is less than 0.05, indicating a near-zero contribution of the CLbody. Therefore, the CLtotal in these four patients was assumed to be equal to CLCRRT in the following the population PK model development.
Population PK Model Development
The concentration-versus-time data of gentamicin were analyzed by a non-linear mixed-effects modeling approach using the NONMEM® software (version 7.3, Icon Development Solutions, Ellicott City, MD, USA). The PK parameters were estimated using the first-order conditional estimation with the interaction (FOCEI) method. The models were executed by the Perl-speaks-NONMEM program (PsN, version 5.0.0, Uppsala University, Uppsala, Sweden) with the Pirana software (version 2.9.9, Pirana Software & Consulting BV) as an interface. The R® software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) was used for the plotting and simulation.
The one-compartment and two-compartment models were examined as the candidate structural model. The clearance (CL) is modeled as a sum of CLbody and CLCRRT. The endogenous drug clearance was estimated, and the extracorporeal clearance was determined as aforementioned. The inter-individual variability (IIV) on the typical population parameter estimates was modeled using a log-normal distribution with a mean of zero and a variance of ω2. For instance, the individual CL for CRRT patients was expressed as CLbody*EXP (ETA (1)) + CLCRRT. For the residual model, which represents the unexplained variability, we explored the additive error model, the proportional error model and the combined (additive + proportional) error model. Model comparison was guided by the objective function value (OFV) and goodness of fit (GOF) plots (including the observed concentrations versus individual predicted concentration, the observed concentrations versus population predicted concentration, the conditional weighted residuals (CWRES) versus population predicted concentration, and the conditional weighted residuals versus time after dose). After determining the base model, the covariates screening was performed. The statistical criterion for addition of a covariate parameter is a drop of OFV by at least 3.84 units (p < 0.05).
For the final model, the bootstrap approach was employed to ascertain the PK parameters uncertainty, and the prediction-corrected visual predictive check (pcVPC) was performed to assess the predictive performance of the model.26 The bootstrap procedure was based on the parameter estimation of different re-sampled dataset (1000 times) executed by the PsN toolkit, and the resulting parameters (eg, the median and confidence intervals of the PK parameters) were compared with those estimated from the original dataset. The pcVPC was based on the predicted concentration–time profiles of 1000 virtual datasets simulated from the final fixed parameters and variances estimates, and the observed and predicted concentrations were binned across time.
Simulation-Based Treatment Optimization
Using the final model, the efficacy and toxicity of gentamicin in CRRT patients under different gentamicin regimens and CRRT effluent doses were evaluated by the Monte Carlo simulation approach. A total of 15 treatment options were proposed based on the combinations of gentamicin regimens (3, 4, 5, 6, and 7 mg/kg q24h with an infusion period of 30 min) and CRRT effluent doses (30, 40, and 50 mL/kg/h). For each simulation, 10,000 virtual critically ill patients were selected from the MIMIC-III database,27 which is publicly available and comprises de-identified health-related data associated with over 40,000 patients who stayed in critical care units of the Beth Israel Deaconess Medical Center between 2001 and 2012. The body weight of patients was restricted to the range of 48–102 kg, which is the observed limit in our included patients. The Sd value was fixed to the observed median value of 0.77. We collected the gentamicin plasma concentration–time data after the first dose and at the steady state (after the fifth dosing), and PK profiles from the first dose to the steady state were plotted to present the accumulation. The peak concentration was defined as 30 min after the infusion was complete.
The Cmax/MIC > 10 and AUC0-24h/MIC > 100 were investigated as surrogate markers for gentamicin’s efficacy. The probability of target attainment (PTA) against pathogens with different MICs (1 and 2 mg/L) was calculated to assess the potential efficacy.13,28,29 Toxicity targets (Cmin > 1 or 2 mg/L) were also evaluated, and the proportion of simulated patients with Cmin >1 or 2 mg/L after the first dose and at steady state was calculated to determine the risk of toxicity.
Results
Patients
Fourteen CRRT patients consisting of nine males and five females were included (Table 1). The median value of weight and age of the included patients were 72 kg and 68.5 years, respectively. The CRRT dose of Petejova et al11 study was 45 mL/kg/h, and the median CRRT dose of D’Arcy et al24 was 42.1 mL/kg/h.
Population PK
The one compartment model was adequate for the PK dataset, and a change to the two-compartment model does not significantly improve the model fit. The unexplained residual variability was well described by a combined (additive + proportional) error model, and the additive and proportional errors were 0.156 mg/L and 8.0%. In the following covariate screening, none of the clinical variables was statistically significant (p < 0.05) for the PK parameters based on the univariate analysis.
The PK parameters and associated uncertainties of the final model are summarized in Table 2. The bootstrap results demonstrated that the estimated PK parameters were robust. The GOF plots (Figure 2) showed an overall good fit of the model to the observed data, although there was slight bias in the plot of CWRES versus time after dose. The pcVPC plot (Figure S1) demonstrated a reasonable agreement between the simulated and observed gentamicin concentrations, which shows that the final PK model is acceptable.
 |
Table 2 Parameter Estimates of the Final Population Pharmacokinetic Model and the Results of the Bootstrap |
Simulation-Based Efficiency and Toxicity Evaluation
At steady state, the simulation results of patients given different gentamicin dosing regimens (3–7 mg/kg q24h) under CRRT doses of 30, 40, and 50 mL/kg/h are presented in Tables 3–5, and the simulation results of the first day are shown in Tables S1–S3. The simulated results demonstrated that the PTAs of Cmax/MIC >10 for gentamicin regimens (6 and 7 mg/kg q24h), regardless of the CRRT dose, achieved desirable target attainment rate (>90%) both at the first day and at steady state for pathogens with MIC ≤ 1 mg/L. The gentamicin regimen of 5 mg/kg q24h achieved near or greater than 90% PTAs at steady state for pathogens with an MIC of 1 mg/L, but the PTAs were less than 80% (between 75.6% and 78.4%) at the first day. For pathogens with an MIC of 2 mg/L, the fractions of patients attaining the Cmax/MIC >10 target were unacceptably low (<52%) for all the studied gentamicin dose regimens. When looking at the PTAs of AUC0-24h/MIC, only the gentamicin regimen of 7 mg/kg q24h under CRRT dose of 30–40 mL/kg/h achieved a good attainment rate (near or greater than 90%) at the first day and at steady state for an MIC ≤ 1 mg/L.
 |
Table 3 The Probability of Target Attainment (PTA) of PK/PD Indices and Non-Toxicity Targets for Patients with Different Gentamicin Dose Regimens Under a CRRT Dose of 30 mL/kg/h at the Steady State |
 |
Table 4 The Probability of Target Attainment (PTA) of PK/PD Indices and Non-Toxicity Targets for Patients with Different Gentamicin Dose Regimens Under a CRRT Dose of 40 mL/kg/h at the Steady State |
 |
Table 5 The Probability of Target Attainment (PTA) of PK/PD Indices and Non-Toxicity Targets for Patients with Different Gentamicin Dose Regimens Under a CRRT Dose of 50 mL/kg/h at the Steady State |
Regarding the risk of toxicity, when the Cmin <1 mg/L safety threshold was applied, the percent patients attaining the non-toxicity target were relatively low for the gentamicin regimens of 6–7 mg/kg q24h (CRRT dose between 30 and 50 mL/kg/h) at the first day and the steady state (31.4–86.8%), and the gentamicin regimen of 5 mg/kg q24h could only achieve a low risk of toxicity (<8%) at a CRRT dose of 50 mL/kg/h. In terms of the Cmin <2 mg/L safety threshold, good safety could be achieved for all gentamicin regimens under CRRT dose of 40–50 mL/kg/h. While at a CRRT dose of 30 mL/kg/h, only gentamicin regimens of 3–4 mg/kg q24h could achieve the low risk of toxicity (1.7–9.7%). Overall, drug accumulation for the dosing interval of 24 h was not apparent, as indicated by the simulated PK profiles in Figure S2 for 7 mg/kg gentamicin under a CRRT dose of 40 mL/kg/h.
Discussion
The PK data to guide appropriate gentamicin dosing in CRRT patient population are limited. Previous studies mainly focused on the summary PK characteristics of gentamicin in CRRT patients using the two-stage compartmental or non-compartmental approach. The PK/PD target attainment of gentamicin in CRRT patients was not reported, and the impact of CRRT on the gentamicin’s PK and PD attainment was unclear. In this study, we used a population modeling and simulation approach to characterize the PK of gentamicin and to inform its optimal dosing regimens in CRRT patients.
One- and two-compartment models were both reported for gentamicin,11,12,30 and our dataset was adequately described by the one-compartment model. The use of a one-compartment model for this dataset can also be visually evident from the gentamicin concentration–time profiles (Figure 1). A concern of using different compartment models is that the peak concentration may be estimated differently by one- and two-compartment models and may therefore have an impact on the target attainment of Cmax/MIC >10. Aminoglycoside antibiotics are known with short distribution phase, and the distribution is expected to be complete by 30 minutes after the end of infusion.31 As a common practice for aminoglycosides, the Cmax is usually defined as the concentration point 30 min after the end of infusion for the calculation of Cmax/MIC index. Therefore, the use of one-compartment model is acceptable for the PK analysis and simulations of the PK/PD target attainment.
The PK of gentamicin in septic critically ill patients with CRRT is quite complicated. On one side, the pathophysiological changes such as capillary leak and renal dysfunction could lead to increased volume of distribution and decreased renal clearance.32 On the other side, the prescribed CRRT could provide increased gentamicin clearance, and titration of CRRT dose is generally empiric. Our study showed that the volume of distribution of gentamicin in critically ill CRRT patients is 0.39 L/kg, which is 56% higher than that of healthy subjects (0.25 L/kg).9 Our finding of increased volume of distribution in CRRT critically ill patients was in good agreement with previous PK study of gentamicin in non-CRRT critically ill patients (volume of distribution: 0.41 L/kg).33 The increase of volume of distribution could lead to a decrease of Cmax. This indicates that a higher loading dose of gentamicin may be needed to achieve the target of Cmax/MIC >10. The residual endogenous clearance of gentamicin in CRRT patients in our study is only 20 mL/min, which is far less than that in normal people (80 mL/min) and also in non-CRRT critically ill patients (57–75 mL/min).9,33,34 This is likely due to the severe renal function damage nature of the included patients, as they are all AKI and septic shock patients. The prescribed CRRT recovered part of the clearance capacity for gentamicin, leading to a typical total clearance of 63.3 mL/min. Nevertheless, the total clearance of gentamicin in our CRRT patients was still lower than that of healthy subjects.
For PK/PD attainment evaluation of gentamicin, the choice of PK/PD index may play an important role for evaluation of dosing regimens. Cmax/MIC > 10 was reported to be desirable for predicting efficacy in several studies,9,12,13,33 but recently the National Antimicrobial Susceptibility Testing Committee for the United States (USCAST) recommended using AUC/MIC rather than Cmax/MIC as the PK/PD index for aminoglycosides.14 In this study, both the Cmax/MIC >10 and AUC0-24h/MIC >100 based target attainment were investigated, and we found that the optimal gentamicin regimen in critically ill CRRT patients differs upon the PK/PD index selected. For example, the optimal regimen of gentamicin in CRRT patients could be a combination of 6–7 mg/kg q24h gentamicin and 40–50 mL/kg/h CRRT dose for an MIC at 1 mg/L based on the PK/PD target of Cmax/MIC >10. However, the optimal regimen would be 7 mg/kg q24h gentamicin and 40 mL/kg/h CRRT dose when the target of AUC0-24h/MIC >100 was used. These findings highlighted that dosing recommendation should be caution with the PK/PD index. The decreased clearance and increased volume of distribution of gentamicin could prolong the elimination half-life of the drug, which may lead to drug accumulation and consequently the toxicities. Delivery of the CRRT in an appropriate dose could increase the drug clearance and reduce the risk of toxicity. In the case of a Cmin <2 mg/L safety threshold, a CRRT dose of 40 mL/kg/h could achieve a good balance between the PK/PD target attainment (both of Cmax/MIC >10 and AUC/MIC >100) and risk of toxicity. If a Cmin <1 mg/L safety threshold was applied, none of the investigated treatment options was successful for simultaneously providing a desirable efficacy and a low risk of toxicity. When this safety threshold is used in the institutions, an extension of the gentamicin dosing interval (eg, 36 or 48 h) combined with drug concentration monitoring is necessary to reduce the risk of toxicity.
Our study has some limitations to acknowledge. First, the sample size of this study is relatively small due to the paucity of available PK data. This prohibited the finding of the influential covariates of gentamicin and may also lead to biased parameter estimates. Second, a classification of the CRRT patients based on the residual renal function was not made in our study due to the lack of daily urine volume or serum creatinine data. This information could inform a more accurate treatment regimens of gentamicin in CRRT patients with different residual renal function. Third, our simulations only considered the CRRT dose range of 30 to 50 mL/kg/h, and lower CRRT doses (eg, 20 to 25 mL/kg/h) were not evaluated. The 20 to 25 mL/kg/h CRRT dose is the recommended option for AKI patients by the KDIGO clinical practice guideline.20 The use of lower CRRT dose is expected to produce desirable PK/PD target attainment, but the associated risk of toxicity would be high for our studied CRRT patient population, as indicated by the simulated results under the CRRT dose of 30 mL/kg/h.
Conclusion
In conclusion, we developed a population PK model for gentamicin in critically ill CRRT patients. Model-based simulations predicted that a combination of 7 mg/kg q24h gentamicin and 40 mL/kg/h CRRT dose might be an optimal treatment option for patients with a pathogen MIC ≤1 mg/L, and drug monitoring is required to manage toxicity.
Data Sharing Statement
All the relevant data are shown in the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 82073940 and 82104306) and Hunan Provincial Natural Science Foundation of China (project number: 2021JJ40815).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Genga KR, Russell JA. Update of sepsis in the intensive care unit. J Innate Immun. 2017;9(5):441–455. doi:10.1159/000477419
2. Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–1562. doi:10.1007/s00134-020-06151-x
3. Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31(12):2742–2751. doi:10.1097/01.CCM.0000098031.24329.10
4. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi:10.1097/01.CCM.0000217961.75225.E9
5. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi:10.1097/CCM.0000000000005337
6. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi:10.1007/s00134-012-2769-8
7. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
8. Becker B, Cooper MA. Aminoglycoside antibiotics in the 21st century. ACS Chem Biol. 2013;8(1):105–115. doi:10.1021/cb3005116
9. Hansen M, Christrup LL, Jarlov JO, Kampmann JP, Bonde J. Gentamicin dosing in critically ill patients. Acta Anaesthesiol Scand. 2001;45(6):734–740. doi:10.1034/j.1399-6576.2001.045006734.x
10. Fuchs A, Guidi M, Giannoni E, et al. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol. 2014;78(5):1090–1101. doi:10.1111/bcp.12444
11. Petejova N, Zahalkova J, Duricova J, et al. Gentamicin pharmacokinetics during continuous venovenous hemofiltration in critically ill septic patients. J Chemother. 2012;24(2):107–112. doi:10.1179/1120009X12Z.0000000006
12. Veinstein A, Venisse N, Badin J, Pinsard M, Robert R, Dupuis A. Gentamicin in hemodialyzed critical care patients: early dialysis after administration of a high dose should be considered. Antimicrob Agents Chemother. 2013;57(2):977–982. doi:10.1128/AAC.01762-12
13. Roberts JA, Field J, Visser A, et al. Using population pharmacokinetics to determine gentamicin dosing during extended daily diafiltration in critically ill patients with acute kidney injury. Antimicrob Agents Chemother. 2010;54(9):3635–3640. doi:10.1128/AAC.00222-10
14. Ambrose PG, Jones RN. Aminoglycoside in vitro Susceptibility Test Interpretive Criteria Evaluations. USCAST 0002; 2019.
15. Chuk AC, Saeed F, Kousar N, et al. Variable pharmacokinetics of extended interval tobramycin or gentamicin among critically ill patients undergoing continuous venovenous hemofiltration. Clin Nephrol. 2015;84(10):214–221. doi:10.5414/CN108559
16. Bos JC, Prins JM, Misticio MC, et al. Population pharmacokinetics with Monte Carlo simulations of gentamicin in a population of severely ill adult patients from Sub-Saharan Africa. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.02328-18
17. Hodiamont CJ, Juffermans NP, Bouman CS, de Jong MD, Mathot RA, van Hest RM. Determinants of gentamicin concentrations in critically ill patients: a population pharmacokinetic analysis. Int J Antimicrob Agents. 2017;49(2):204–211. doi:10.1016/j.ijantimicag.2016.10.022
18. Ernest D, Cutler DJ. Gentamicin clearance during continuous arteriovenous hemodiafiltration. Crit Care Med. 1992;20(5):586–589. doi:10.1097/00003246-199205000-00007
19. Tang GJ, Tang JJ, Lin BS, Kong CW, Lee TY. Factors affecting gentamicin pharmacokinetics in septic patients. Acta Anaesthesiol Scand. 1999;43(7):726–730. doi:10.1034/j.1399-6576.1999.430707.x
20. Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi:10.1007/s00134-017-4755-7
21. Tandukar S, Palevsky PM. Continuous renal replacement therapy who, when, why, and how. Chest. 2019;155(3):626–638. doi:10.1016/j.chest.2018.09.004
22. Radigan EA, Gilchrist NA, Miller MA. Management of aminoglycosides in the intensive care unit. J Intensive Care Med. 2010;25(6):327–342. doi:10.1177/0885066610377968
23. Xie F, Li S, Cheng Z. Population pharmacokinetics and dosing considerations of daptomycin in critically ill patients undergoing continuous renal replacement therapy. J Antimicrob Chemother. 2020;75(6):1559–1566. doi:10.1093/jac/dkaa028
24. D’Arcy DM, Corrigan OI, Deasy E, Gowing CM, Donnelly MB. Gentamicin pharmacokinetics in critically ill patients during treatment with continuous venovenous haemodiafiltration (CVVHDF). Eur J Clin Pharmacol. 2015;71(3):377–378. doi:10.1007/s00228-014-1765-z
25. Baud FJ, Houze P, Carli P, Lamhaut L. Alteration of the pharmacokinetics of aminoglycosides by adsorption in a filter during continuous renal replacement therapy. An in vitro assessment. Therapie. 2020;76(5):415–424.
26. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–151. doi:10.1208/s12248-011-9255-z
27. Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3(1):160035. doi:10.1038/sdata.2016.35
28. Boisson M, Mimoz O, Hadzic M, et al. Pharmacokinetics of intravenous and nebulized gentamicin in critically ill patients. J Antimicrob Chemother. 2018;73(10):2830–2837. doi:10.1093/jac/dky239
29. Rea RS, Capitano B, Bies R, Bigos KL, Smith R, Lee H. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30(6):674–681. doi:10.1097/FTD.0b013e31818b6b2f
30. Bukkems LH, Roger C, Hodiamont CJ, et al. Predictive performance of a gentamicin population pharmacokinetic model in two western populations of critically ill patients. Int J Antimicrob Agents. 2018;52(2):218–225. doi:10.1016/j.ijantimicag.2018.04.016
31. D’Arcy DM, Casey E, Gowing CM, Donnelly MB, Corrigan OI. An open prospective study of amikacin pharmacokinetics in critically ill patients during treatment with continuous venovenous haemodiafiltration. BMC Pharmacol Toxicol. 2012;13:14. doi:10.1186/2050-6511-13-14
32. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi:10.1016/j.addr.2014.07.006
33. Goncalves-Pereira J, Martins A, Povoa P. Pharmacokinetics of gentamicin in critically ill patients: pilot study evaluating the first dose. Clin Microbiol Infect. 2010;16(8):1258–1263. doi:10.1111/j.1469-0691.2009.03074.x
34. Hodiamont CJ, Janssen JM, de Jong MD, Mathot RA, Juffermans NP, van Hest RM. Therapeutic drug monitoring of gentamicin peak concentrations in critically ill patients. Ther Drug Monit. 2017;39(5):522–530. doi:10.1097/FTD.0000000000000432
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.