Back to Journals » Journal of Inflammation Research » Volume 16
Platelet-to-Lymphocyte Ratio Improves the Predictive Ability of the Risk Score for Atrial Fibrillation Recurrence After Radiofrequency Ablation
Authors Huang W , Sun H, Tang Y, Luo Y, Liu H
Received 12 October 2023
Accepted for publication 3 December 2023
Published 11 December 2023 Volume 2023:16 Pages 6023—6038
DOI https://doi.org/10.2147/JIR.S440722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Wenchao Huang,* Huaxin Sun,* Yan Tang,* Yan Luo, Hanxiong Liu
Department of Cardiology, The Third People’s Hospital of Chengdu, College of Medicine, Southwest Jiaotong University, Chengdu, Sichuan, 610031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hanxiong Liu, Affiliated Hospital of Southwest Jiaotong University, The Third People’s Hospital of Chengdu, Chengdu Cardiovascular Disease Research Institute, 82 Qinglong St. Chengdu, Sichuan, People’s Republic of China, Tel +86 18897966045, Email [email protected]
Purpose: To investigate the effect and comprehensive predictive value of the platelet-to-lymphocyte ratio (PLR) for long-term recurrence in patients with atrial fibrillation (AF) post ablation.
Patients and Methods: We retrospectively analysed 638 consecutive AF patients who underwent ablation, including 302 (47.3%) with paroxysmal AF and 336 (52.7%) with nonparoxysmal AF. Patients were grouped into the recurrence and nonrecurrence groups.
Results: After a mean follow-up of 15.1± 9.3 months, 175 patients (27.4%) with AF had long-term recurrence, including 114 patients (33.9%) with nonparoxysmal AF and 61 patients (20.2%) with paroxysmal AF. In the entire cohort and in patients with nonparoxysmal AF, but not in those with paroxysmal AF, the PLR was significantly higher in the recurrence group than in the nonrecurrence group (P< 0.05). After adjusting for the APPLE score, the PLR as a continuous variable independently predicted AF recurrence (hazard ratio [HR], 1.003; 95% confidence interval [CI], 1.001– 1.005; P< 0.01). The addition of the PLR to the APPLE score improved its predictive ability for recurrence (the C-statistic value increased from 0.645 to 0.675, P=0.02; the net reclassification improvement was 0.221, 95% CI 0.049– 0.394, P=0.01; and the integrated discrimination improvement was 0.029, 95% CI 0.013– 0.045, P< 0.01). For nonparoxysmal AF, the PLR was stratified into tertiles, the PLR independently increased the nonparoxysmal AF recurrence risk after adjusting for multiple confounding factors (HR, 1.393; 95% CI, 1.102– 1.762; P< 0.01), and the addition of the PLR to the left atrial diameter improved its predictive ability for arrhythmia recurrence (the C-statistic value increased from 0.601 to 0.667, P< 0.01).
Conclusion: The PLR is an independent predictive factor of long-term AF recurrence post ablation after adjusting for the APPLE score and can improve the predictive ability and clinical usefulness of the APPLE score. However, the PLR is an effective predictor of recurrence in patients with nonparoxysmal AF rather than in paroxysmal AF.
Plain Language Summary: AF is a common cardiac arrhythmia that can be treated by catheter ablation. Even though the application of ablation has increased in clinical practice, the AF recurrence rate remains stubbornly high. Predicting the risk of recurrence would allow the optimization of AF treatment and management strategies. The impact of inflammation on atrial fibrillation recurrence is unperceived in the existing AF risk scores. Therefore, we investigated the predictive ability of the platelet-to-lymphocyte ratio, a marker of systemic inflammation that can be easily calculated following routine blood analysis, in estimating the risk of AF recurrence. We found that in the entire cohort and in patients with nonparoxysmal AF, but not in those with paroxysmal AF, the PLR ratio was significantly higher in the recurrence group than in the nonrecurrence group. Adding the platelet-to-lymphocyte ratio to the APPLE risk score improved its ability to predict AF in the entire cohort. In patients with nonparoxysmal AF, the addition of the RLR to the left atrial diameter improved its predictive accuracy. However, the PLR ratio did not predict recurrence of paroxysmal AF. Interestingly, we found that recurrence risk scores, such as the APPLE score and CHA2DS2-VASc, had no predictive value for nonparoxysmal AF. Therefore, the addition of the PLR to the APPLE score should be considered in evaluating AF recurrence risk for patients with an unknown type, and the addition of the PLR to the left atrial diameter should be performed to predict AF recurrence in patients with nonparoxysmal AF.
Keywords: platelet-to-iymphocyte ratio, atrial fibrillation, recurrence, prediction model, inflammation, ablation
Introduction
Atrial fibrillation (AF) is a heart arrhythmia that is common worldwide and causes stroke, heart failure, dementia, and multiple complications; it is associated with mortality and disability.1 Compared with heart rate control, the incidence of new ischaemic cerebrovascular disease or transient ischaemic attack was lower in patients with heart rhythm control.2 As a means of heart rhythm control, catheter ablation is recommended as the first-line therapy to restore sinus rhythm, and millions of patients with AF have benefited from this treatment.3 However, AF recurrence rates post ablation remain stubbornly high, especially in patients with persistent AF.4 AF recurrence can be treated by a second, potentially modified, ablation procedure, increasing medical costs and the health care burden.5 Therefore, clinically applicable models are needed to predict AF recurrence before or after catheter ablation and allow the optimization of therapeutic strategies.6 Numerous such risk scores have been established in clinical settings, including APPLE, CHA2DS2-VASc, DR-FLASH, and HATCH.7 Of these, the APPLE score is a noninvasive and easily available clinical predictor of AF recurrence and even atrial remodelling.8,9 However, this score is limited by its focus on clinical and procedural parameters; it does not consider the contribution of pathophysiology and atrial substrates. It has been shown that systemic or local inflammation plays a vital role in accelerating atrial electrical and structural remodelling and promotes AF recurrence.10 Moreover, some studies have suggested that the proinflammatory microenvironments of the atria are partly caused by tissue damage and cell death induced by catheter ablation.11 This evidence indicates that inflammation should be considered when evaluating the risk of AF recurrence. Recently, the platelet-to-lymphocyte ratio (PLR) has been established as a substitute marker of systemic inflammation in cardiovascular disease. An elevated PLR has been associated with poor clinical outcomes, especially in acute coronary syndromes;12 however, the predictive ability of the PLR in AF recurrence remains unknown. Here, we aimed to investigate the impact and predictive value of the PLR in AF recurrence and determine whether the addition of the PLR can improve predictive models for the recurrence of specific AF subtypes.
Materials and Methods
Study Cohorts
We conducted a single-centre, retrospective, observational cohort study in consecutive AF populations selected for catheter ablation from Chengdu Third People’s Hospital (Sichuan, China) between February 2017 and August 2022. All study protocols were approved by the ethics committee of The Third People’s Hospital of Chengdu (No. 2023-S-38) and were conducted according to the Declaration of Helsinki and institutional guidelines. The requirement for informed consent was cancelled due to the retrospective nature of the study. We declared that the privacy of the participants was covered and that all data derived from the medical record system were anonymized to maintain the confidentiality of the patient information.
The exclusion criteria included the following information: (1) presence of absolute contraindication for catheter ablation; (2) prior left atrial ablation or surgical procedure; (3) a variety of secondary atrial fibrillation, such as hyperthyroidism, acute alcoholism, electrolyte imbalance; (4) severe structural cardiac diseases and cardiac valvular disease; and (5) incomplete follow-up data. Individuals with incomplete key variables, including the APPLE score and the PLR, were excluded. After excluding 109 patients with failed follow-up and incomplete clinical data, 638 consecutive patients were finally included.
Data Collection and Definitions
Data on demographic parameters, previous medical history, medical diagnosis, laboratory detections, and medications were obtained from the electronic medical management system. The demographics and anthropometric data included age, sex, body mass index (BMI), smoking, AF duration, systolic blood pressure (SBP), heart rate (HR), previous history of hypertension, diabetes mellitus, coronary heart disease (CHD), chronic heart failure (CHF), stroke, peripheral artery disease (PAD) and chronic obstructive pulmonary disease (COPD). The clinical data included the diagnosis of AF type, blood parameters on admission including fasting blood glucose (FBG), homocysteine, brain natriuretic peptide (BNP), estimated glomerular filtration rate, albumin (Alb), blood uric acid (BUA), triglycerides, total cholesterol, platelets and lymphocytes, imaging data on admission (left atrial diameter, LAD, left ventricular end diastolic diameter, LVED and left ventricular ejection fraction, LVEF), perioperative complications and medical treatment after discharge. Perioperative complications included respiratory tract infection, pleural effusion, haematoma, lower limb infection, pericardial tamponade, and loss of consciousness within 7 days after surgery. All blood parameters of AF patients were measured during hospitalization as part of their treatment.
Paroxysmal AF was determined by whether episodes lasted <7 days and self-terminated. Nonparoxysmal AF was defined as any AF episode either lasting longer than 7 days or requiring any current cardioversions for termination. The ratio of platelets to lymphocytes was calculated as the PLR.
Recurrence Risk Scoring System
APPLE and CHA2DS2-VASc scores were used to predict the outcomes of AF patients post ablation. The APPLE score was calculated through 5 variables (1 point for each), including age >65 years, persistent AF, eGFR <60 mL/min/1.73 m2, LAD ≥43 mm, and LVEF <50%. The CHA2DS2-VASc score was comprised of heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74, and female sex.
Preoperative Preparation and Ablation Strategy
All patients were treated with anticoagulants and underwent transesophageal echocardiography before surgery to exclude left atrial thrombosis. Anatomical models of the left atrial and pulmonary veins were established under the guidance of a three-dimensional imaging system (CARTO-3 System, Version 6; Biosense Webster Inc., Irvine, CA, USA). Radiofrequency ablation was performed in all patients under general anaesthesia, with continuous intraoperative monitoring of their vital signs. Patients with paroxysmal AF underwent annular ablation along the left and right pulmonary veins to achieve pulmonary vein isolation (PVI). Patients with nonparoxysmal AF underwent further substrate modifications based on PVI. The low-voltage area of the left atrium was located under the guidance of the CARTO-3 navigation system, and the ablation circuit was designed accordingly. All patients underwent ablation in the AF state; the procedure was terminated upon cessation of AF. If atrial flutter or tachycardia occurred during ablation, mapping and ablation were continued. If sinus rhythm could not be restored after ablation, electrical cardioversion was performed. The ablation-related parameters were as follows: power, 50 W (left atrial and pulmonary vein isolation) and 35–40 W (ridge and right atrial); perfusion rate of cold saline, 15 mL/min.
Postoperative Management and Follow-Up
All patients were treated with oral antiarrhythmic drugs for at least 3 months after ablation. Amiodarone was the preferred antiarrhythmic drug, but if oral amiodarone was contraindicated, it was changed to dronedarone. In the absence of contraindications, anticoagulation (warfarin or rivaroxaban) was continued. All patients were followed up regularly as outpatients at 3-, 6-, and 12-months post ablation using 24-hour Holter monitoring and were asked about symptoms associated with arrhythmias. In addition, when patients’ symptoms were consistent with tachycardia during the follow-up period, a surface 12-lead electrocardiogram and 24-hour Holter monitoring were performed at the nearest hospital. Long-term recurrence was defined as all 30-second atrial tachyarrhythmias (including AF, atrial flutter, atrial tachycardia) continuously as recorded after the 3-month blank period.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. The Kolmogorov–Smirnov test was used to determine data normality. An unpaired t-test or one-way analysis of variance was used to compare continuous variables with a normal distribution, and a nonparametric test was used to compare those with a skewed distribution. Categorical variables were expressed as counts or proportions (%) and were compared using the chi-squared or Fisher’s exact test, as appropriate.
The Cox proportional hazards regression model was used to analyse the association between the PLR, risk scoring system, and recurrence. All variables known to affect recurrence, including age, sex, BMI, AF duration, BNP, eGFR, LAD, LVEF, antiarrhythmic drugs, history of hypertension, diabetes mellitus, CHD, CHF, stroke, and PAD, CHA2DS2‑VASc score (including AF types), APPLE score (without AF type), and PLR, were included in the univariate Cox regression analysis. Variables with an unadjusted P value of <0.1 were included in the multivariate model. When the CHA2DS2-VASc and APPLE scores were included in the multivariate regression analysis, the variables comprising these scores were no longer entered separately. The results are presented as hazard ratios (HRs) with associated 95% confidence intervals (CIs). A receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to determine the ability of the PLR and risk score system to predict AF recurrence in patients after ablation. The dose‒response relationship between baseline PLR and AF recurrence was evaluated by restricted cubic spline (RCS). The DeLong test was used to compare whether the AUCs of the different models were significantly different. The incremental predictive value of PLR on the recurrence risk score was further analysed by net reclassification improvement (NRI) and integrated discrimination improvement (IDI). Calibration curves were established to evaluate the performance of the model, and decision curve analysis was used to evaluate clinical usefulness and net benefit.
The closer the Brier score is to zero, the greater the degree of correction. As a calibration index, the closer the Brier score is to zero, the higher the calibration value is.
All tests were two-sided, and a P value <0.05 was considered to indicate statistical significance. Analyses were performed using R software version 4.3.1 (R Project for Statistical Computing, Vienna, Austria), SPSS software version 26.0 (IBM Corporation, San Diego, CA, USA) and GraphPad Prism v9.5.0 (GraphPad Software, San Diego, California, USA).
Results
Clinical Characteristics of Patients with AF
A total of 638 patients with AF were enrolled in this study, including 302 with paroxysmal AF and 336 with nonparoxysmal AF. After 15.1±9.3 months of follow-up, 175 patients (27.4%) with AF had long-term recurrence; 114 (33.9%) of these had nonparoxysmal AF, and 61 (20.2%) had paroxysmal AF. The baseline characteristics between the AF recurrence and nonrecurrence groups are shown in Table 1. Patients with AF recurrence were more likely to be female and to have nonparoxysmal AF for a longer duration, a higher BNP level, lower eGFR, lower lymphocyte count, larger LAD, higher CHA2DS2‑VASc score, higher APPLE score, and higher PLR. They were also more likely to have stroke and PAD and to not take antiarrhythmic drugs after ablation.
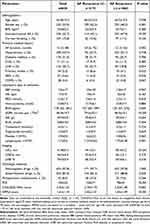 |
Table 1 Baseline Characteristics of the Study Population |
Compared to patients without recurrence, patients with nonparoxysmal AF recurrence had a larger LAD and lower preoperative eGFR, which is consistent with paroxysmal AF (Table 2). In addition, the recurrence group of patients with nonparoxysmal AF had a longer AF duration, lower lymphocyte count, and lower PLR than the nonrecurrence group. For paroxysmal AF, patients with recurrence were more likely to be female, older, and have a higher heart rate, BNP level, CHA2DS2-VASc score, and APPLE score. They were also more likely to have a history of stroke and PAD and to not take antiarrhythmic drugs after ablation.
 |
Table 2 Baseline Characteristics of the Patients Stratified by the Different AF Types |
Predictive Value of the PLR, CHA2DS2-VASc Score, and APPLE Score for AF Recurrence
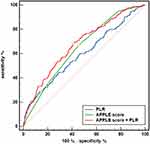
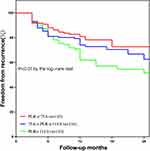
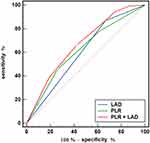
All clinical and echocardiographic variables of AF recurrence were assessed using univariate and multivariate analyses (Table 3). Following multivariate analysis, female sex, longer AF duration, previous stroke, lower eGFR, larger LAD, and APPLE score (HR, 1.334; 95% CI, 1.149–1.550; P<0.001), but not the CHA2DS2-VASc score, remained significant predictors of AF recurrence. The PLR was an independent predictor of AF recurrence when separately included in models 1 (HR, 1.003; 95% CI, 1.001–1.005; P=0.001) and 2 (HR, 1.003; 95% CI, 1.001–1.005; P=0.004). Additionally, the RCS results showed a linear association between baseline PLR levels and the risk of recurrence (Figure 1; nonlinear P = 0.578). Figure 2 shows the ROC curves of the APPLE score and the PLR for predicting AF recurrence, which had AUCs of 0.645 (95% CI, 0.601–0.690; P<0.001) and 0.596 (95% CI, 0.557–0.634; P=0.006), respectively. Patients with nonparoxysmal AF were stratified into three groups according to PLR tertiles (T1, PLR ≤75.6; T2, 75.6< PLR ≤114.9; T3, PLR >114.9) (Table 4). Patients with higher PLR tended to have higher platelet counts and lower lymphocyte counts. In addition, the highest PLR group had higher FBG levels than the other two groups. The Kaplan–Meier curves of the incidence of AF recurrence for the different PLR tertiles are shown in Figure 3. The incidence of long-term AF recurrence increased progressively with a higher PLR (Log rank test, P<0.01). A higher PLR, as a categorical variable, was associated with a high risk of AF recurrence after adjustment for multiple confounders (HR, 1.393; 95% CI, 1.102–1.762; P=0.006) in patients with nonparoxysmal AF (Table 5). The multivariate analysis revealed that female sex, longer AF duration, and larger LAD were significant predictors of AF recurrence. The APPLE and CHA2DS2‑VASc scores were not significant predictors of AF recurrence in patients with nonparoxysmal AF. Figure 4 presents the ROC curves of the ability of the PLR and LAD to predict AF recurrence, with AUCs of 0.621 (95% CI, 0.566–0.673; P<0.001) and 0.601 (95% CI, 0.546–0.654; P<0.001), respectively.
 |
Table 3 Univariate and Multivariate Cox Regression Analysis for Predicting Recurrence in All Patients |
 |
Table 4 The Baseline Characteristics Based on Tertiles of the PLR for Patients with Nonparoxysmal AF |
 |
Table 5 Univariate and Multivariate Cox Regression Analysis for Predicting Recurrence in Patients with Nonparoxysmal AF |
 |
Figure 1 RCS for the odds ratio of risk of recurrence. Abbreviations: RCS, restricted cubic spline, PLR, platelet to lymphocyte ratio. |
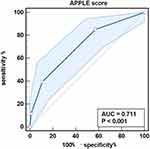
For patients with paroxysmal AF, the multivariate analysis identified female sex, previous stroke, history of PAD, lower eGFR, larger LAD, APPLE score (HR, 1.89; 95% CI, 11.37–2.60; P<0.001) and CHA2DS2‑VASc score (HR, 1.19; 95% CI, 1.00–1.40; P=0.047), but not PLR, as significant predictors of AF recurrence (Table 6). Figure 5 presents the ROC curve of the ability of the APPLE score to predict AF recurrence, with an AUC of 0.711 (95% CI, 0.656–0.762; P<0.001). The AUCs of the CHA2DS2‑VASc score and PLR for predicting AF recurrence were 0.645 (95% CI, 0.588–0.699; P<0.001) and 0.547 (95% CI, 0.489–0.604; P=0.288), respectively (Figures S1 and S2).
 |
Table 6 Univariate and Multivariate Cox Regression Analysis for Predicting Recurrence in Patients with Paroxysmal AF |
Incremental Predictive Value of PLR for AF Recurrence
In the total patient cohort, adding the PLR to the APPLE score to build a new model significantly improved the C-statistic of the APPLE score, from 0.645 (95% CI, 0.601–0.690) to 0.675 (95% CI, 0.629–0.722) (DeLong test, P=0.019). The model performance is presented in Figure 2 (sensitivity, 68.6%; specificity, 59.2%). The addition of the PLR significantly improved the APPLE score, as assessed by the NRI (0.221; 95% CI 0.049–0.394; P=0.012) and the IDI (0.029; 95% CI 0.013–0.045; P<0.001) (Table S1). Calibration plots for recurrence presented good agreement between the predicted and actual observation possibilities for the model after adding PLR (Figure S3). With a Brier score of 0.183, the model was acceptable. In this analysis, decision curve analysis showed a greater net benefit in the risk of AF recurrence with the addition of PLR to the APPLE score than with the APPLE score alone (Figure S4).
For patients with nonparoxysmal AF, as shown in Figure 4, adding PLR into the model containing LAD alone significantly improved the C-statistic from 0.601 (95% CI, 0.546–0.654) to 0.667 (95% CI, 0.614–0.717), with a statistically significant difference (DeLong test, P=0.001).
Discussion
The main findings of our study were as follows: 1) the PLR and the APPLE score were elevated in patients with AF recurrence regardless of the clinical classification; 2) the PLR represented an independent predictor of AF recurrence by multivariate analysis and showed a linear dose‒response association with AF recurrence risk; 3) the PLR was a significant three-grade classifier with increased long-term recurrence risk in patients with nonparoxysmal AF; 4) the PLR in combination with LAD increased the predictive accuracy in patients with nonparoxysmal AF; and 5) the addition of the PLR to the APPLE score improved the risk prediction capacity of AF recurrence in the entire cohort with favourable clinical availability.
Atrial inflammation is recognized as the pathophysiological basis of AF initiation, progression, and maintenance.13 Inflammation is also associated with supraventricular tachycardias, which further confirms the effect of inflammation on cardiac substrates.14 In addition, increasing evidence suggests that surgery-induced inflammation creates a substrate for postoperative AF.15 In addition, the moderate inflammation caused by catheter ablation or a preexisting substrate may increase trigger-related ectopic activities and promote AF recurrence.16 Therefore, it is important to consider the role of inflammation in AF recurrence. The PLR is a biomarker based on lymphocyte and platelet numbers, which reflect the systemic inflammation mediated by the activation of platelets and the immune response.17 In this study, we chose the PLR as a systemic inflammatory marker because of its wide availability and simple calculation. We found a reduction in lymphocyte numbers and an increase in the PLR in all patients with AF recurrence compared to patients without recurrence; the PLR was also increased in patients with nonparoxysmal AF recurrence. Lymphocyte numbers differed between the paroxysmal and nonparoxysmal AF groups without any distinction between platelet and anticoagulant therapy. Our results differ from those of a recent study indicating that the PLR was increased and platelet numbers were reduced in patients with permanent AF compared with those in patients with paroxysmal AF.18 Discrepancies in AF severity may explain the differences between the studies. In addition, we utilized APPLE and CHA2DS2‑VASc scores as positive controls to highlight the characteristic differences between paroxysmal and nonparoxysmal AF and to exclude the effect of patient selection bias on the results.
One key finding of our study was that the PLR is an independent predictor of AF recurrence after adjusting for APPLE or CHA2DS2‑VASc scores and the dose‒response relationship of the association between the PLR and AF recurrence risk. Although the HR of the PLR (1.003; 95% CI, 1.001–1.005) was slightly smaller than its value in the prediction of AF onset (1.257; 95% CI, 1.138–1.376) in the study by Li et al,19 the enrolled subjects in that study were older patients with extensive hypertension. In terms of predictive accuracy, the PLR enhances the AUC in combination with the APPLE score compared with the APPLE score alone for the prediction of AF recurrence. Regarding the continuous variable, although the risk degree of PLR for AF recurrence was slightly inferior to the APPLE or CHA2DS2‑VASc scores, it was still a significant predictor of recurrence in the total AF population. After division of the PLR into tertiles, a good risk stratification capacity was observed; patients with a higher PLR had a higher AF recurrence post ablation, especially when diagnosed with persistent or permanent AF. Similar to our results, Li et al used PLR tertiles to demonstrate significant differences in the PLR in cumulative new-onset AF in a community-based cohort.19 Nevertheless, our study is the first to demonstrate the risk stratification potential of the PLR for AF recurrence using tripartite data. In the clinical setting, our findings and those of other studies20,21 support the use of the PLR as a risk stratification metric to identify patients suitable for catheter ablation.
We also determined the predictive value of the PLR for the recurrence of different AF types. The addition of the PLR to the LAD improves the nonparoxysmal AF predictive accuracy. However, the predictive ability of the PLR for AF outcomes may partly depend on the subject population. One study found that a lower PLR (<88.16) was associated with weakened left atrial strain and slowed left atrial appendage flow velocity in non-valvular AF22 and can therefore be a predictor of atrial thrombogenesis. Another study revealed that an elevated preoperative PLR (118 [91–152]) was not independently associated with postoperative AF.23 These data suggest that it is necessary to consider specific AF populations and clinical conditions when using the PLR as a risk factor for predicting clinical outcomes.
In the subgroup analysis of patients with paroxysmal AF in our study, the PLR was not an independent risk factor for AF recurrence following multivariate analysis. The PLR therefore had a better predictive value in patients with nonparoxysmal AF, whereas APPLE scores were more suitable for the prediction of paroxysmal AF recurrence. Consistent with these results, previous studies have demonstrated that the APPLE score is superior to the CHA2DS2-VASc score in predicting AF recurrence after single and repeat ablation.8,24 Interestingly, the APPLE score also performed better than the CHA2DS2-VASc score in predicting recurrence after repeat ablation.25 Notably, our study identified that sex can enhance the predictive value of the APPLE score in paroxysmal AF. Although the APPLE score can be widely applied in clinical settings, the absence of an inflammatory component limits its application.
Our study has some limitations. First, this was a retrospective analysis with a small sample size from a single centre; external validation should be performed to exclude selection bias. Second, the PLR was calculated using quantitative platelet and lymphocyte numbers at admission; the same type of data should be recorded at discharge to provide time-dependent variables. Third, the mean follow-up period was approximately one year, and the primary endpoint was AF recurrence. A longer follow-up period and additional secondary endpoints are needed to determine the predictive value of the PLR for long-term prognosis.
Conclusion
The preoperative PLR was associated with AF recurrence after radiofrequency ablation in patients with nonparoxysmal AF but not in those with paroxysmal AF. The APPLE and CHA2DS2‑VASc risk scores for AF recurrence after radiofrequency ablation were mainly relevant for patients with paroxysmal AF. Therefore, for the prediction of AF recurrence after radiofrequency ablation, the addition of the PLR to the risk score should be considered for patients with unknown types of AF. LAD combined with PLR should be considered for patients with nonparoxysmal AF, and the risk score alone can be used for paroxysmal AF.
Abbreviations
AF, atrial fibrillation; AUC, area under the curve; BMI, body mass index; BNP, brain natriuretic peptide; CHD, coronary heart disease; CHF, chronic heart failure; CI, confidence interval; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HR, hazard ratio; IDI, integrated discrimination improvement; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NRI, net reclassification improvement; PAD, peripheral artery disease; PLR, platelet-to-lymphocyte ratio; RCS, restricted cubic splines; ROC, receiver operating characteristic.
Data Sharing Statement
The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Ethics Committee of The Third People’s Hospital of Chengdu, with informed consent waived due to its retrospective nature. The privacy of the participants was covered, and all data derived from the medical record system were anonymized to maintain the confidentiality of the patient information.
Funding
This work was supported by the Chengdu High-level Key Clinical Specialty Construction Project [2021YJ0215] and the Sichuan Province Science and Technology Support Program [2020YJ0483].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi:10.1161/CIRCULATIONAHA.113.005119
2. Kılıç R, Güzel T, Aktan A, et al. The effect of treatment strategy on long-term follow-up results in patients with nonvalvular atrial fibrillation in Turkey: AFTER-2 subgroup analysis. Aging Clin Exp Res. 2023;35(8):1695–1704. doi:10.1007/s40520-023-02467-y
3. Schwennesen HT, Andrade JG, Wood KA, Piccini JP. Ablation to reduce atrial fibrillation burden and improve outcomes: JACC review topic of the week. J Am Coll Cardiol. 2023;82(10):1039–1050. doi:10.1016/j.jacc.2023.06.029
4. Pallisgaard JL, Gislason GH, Hansen J, et al. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J. 2018;39(6):442–449. doi:10.1093/eurheartj/ehx466
5. Mansour M, Karst E, Heist EK, et al. The impact of first procedure success rate on the economics of atrial fibrillation ablation. JACC Clin Electrophysiol. 2017;3(2):129–138. doi:10.1016/j.jacep.2016.06.002
6. Bisson A, Fawzy AM, El-Bouri W, et al. Clinical phenotypes and atrial fibrillation recurrences after catheter ablation: an unsupervised cluster analysis. Curr Probl Cardiol. 2023;48(8):101732. doi:10.1016/j.cpcardiol.2023.101732
7. Mulder MJ, Kemme MJB, Hopman LHGA, et al. Comparison of the predictive value of ten risk scores for outcomes of atrial fibrillation patients undergoing radiofrequency pulmonary vein isolation. Int J Cardiol. 2021;344:103–110. doi:10.1016/j.ijcard.2021.09.029
8. Kornej J, Hindricks G, Shoemaker MB, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104(10):871–876. doi:10.1007/s00392-015-0856-x
9. Parameswaran R, Kalman JM, Savelieva I. APPLE score in atrial fibrillation: a simple solution to predict a complex process? Europace. 2019;21(1):1–2. doi:10.1093/europace/euy157
10. Hu D, Barajas-Martinez H, Zhang ZH, et al. Advances in basic and translational research in atrial fibrillation. Philos Trans R Soc Lond B Biol Sci. 2023;378(1879):20220174. doi:10.1098/rstb.2022.0174
11. Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66. doi:10.1148/radiol.2511072175
12. Paolisso P, Foà A, Bergamaschi L, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20(1):33. doi:10.1186/s12933-021-01222-9
13. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. 2020;127(1):51–72. doi:10.1161/CIRCRESAHA.120.316363
14. Aydın M, Yıldız A, Yüksel M, et al. Assessment of the neutrophil/lymphocyte ratio in patients with supraventricular tachycardia. Anatol J Cardiol. 2016;16(1):29–33. doi:10.5152/akd.2015.5927
15. Dobrev D, Aguilar M, Heijman J, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16(7):417–436. doi:10.1038/s41569-019-0166-5
16. Liu D, Li Y, Zhao Q. Effects of inflammatory cell death caused by catheter ablation on atrial fibrillation. J Inflamm Res. 2023;16:3491–3508. doi:10.2147/JIR.S422002
17. Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep. 2017;7(1):40426. doi:10.1038/srep40426
18. Ömür SE, Zorlu Ç, Yılmaz M. Comparison of the relationship between inflammatory markers and atrial fibrillation burden. Anatol J Cardiol. 2023;27(8):486–493. doi:10.14744/AnatolJCardiol.2023.2927
19. Li J, Hu Z, Hou L, et al. Mediating effect of subclinical inflammation on the process of morning hypertension leading to atrial fibrillation in community-based older adults. Clin Exp Hypertens. 2023;45(1):2253381. doi:10.1080/10641963.2023.2253381
20. Tamaki S, Nagai Y, Shutta R, et al. Combination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as a novel predictor of cardiac death in patients with acute decompensated heart failure with preserved left ventricular ejection fraction: a Multicenter Study. J Am Heart Assoc. 2023;12(1):e026326. doi:10.1161/JAHA.122.026326
21. Zhao Z, Jiang B, Zhang F, et al. Association between the systemic immune-inflammation index and outcomes among atrial fibrillation patients with diabetes undergoing radiofrequency catheter ablation. Clin Cardiol. 2023;46(11):1426–1433. doi:10.1002/clc.24116
22. Zuo K, Yang X. Decreased platelet-to-lymphocyte ratio as predictor of thrombogenesis in nonvalvular atrial fibrillation. Herz. 2020;45(7):684–688. doi:10.1007/s00059-018-4770-7
23. Navani RV, Baradi A, Colin Huang KL, et al. Preoperative platelet-to-lymphocyte ratio is not associated with postoperative atrial fibrillation. Ann Thorac Surg. 2020;110(4):1265–1270. doi:10.1016/j.athoracsur.2020.02.008
24. Kornej J, Hindricks G, Kosiuk J, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF ablation registry. Circ Arrhythm Electrophysiol. 2014;7(2):281–287. doi:10.1161/CIRCEP.113.001182
25. Kornej J, Hindricks G, Arya A, et al. The APPLE score - a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One. 2017;12(1):e0169933. doi:10.1371/journal.pone.0169933
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.