Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Plasma miR-150-5p as a Biomarker for Chronic Obstructive Pulmonary Disease
Authors Ding Y , Tang S, Zhou Z , Wei H, Yang W
Received 20 December 2022
Accepted for publication 15 March 2023
Published 23 March 2023 Volume 2023:18 Pages 399—406
DOI https://doi.org/10.2147/COPD.S400985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yichuan Ding,1– 3,* Sihui Tang,1,2,4,* Zihan Zhou,1– 3 Hui Wei,1– 3 Wanchun Yang1– 3
1Department of Respiratory and Critical Care Medicine, the Second People’s Hospital of Hefei, Hefei, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Hefei Hospital Affiliated to Anhui Medical University, Hefei, People’s Republic of China; 3The Fifth Clinical College of Anhui Medical University, Hefei, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, the Second People’s Hospital of Hefei Affiliated to Bengbu Medical University, Bengbu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wanchun Yang, Department of Respiratory and Critical Care Medicine, the Second People’s Hospital of Hefei, Hefei, Anhui, 230011, People’s Republic of China, Tel +8662965684, Fax +8662965684, Email [email protected]
Purpose: To investigate the potential of plasma microRNA-150-5p (miR-150-5p) as a biomarker for chronic obstructive pulmonary disease (COPD) and its relationship with clinical indicators such as pulmonary function.
Patients and Methods: Fifty-nine patients with COPD and twenty-six healthy control individuals were recruited in the Second People’s Hospital of Hefei from September 2021 to September 2022. The plasma expression level of miR-150-5p was measured by quantitative real-time polymerase chain reaction.
Results: The miR-150-5p level in the COPD group was significantly lower than that in the control group, and the relative expression was lower in patients with severe airflow limitation than those with mild limitation. Plasma miR-150-5p levels were positively correlated with pulmonary function indicators and negatively correlated with the white blood cell count and C-reactive protein level. The receiver operating characteristic curve suggested that plasma miR-150-5p had predictive value for COPD (area under curve = 0.819, sensitivity 64.4%, specificity 92.3%).
Conclusion: MiR-150-5p can be useful for the diagnosis and disease assessment of COPD, and has value as a biomarker for COPD.
Keywords: chronic obstructive pulmonary disease, miR-150-5p, biomarker, miRNA
Introduction
Chronic obstructive pulmonary disease (COPD) is a commonly-occurring disease of the respiratory system characterized by persistent and incomplete reversible airflow limitation.1 The incidence of COPD may continue to increase each year in the next decade.2 Therefore, early diagnosis, timely evaluation of the disease, and formulation of individualized treatment plans are crucial to improving the patient condition and clinical efficacy.3 The causes of COPD are complex, the most common of which is smoking. Other factors that may cause or aggravate COPD include environmental exposures and genetic risks.4 COPD, as a progressive inflammatory disease of the respiratory tract, alveoli, and microvessels,5 is related to the airway and systemic inflammatory response caused by inflammatory factors such as tumor necrosis factor-α and interleukin-8,6,7 although its specific mechanism still needs further study.
Biomarkers are a current research hotspot and may play a helpful role in early diagnosis, evaluation, treatment, and prognosis of various diseases. Biomarkers can come from many sources including tissues, serum, plasma, sputum, and bronchoalveolar lavage fluid.8 Among them, plasma has the characteristic of relatively easy clinical collection making it convenient for repeated detection and comparative analyses during the occurrence and development of diseases.
MicroRNAs (miRNAs) are small non-coding RNAs that can degrade target mRNAs, thereby inhibiting translation, and regulate post-transcriptional gene expression.9 Dysregulation of their expression is closely related to the occurrence and development of various diseases.10 Recent research suggests that altering the expression of miRNAs in respiratory disorders can modulate their pathogenesis.11 It has been reported that miRNAs can aggravate lung diseases by regulating the release of proinflammatory factors in smooth muscle cells.12 Among them, miR-150-5p is a small non-coding RNA composed of 22 nucleotides that participates in biological processes such as cell differentiation, proliferation, apoptosis, and autophagy.11,13 Current research on miR-150-5p has mainly focused on its association with tumors,14,15 rheumatic immune diseases,16,17 and other systemic diseases.18,19 Basic studies have confirmed the relationship between miR-150-5p and lung inflammation and injury,14,20 but there are still few studies on its function in the pathogenesis of COPD. The current study detected miR-150-5p in the plasma of COPD patients by quantitative real-time polymerase chain reaction (qRT-PCR) to explore whether it has the potential of being used as a biomarker for COPD.
Materials and Methods
Study Population
We included 59 patients who were admitted to The Second People’s Hospital of Hefei from September 2021 to September 2022 and were diagnosed with COPD. We also recruited 26 healthy controls without pulmonary disorders and non-smoking. The inclusion criteria of COPD patients were: (1) met the guidelines for diagnosis and treatment of COPD; (2) no history of thoracic surgery or injection of immunosuppressants in the past 3 months; and (3) clinical data were complete. Exclusion criteria were: (1) patients with severe heart, liver, and kidney dysfunction; (2) patients with bronchial asthma, bronchiectasis, or other lung diseases; (3) patients with infections in other parts of the body; (4) patients with a history of malignant tumors; and (5) patients with an abnormal mental state who could not cooperate.
Of the 59 patients with COPD selected, 50 were males and 9 were females, aged 75.12 ± 7.85 years. The 26 healthy control individuals that were randomly selected included 20 males and 6 females, aged 71.65 ± 6.35 years. There was no significant difference between the sex ratios and age composition of the COPD group and the healthy controls (P > 0.05). This study complied with the ethical guidelines of the Declaration of Helsinki and was accredited by the Medical Ethics Committee of our hospital; informed consent was provided by all patients and healthy controls.
Data Collection
Patients’ age, sex, smoking history, body mass index (BMI), white blood cell (WBC) count, procalcitonin (PCT) level, C reactive protein (CRP) level, and pulmonary function indexes were collected. Functional data included forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and FEV1 as a percentage of the predicted value (FEV1%pred). Based on the pulmonary function results, COPD patients were divided into a severe group (FEV1%pred < 50%) of 36 cases and a mild group (FEV1%pred ≥ 50%) of 23 cases.
qRT-PCR to Detect the Expression of Plasma miR-150-5p
Five milliliters of fasting peripheral blood was collected in an EDTA-K2 purple anticoagulant tube in the morning from healthy controls and before each COPD patient was admitted to the hospital for treatment, then centrifuged at 4 °C at 3000 r/min for 15 min. After centrifugation, the upper plasma layer was transferred to a sterilized Eppendorf tube and stored at −80 °C. The qRT-PCR protocol was as follows: RNA extraction using an miRcute Plasma miRNA Isolation Kit (DP503, Tiangen Biotech, Beijing, China), reverse transcription using a riboSCRIPT Reverse Transcription Kit (C11027, RiboBio, Guangzhou, China), and finally qRT-PCR using an miRNA qPCR Starter Kit (C10211, RiboBio) with amplification in a LC480 fluorescent quantitative PCR instrument (Roche, Basel, Switzerland). The reaction conditions were 95 °C for 10 min, followed by 45 cycles of 95 °C for 10s, 60 °C for 25s, and 70 °C for 25s. The primers (RiboBio) were designed and synthesized using the stem-loop method. The miR-150-5p sequence is 5′-UCUCCCAACCCUUGUACCAGUG-3′. Cel-miR-39-3p was used as the internal reference gene. qRT-PCR analyses were repeated 3 times and averaged. Relative results were calculated using the 2−ΔΔCt method.
Statistical Analyses
Data were analyzed using SPSS 25.0 and GraphPad Prism 9.0 software. Normally distributed data are expressed as means ± standard error of the mean. Student’s t-test was used for comparisons between two groups. Non-normally distributed data are expressed as interquartile ranges, and the Mann–Whitney U-test was used for comparisons between two groups. Count statistics are expressed as n (%), and the chi-square test was used for comparisons. Pearson’s or Spearman correlation analysis was used to assess correlations between the miR-150-5p expression level and lung function indexes in COPD patients. The receiver operating characteristic (ROC) curve was used to assess the predictive value of plasma miR-150-5p in the diagnosis of COPD. P < 0.05 indicated a statistically significant difference.
Results
Comparisons of Clinical Data
COPD patients were divided into low- and high-expression groups with the median miR-150-5p expression level of 0.16 as the boundary value. Clinical data of the two groups are compared in Table 1. The results showed that there was no significant difference in sex, BMI, FVC, and smoking history between the two groups (P > 0.05). There was, however, a significant difference in age, WBC count, CRP level, PCT, FEV1/FVC, and FEV1%pred (P < 0.05).
 |
Table 1 Basic Clinical Information of the High- and Low-miR-150-5p Expression COPD Patients |
Expression of miR-150-5p
Comparing the expression of plasma miR-150-5p between the COPD and control groups showed that it was significantly lower in the COPD group than in the healthy control group (P < 0.05). We also compared the expression of miR-150-5p between the mild and severe COPD groups and found that the expression level in COPD patients with severe airflow limitation was significantly lower (P < 0.05; Figure 1).
Correlation Analyses of miR-150-5p with Pulmonary Function Indexes
The relationship between plasma miR-150-5p expression and pulmonary function in COPD patients is shown in Figure 2. Correlation analyses showed that the plasma miR-150-5p expression level in COPD patients was positively correlated with FEV1/FVC, FEV1, and FEV1%pred. Because there were significant differences in the WBC count, CRP level, and age between the low- and high-expression groups, we also analyzed the correlation between plasma miR-150-5p expression and WBCs, CRP level, and age. The results are shown in Figure 2. Correlation analyses showed that the plasma miR-150-5p expression level in COPD patients was negative correlated with WBC count, CRP level, and age.
The Plasma miR-150-5p Level is a Diagnostic Biomarker for COPD
The predictive value of plasma miR-150-5p for COPD is shown in Figure 3. The ROC analysis showed that plasma miR-150-5p levels were valuable in the clinical diagnosis of COPD (area under the curve = 0.819, sensitivity 64.4%, specificity 92.3%).
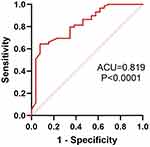 |
Figure 3 Receiver operating characteristic curves for plasma miR-150-5p. AUC, area under the curve. |
Discussion
COPD is expected to become the third most common cause of death globally by 2030.2 At present, the therapy and prognosis of COPD have become more challenging in the situation of COVID-2019, and its incidence will continue to rise in the future.21 COPD is a complicated and heterogeneous disease at the genetic, cellular, and molecular levels.5 At the same time, as a progressive disease, COPD may only have clinical significance at specific stages.22 Therefore, the use of biomarkers that may alter the pathophysiological pathway of the disease has the potential to improve the accuracy of clinical diagnosis and the effectiveness of treatment.23
MiRNAs can be detected in various body fluids of COPD patients and are very stable, thus having value as potential disease diagnostic markers.24 Recently, the function of miRNAs in the pathogenesis of COPD was confirmed, and an increasing number of miRNAs, such as miR-146a, miR-199a-5p, miR-106b, and miR-638, have proven their value in the diagnosis and treatment of COPD,25–29 providing new ideas for the early diagnosis, screening, and prognosis of this disease.
Studies have found that the miR-150-5p level is heightened in blood samples.20 Thus, we speculated that miR-150-5p had the potential to be a biomarker. For example, researchers have reported that the expression of miR-150-5p is associated with the severity and outcome of advanced heart failure, and is a novel circulatory biomarker.19 MiR-150-5p levels vary greatly in patients with myasthenia gravis and are significantly decreased after thymectomy, suggesting that its expression may be associated with disease remission.30 In studies of acute ischemic stroke, miR-150-5p was highly associated with patient mortality within 90 days, outperforming traditional classifications for risk factors.31 Plasma miR-150-5p is related with disease activity in ankylosing spondylitis and is an important non-invasive biomarker candidate.32 Prospective studies have also found that the pathogenesis of chronic lymphocytic leukemia is associated with the dysregulation of miR-150-5p.33 In addition, the expression of miR-150-5p in the blood of COPD patients predicts the development of cancer, and miR-150-5p was found to be dysregulated in patients with a variety of cancers, including non-small cell lung cancer.34,35 In basic research, the possible mechanism of miR-150-5p involved in the occurrence and development of diseases has also attracted the attention of many scholars. For example, the level of miR-150-5p is negatively correlated with metastasis-associated lung adenocarcinoma transcript 1 (MALAT1). Overexpression of MALAT1 inhibits apoptosis and degradation of the extracellular matrix by down-regulating miR-150-5p.36 In aortic endothelial cells, MALAT1 can competitively bind to miR-150-5p and upregulate endothelin-1 expression, resulting in an imbalance of oxidative stress and increased release of proinflammatory cytokines.37 Chen’s study identified vascular endothelial growth factor A as a target gene of miR-150-5p, which is associated with decreased cell proliferation, cell invasion, and angiogenesis through multiple sets of evidence.15 It is worth noting that the above pathological processes are also inseparable from the pathogenesis of COPD, and miR-150-5p must affect COPD through some pathway.
Currently, there are few studies on miR-150-5p and diseases of the respiratory tract. In basic studies, it was found that upregulation of miR-150-5p can reduce the overexpression of MALAT1, thereby inhibiting the apoptosis and the inflammatory response of human pulmonary microvascular endothelial cells, and alleviating the lung injury that occurs in acute respiratory distress syndrome.14 A study found that down-regulation of miR-150-5p promotes the expression of inflammatory cytokines by targeting SLC38A1.38 In extracellular vesicles secreted by hypoxic lung cancer cells, miR-150-5p has immunomodulatory effects that reprogram natural killer cells into dysfunctional pro-inflammatory and pro-angiogenic phenotypes.39 Inhibition of oxidative stress by increasing the expression of protective miRNAs, such as miR-150-5p, can prevent the occurrence and development of COPD.20 A clinical study showed that COPD patients with high miR-150-5p expression survived longer than those with low expression. Thus, overexpression of miR-150-5p may reduce tissue damage by weakening the body’s immune response.40 Among them, the relationship between MALAT1 and miR-150-5p deserves further study. In vascular endothelial cells, the expression of MALAT1 triggers the expression of pro-inflammatory factors and the increase of endothelial cell permeability.41 MiR-150 is the target of MALAT1 in airway smooth muscle cells.14 And bioinformatics analysis has predicted the binding of MALAT1 and miR-150-5p.42 MALAT1 is abnormally expressed in a variety of diseases including respiratory diseases, possibly aggravating the development of COPD by regulating inflammatory responses, inhibiting apoptosis, and leading to lung injury through various mechanisms.
In the current study, the plasma miR-150-5p level, as a novel biomarker, was significantly higher in healthy people compared with COPD patients. The ROC curve suggested the value of miR-150-5p in the clinical diagnosis of COPD patients. Plasma miR-150-5p was not only significantly different between COPD patients and healthy controls, but also relatively lower in patients with low FEV1%pred, indicating that miR-150-5p is closely related to the severity of clinical airflow limitation in COPD patients and has the potential to be used as a marker of disease severity. In addition, the WBC number was negatively correlated with miR-150-5p expression in the correlation analysis suggesting that miR-150-5p may be related to negative feedback regulation of inflammation.
The development of chronic inflammation and emphysema is one of the hallmarks of COPD, and various immune cells play a crucial role in the pathogenesis of this disease.43 Previous studies have confirmed that the dysregulation of miR-150-5p has a regulatory relationship with the expression of inflammatory factors.38,39 Therefore, down-regulation of miR-150-5p may lead to further exacerbation of the disease by aggravating the inflammatory response and lung injury. Previous genome-wide association studies found that the binding checkpoint of miR-150 was significantly enriched in genes related to lung function decline,44 which is consistent with our results. Specifically, we found that the plasma miR-150-5p expression level of COPD patients was positively correlated with FEV1, FEV1/FVC, and FEVI%pred, the latter of which is generally used to formulate the grouping of COPD patients clinically. Whether it can become a marker of COPD disease severity is worth further exploration in the future.
Our study has two major limitations. First, this was a single-center study. Thus, to verify the value of miR-150-5p as a biomarker, further validation in a larger COPD cohort will be required. Second, among hospitalized patients with COPD, relatively few have mild or moderate pulmonary function scores, especially those with mild disease exhibiting inconspicuous early clinical symptoms. Identifying COPD at an early stage may better prevent progression of the disease and morbidity. However, confirming the possibility that miR-150-5p can be used for this purpose requires recruiting more patients in outpatient clinics and the community to verify its value in early screening and to judge disease severity.
Conclusion
This study confirmed that the plasma miR-150-5p level has value in the diagnosis and disease assessment of COPD patients. Its expression may be associated with clinical indicators such as the WBC number, CRP level, and age, and is positively correlated with pulmonary function indicators. In conclusion, plasma miR-150-5p has the potential of becoming a beneficial biomarker of COPD.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the medical ethics committee of the Second People’s Hospital of Hefei (Approval 2021-research-052) and conformed to the ethical guidelines of the Declaration of Helsinki.
Funding
This work was supported by the application of medical research projects in Hefei (no. Hwk2021yb010), the Basic and Clinical Cooperative Research Promotion Program of Anhui Medical University (no. 2021xkjT040), the Ph.D. Special Funding Project in the Second People’s Hospital of Hefei (no. 2020bszx05), the Youth Research Fund of The Second People’s Hospital of Hefei (no. 2021ynq02), and the Natural Science Project of Bengbu Medical College (no. 2020byzd286).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agustí AG, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. Plos Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
3. Hu H, Nie Z, Lu Y, et al. Circulating miR-125b but not miR-125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine. 2017;96(51):e9059. doi:10.1097/MD.0000000000009059
4. Lareau SC, Fahy B, Meek P, Wang A. Chronic Obstructive Pulmonary Disease (COPD). Am J Resp Crit Care. 2019;199(1):P1–P2. doi:10.1164/rccm.1991P1
5. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
6. Lin TL, Chen WW, Ding ZR, Wei SC, Huang ML, Li CH. Correlations between serum amyloid A, C‐reactive protein and clinical indices of patients with acutely exacerbated chronic obstructive pulmonary disease. J Clin Lab Anal. 2019;33(4):e22831. doi:10.1002/jcla.22831
7. Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–1022. doi:10.1183/09031936.99.14510159
8. Farrell O. Plasma Extracellular Vesicle miRNAs Can Identify Lung Cancer, Current Smoking Status, and Stable COPD. Int J Mol Sci. 2021;22(11):5803. doi:10.3390/ijms22115803
9. Zhang J, Li S, Li L, et al. Exosome and Exosomal MicroRNA: trafficking, Sorting, and Function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
10. Ezzie ME, Crawford M, Cho J, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67(2):122–131. doi:10.1136/thoraxjnl-2011-200089
11. Wang J, Chen J, Sen S. MicroRNA as Biomarkers and Diagnostics. J Cell Physiol. 2016;231(1):25–30. doi:10.1002/jcp.25056
12. O’Leary L, Sevinc K, Papazoglou IM, et al. Airway smooth muscle inflammation is regulated by microRNA-145 in COPD. FEBS Lett. 2016;590(9):1324–1334. doi:10.1002/1873-3468.12168
13. Bartel DP. MicroRNAs: genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
14. Yao MY, Zhang WH, Ma WT, Liu QH, Xing LH, Zhao GF. Long non-coding RNA MALAT1 exacerbates acute respiratory distress syndrome by upregulating ICAM-1 expression via microRNA-150-5p downregulation. Aging. 2020;12(8):6570–6585. doi:10.18632/aging.102953
15. Chen X, Zeng K, Xu M, et al. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 2018;9(10). doi:10.1038/s41419-018-0962-6
16. Kakan SS, Edman MC, Yao A, et al. Tear miRNAs Identified in a Murine Model of Sjögren’s Syndrome as Potential Diagnostic Biomarkers and Indicators of Disease Mechanism. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.833254
17. Chen J, Papp G, Póliska S, et al. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PLoS One. 2017;12(3):e0174585. doi:10.1371/journal.pone.0174585
18. Hosokawa K, Kajigaya S, Feng X, et al. A plasma microRNA signature as a biomarker for acquired aplastic anemia. Haematologica. 2017;102(1):69–78. doi:10.3324/haematol.2016.151076
19. Scrutinio D, Conserva F, Passantino A, Iacoviello M, Lagioia R, Gesualdo L. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: a genome-wide prospective study. J Heart Lung Transplant. 2017;36(6):616–624. doi:10.1016/j.healun.2017.02.008
20. Zhu M, Ye L, Zhu G, et al. ROS-Responsive miR-150-5p Downregulation Contributes to Cigarette Smoke-Induced COPD via Targeting IRE1α. Oxid Med Cell Longev. 2022;2022:1–23. doi:10.1155/2022/5695005
21. Halpin DMG, Criner GJ, Papi A, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
22. Savarimuthu FS, Davidson MR, Tan ME, et al. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. Bmc Genomics. 2014;15:88. doi:10.1186/1471-2164-15-88
23. Celli BR, Locantore N, Yates J, et al. Inflammatory Biomarkers Improve Clinical Prediction of Mortality in Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care. 2012;185(10):1065–1072. doi:10.1164/rccm.201110-1792OC
24. Pattarayan D, Thimmulappa RK, Ravikumar V, Rajasekaran S. Diagnostic Potential of Extracellular MicroRNA in Respiratory Diseases. Clin Rev Allerg Immu. 2018;54(3):480–492. doi:10.1007/s12016-016-8589-9
25. Soeda S, Ohyashiki JH, Ohtsuki K, Umezu T, Setoguchi Y, Ohyashiki K. Clinical relevance of plasma miR-106b levels in patients with chronic obstructive pulmonary disease. Int J Mol Med. 2013;31(3):533–539. doi:10.3892/ijmm.2013.1251
26. Mizuno S, Bogaard HJ, Gomez-Arroyo J, et al. MicroRNA-199a-5p Is Associated With Hypoxia-Inducible Factor-1α Expression in Lungs From Patients With COPD. Chest. 2012;142(3):663–672. doi:10.1378/chest.11-2746
27. Hassan T, Carroll TP, Buckley PG, et al. miR-199a-5p Silencing Regulates the Unfolded Protein Response in Chronic Obstructive Pulmonary Disease and α1-Antitrypsin Deficiency. Am J Resp Crit Care. 2014;189(3):263–273. doi:10.1164/rccm.201306-1151OC
28. Christenson SA, Brandsma CA, Campbell JD, et al. miR-638 regulates gene expression networks associated with emphysematous lung destruction. Genome Med. 2013;5(12):114. doi:10.1186/gm519
29. Sato T, Liu X, Nelson A, et al. Reduced miR-146a Increases Prostaglandin E2 in Chronic Obstructive Pulmonary Disease Fibroblasts. Am J Resp Crit Care. 2010;182(8):1020–1029. doi:10.1164/rccm.201001-0055OC
30. Punga T, Panse R, Andersson M, Truffault F, Berrih Aknin S, Punga AR. Circulating miRNAs in myasthenia gravis: miR‐150‐5p as a new potential biomarker. Ann Clin Transl Neur. 2014;1(1):49–58. doi:10.1002/acn3.24
31. Scherrer N, Fays F, Mueller B, et al. MicroRNA 150-5p Improves Risk Classification for Mortality within 90 Days after Acute Ischemic Stroke. J Stroke. 2017;19(3):323–332. doi:10.5853/jos.2017.00423
32. Perez-Sanchez C, Font-Ugalde P, Ruiz-Limon P, et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Hum Mol Genet. 2018;27(5):875–890. doi:10.1093/hmg/ddy008
33. Casabonne D, Benavente Y, Seifert J, et al. Serum levels of hsa-miR-16-5p, hsa-miR-29a-3p, hsa-miR-150-5p, hsa-miR-155-5p and hsa-miR - 223-3p and subsequent risk of chronic lymphocytic leukemia in the EPIC study. Int J Cancer. 2020;147(5):1315–1324. doi:10.1002/ijc.32894
34. Lu W, Zhang H, Niu Y, et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol Cancer. 2017;16(1). doi:10.1186/s12943-017-0685-9
35. Yan L, Jiao D, Hu H, et al. Identification of lymph node metastasis-related microRNAs in lung adenocarcinoma and analysis of the underlying mechanisms using a bioinformatics approach. Exp Biol Med. 2017;242(7):709–717. doi:10.1177/1535370216677353
36. Zhang Y, Wang F, Chen G, He R, Yang L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019;9:54. doi:10.1186/s13578-019-0302-2
37. Ou M, Zhao H, Ji G, Zhao X, Zhang Q. Long noncoding RNA MALAT1 contributes to pregnancy-induced hypertension development by enhancing oxidative stress and inflammation through the regulation of the miR-150-5p/ET-1 axis. FASEB J. 2020;34(5):6070–6085. doi:10.1096/fj.201902280R
38. Yang Y, Tai W, Lu N, et al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging. 2020;12(10):9085–9102. doi:10.18632/aging.103176
39. Chang W, Tsai M, Hung J, et al. miR-150-5p-Containing Extracellular Vesicles Are a New Immunoregulator That Favor the Progression of Lung Cancer in Hypoxic Microenvironments by Altering the Phenotype of NK Cells. Cancers. 2021;13(24):6252. doi:10.3390/cancers13246252
40. Keller A, Ludwig N, Fehlmann T, et al. Low miR-150-5p and miR-320b Expression Predicts Reduced Survival of COPD Patients. Cells-Basel. 2019;8(10):1162. doi:10.3390/cells8101162
41. Vimalraj S, Subramanian R, Dhanasekaran A. LncRNA MALAT1 Promotes Tumor Angiogenesis by Regulating MicroRNA-150-5p/VEGFA Signaling in Osteosarcoma: in-Vitro and In-Vivo Analyses. Front Oncol. 2021;11:742789. doi:10.3389/fonc.2021.742789
42. Jiang H, Zhu M, Wang H, Liu H. Suppression of lncRNA MALAT1 reduces pro-inflammatory cytokines production by regulating miR-150-5p/ZBTB4 axis through JAK/STAT signal pathway in systemic juvenile idiopathic arthritis. Cytokine. 2021;138:155397. doi:10.1016/j.cyto.2020.155397
43. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
44. Pottelberge GRV, Mestdagh P, Bracke KR, et al. MicroRNA Expression in Induced Sputum of Smokers and Patients with Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care. 2011;183(7):898–906. doi:10.1164/rccm.201002-0304OC
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


