Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Plasma Lipoprotein-Associated Phospholipase A2 Affects Cognitive Impairment in Patients with Cerebral Microbleeds
Authors Liu L, Zhang X, Jiang N, Liu Y, Wang Q, Jiang G, Li X, Zhao L, Zhai Q
Received 25 December 2022
Accepted for publication 8 March 2023
Published 22 March 2023 Volume 2023:19 Pages 635—646
DOI https://doi.org/10.2147/NDT.S401603
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Richard J Porter
Lu Liu,1,* Xiaojiu Zhang,1,2,* Nan Jiang,3,* Yufeng Liu,1 Qing Wang,1 Guanghui Jiang,1 Xuejing Li,4 Liandong Zhao,1 Qijin Zhai1
1Department of Neurology, Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China; 2Department of Neurology, Hongze People’s Hospital, Huai’an, Jiangsu, People’s Republic of China; 3Department of Neurology, Lianshui PEople’s Hospital Affiliated to Kangda College of Nanjing Medical University, Huai’an, Jiangsu, People’s Republic of China; 4Rehabilitation Centre, Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liandong Zhao; Qijin Zhai, Email [email protected]; [email protected]
Purpose: The plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) is an inflammatory biomarker of cerebral microbleeds (CMBs) and may be related to the occurrence, development, and prognosis of cognitive impairment. The present study aimed to investigate the impact of plasma Lp-PLA2 level on the cognitive impairment in patients with CMBs.
Methods: In this study, 213 patients with CMBs confirmed by 3.0 T brain magnetic resonance imaging (MRI) were analyzed. Lp-PLA2 levels were determined by magnetic particle chemiluminescence immunoassay technology, and cognitive function was assessed using the Montreal Cognitive Assessment Scale (MoCA). The cognitive functions of patients with CMBs were divided into three groups according to the MoCA scale, including normal cognition (NC), mild cognitive impairment (MCI), and moderate-severe cognitive impairment (MSCI). Clinical, laboratory and radiological data of the three groups were analysed. The relationship between plasma Lp-PLA2 and MoCA score in patients with CMBs was investigated through rank correlation analysis and multivariate regression analysis, and receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of Lp-PLA2.
Results: CMBs were detected in 213 (30.2%) of 705 patients who underwent 3.0 T MRI. Multiple comparisons showed that plasma Lp-PLA2 in patients with CMBs with normal cognitive scores was significantly lower than that in the other two groups with cognitive impairment (p < 0.05). In the single factor correlation analysis, high level of plasma Lp-PLA2 was negatively correlated with the decrease of MoCA score in patients with CMBs (r =− 0.389, p < 0.01). Multivariate regression analysis showed that high plasma Lp-PLA2 was an independent risk factor for a low MoCA score in patients with CMBs (odds ratio [OR]=1.014; 95% confidence interval [CI], 1.002– 1.026; p=0.025).
Conclusion: A high level of plasma Lp-PLA2 is positively correlated with the generation of cognitive impairment in patients with CMBs and negatively correlated with the degree of impairment. Plasma Lp-PLA2 is an important indicator of cognitive impairment in patients with CMBs and may provide a therapeutic target for preventing CMB-induced cognitive impairment.
Keywords: cerebral microbleeds, lipoprotein-associated phospholipase A2, cognitive impairment, risk factors, cerebral small vessel disease
Introduction
The cerebral microbleed (CMB) is a sub-clinical lesion of brain parenchyma caused by small vascular lesions in the brain. In T2-weighted magnetic resonance imaging (MRI) or susceptibility weighted imaging (SWI), a small (2–10 mm in diameter) signal loss area indicates CMBs. Existing studies argue that aging, hypertension and the apolipoprotein E (ApoE) genotype may increase the risk of subsequent lobar CMBs,1 but the presence of baseline CMBs is the most important risk factor for future CMBs. The hypertensive vasculopathy – deep regions and cerebral amyloid angiopathy (CAA) – lobar regions are typically involved in the pathogenesis of CMBs. As such, it has been suggested that CMBs may cause the cognitive dysfunction and dementia through certain cerebrovascular pathology and neurodegenerative pathology.2,3 Some studies showed that a high number of microbleeds was associated with lower scores on the MiniMental State Examination (MMSE).4 Based on large-scale population studies, the prevalence of CMBs has reached 7–25%,5 which is associated with overall cognitive impairment in all test domains, especially in reducing orientation, dependence on executive function, attention, numeracy, and delayed recall.3,6
Both CMBs and inflammation play a role in promoting neurodegeneration and deterioration, and inflammation is one of the risk factors for CMBs.7 Although the potential inflammatory mechanism is not yet clear, more and more studies support that inflammatory indicators can affect the severity and progress of CMBs.8 Additionally, compared with systemic inflammatory factors, markers of vascular inflammation/endothelial dysfunction are more strongly and consistently associated with CMBs.9 As an important factor causing cognitive impairment, the specific mechanism of CMBs may be related to impairment of the blood–brain barrier caused by intracerebral microbleeds, blood circulation disorders in the brain tissue, and the damage of brain tissue caused by the release of various biological inflammatory mediators by blood decomposition products.9,10 Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2), as a vascular inflammatory factor, is an enzyme that hydrolyzes oxidized phospholipids to produce potential atherosclerotic granules.11 It has high specificity and low biological variability and is directly involved in the occurrence of plaque inflammation.12 It is a known biomarker of vascular and neurological inflammation as well as cerebral and cardiovascular risk and has been used as one of the important targets for the increased burden of cerebrovascular disease. Our current research has found that Lp-PLA2 seems to be closely related to the occurrence and development of CMBs.13 Other studies have already found that there is a relationship between the occurrence of inflammation and cognitive decline,14,15 but whether Lp-PLA2, as an inflammatory factor, drives the occurrence and aggravation of CMBs and cognitive impairment is unclear.
There is a significant correlation between CMBs and dementia, and Lp-PLA2 plays a role in both cerebral small vessel disease (SVD) and cognitive impairment.10 However, the potential causal mechanism between brain microbleeds and cognitive impairment remains unclear. In light of these correlations among the three, we measured cognitive function and the levels of plasma Lp-PLA2 in patients with CMBs to elucidate their relationship. Leveraging 213 patient samples with available brain MRI, we hypothesized that plasma Lp-PLA2 is associated with cognitive impairment of CMBs. This may provide a theoretical basis for the early intervention and treatment of cognitive impairment in patients with CMBs.
Materials and Methods
Patients
We identified patients with CMBs who underwent 3.0 T MRI examination in our center from April 2018 to December 2021. The inclusion criteria were: (a) Voluntarily agree to participate in the project. (b) In line with diagnostic criteria of cerebral microbleed. (c) Able to accept 3.0 T MRI examination and neuropsychological scale assessment. The exclusion criteria were: (a) Patients with infectious diseases, autoimmune diseases, psychiatric disorders, malignant tumors, thyroid disorders, hematological diseases, or heart, kidney, and other organ dysfunctions. (b) History of intracranial hemorrhage or craniocerebral trauma. (c) MRI of the brain cannot be performed because of the presence of metallic materials in the body. (d) Patients who were unable to cooperate with neuropsychological examination for intelligence, consciousness, spirit, and speech communication disorders. (e) Patients with Parkinson’s disease, multiple sclerosis, and other diseases known to cause cognitive impairment.
All patients were admitted to the hospital through outpatient department or emergency department, and underwent MRI after admission. A total of 213 people were included in this study. The recruitment, exclusions, and flow of patients through the study are depicted in the flow chart (Figure 1). Among 213 patients, 148 showed dizziness, 87 showed weakness of limbs (unilateral or bilateral), 41 showed numbness or sensory abnormalities of limbs,39 felt headache, 25 showed blurred vision or hypoacusis, 12 show hypomnesis, and others included unclear speech, choking cough, walking instability, insomnia, etc. This project was approved by Ethics Committee of Affiliated Huai’an Hospital of Xuzhou Medical University (Approval No. HEYLL201827 of Institutional Review Committee). All study procedures were conducted in accordance with the principles of the Declaration of Helsinki and its later amendments. Written informed consent was provided by the participants or their legal guardians.
MRI Data Acquisition and CMBs Evaluation
In this study, 3.0 T MRI was used to examine the participants. The instrument was produced by GE Company from the USA (Signa EXCITE HD). The MRI sequence includes: T1-weighted imaging (T1WI) repetition time (TR)/echo time (TE)/field of view (FOV)=1750 ms/24 ms/24×18 cm; matrix, 320×224; slice thickness, 5 mm), T2-weighted imaging (T2WI) (TR/TE/FOV=4841 ms/102 ms/24×240 cm; matrix, 256 ×256; slice thickness, 5 mm), fluid-attenuated inversion recovery (FLAIR) (TR/TE/FOV=9000 ms/130 ms/24×24 cm; matrix, 192×256; slice thickness, 5 mm), Diffusion weighted imaging (DWI) (TR/TE/FOV=4880 ms/65 ms/24× 24 cm, b = 0/1000; matrix, 130×160; slice thickness, 5 mm), SWI adopting three-dimensional susceptibility weighted angiography sequence (TR/TE/FOV=86 ms/45 ms/24×22 mm; flip angle, 15°; matrix, 384×320; slice thickness, 2 mm; slice gap, 0 mm). The original SWI data obtained with scanning were transmitted to the AW46 workstation to reconstruct the post-processing and the phase map, amplitude map, and vascular map were obtained.
Two senior imaging physicians (CWH with 10 years of neuroradiology experience and YDY with 6 years of neuroradiology experience) used the blind method to analyze the images. When the opinions were inconsistent, the two physicians and the third physician (LD) jointly determined the results by a vote. CMBs were defined as follows: (a) SWI sequence showed round or oval uniform signal loss of 2–10 mm in diameter with no edema or mass around it. (b) Exclusion of bilateral symmetry in the globus pallidus representing calcification of low signal shadow, cerebral small vessels distal branch cross-sectional flow empty shadow, vascular malformation (including cavernous hemangioma and telangiectasia) showed low signal changes. The location, size, and number of CMBs and white matter lesions were also recorded. The following MRI markers were rated: For the CMBs, we used the Microbleed Anatomical Rating Scale (MARS)16 to record information of its presence, distribution and number. Microbleeds were classified into deep, lobar/infratentorial and mixed categories. Severity of CMBs was rated from 0 to 3 based on the number of CMBs: No CMBs = grade 0, 1–2 = grade 1, 3–10 = grade 2, and 10 or more = grade 3. The Fazekas scale was used for periventricular and deep WMH evaluation (1) Periventricular WMH: 0 = absent; 1 = caps or pencil-thin; 2 = smooth halo; 3 = irregular extending to deep WM; Deep WMH. (2) Deep WMH: 0 = absent; 1 = punctate foci; 2=beginning confluence; 3 = large confluent areas. The modified Fazekas scale was used to categorize WMH severity (Grade I: Fazekas 0–1; Grade II: Fazekas 2; Grade III: Fazekas 3).17,18
Lp-PLA2 Collection and Determination
An EDTA anticoagulant tube was used to collect 4 mL fasting venous blood from all subjects in a quiet state in the early morning. The blood was centrifuged at 2500 rpm for 15 minutes and the supernatant samples were collected. Each supernatant sample was placed in a magnetic particle automatic chemiluminescence immunoassay analyzer (MQ60pro) for the determination of the concentration of Lp-PLA2. Reagents were provided by Beijing Rejing Biotechnology Co., Ltd. The experimental procedures and judgment results were carried out in strict accordance with the Lp-PLA2 standard operating procedure.
Cognitive Function Assessment and Criteria
The Montreal Cognitive Assessment (MoCA) of each patient was performed by an experienced neurodiagnostician (ZQJ) in a quiet setting. The MoCA is a neuropsychological test used to assess the change of overall cognitive function. Each MoCA scale was completed within 10 minutes with a total possible score of 30 points. To ensure the validity and reliability of the results, the MoCA scale evaluation of all participants was based on the MoCA inspection operation guide. One point was added to the total MoCA score for participants with ≤12 years of education (if total MoCA score was <30). The participants whose cognition was assessed were divided into three groups: (a) normal cognition (NC), within the normal range of MoCA ≥26 points, (b) mild cognitive impairment (MCI), MoCA of 18–26 points. (c) moderate-severe cognitive impairment (MSCI), MoCA score <8. On the second day after the MRI diagnosis of CMBs, 213 participants completed the MoCA score evaluation.
Acquisition of Remaining Risk Factors
Blood pressure (BP) was measured three times in patients who had been seated for 10 minutes in a quiet environment. The average of the three measurements was recorded. Blood (10 mL) was collected in the morning while fasting. After the blood sample stood at room temperature for 30 minutes, the supernatant was centrifuged at 3500 rpm for 15 minutes. Fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (Cr), homocysteine (HCY), high-sensitivity C-reactive protein (Hs-CRP), and fibrinogen (FIB) were measured with an automatic biochemical analyzer (TBA 40FR, Toshiba, Tokyo, Japan).
Statistical Analysis
All data were processed with SPSS 25.0 statistical software (version 25.0, IBM) and GraphPad prism 8.0.2 (version 8, GraphPad Prism Software Inc.) was used to make the statistical graphs. The normally distributed continuous variables are represented as mean±standard deviation, and the non-normally distributed continuous variables are represented by median and quartile spacing. In addition, the classification variables are expressed in the form of numbers (percentage). For the comparison of baseline data for patients grouped according to three levels of cognitive function, the Kruskal–Wallis H-test was used for non-normally distributed measurement data, and the Bonferroni method was used for multiple comparisons to correct the α test level. The χ2 test was used for comparison of enumeration data. The relationship between plasma Lp-PLA2 level and MoCA score in patients with CMBs was tested with Spearman rank correlation analysis. We assessed the possible predictive factors of cognitive impairment in patients with cerebral microbleeds using univariable logistic regression models. Variables were included in the multivariate analysis based on the results of the univariate analysis. We drew the receiver operating characteristic (ROC) curve and evaluated the diagnostic value and prognosis model of Lp-PLA2 by calculating the area under the ROC curve (AUC) and the cut-off value. P < 0.05 indicated that the difference was statistically significant.
Results
Comparison of Baseline Characteristics of Patients with CMBs with Different Degrees of Cognitive Impairment
According to the Kruskal–Wallis H-test, there were statistically significant differences in age (p < 0.01), FPG level (p < 0.01), creatinine level (p < 0.01), HCY level (p < 0.01), number of CMBs (p < 0.01), and Lp-PLA2 level (p < 0.01) among three groups of patients with CMBs with different cognitive functions (Table 1). According to the χ2 test, the differences in gender (p = 0.008), hypertension (p = 0.031), diabetes (p = 0.028), history of stroke (p < 0.01), presence of drinking (p = 0.039), usage of statins (p = 0.044), usage of platelet aggregation inhibitors (p = 0.016), severity of CMBs (p < 0.01), and white matter lesions (p < 0.01) were statistically significant among the three groups of subjects with different cognitive levels (Table 1). A significant difference in cognitive function was found between patients with and without the mixed CMBs (p < 0.01); When the mixed CMBs did not, patients with the deep CMBs had significantly lower MoCA scores than patients with the lobar CMBs, and more likely to be diagnosed as MSCI (p < 0.01). The number of CMBs were higher in patients with MSCI (p < 0.01, Table 1). Other clinical characteristics, cerebrovascular disease-related risk factors, and biomarkers were not significantly different between the three groups (p > 0.05, Table 1).
 |
Table 1 Comparison of Clinical Characteristics Between the Patients with Different Degrees of Cognitive Impairment |
It is noteworthy in Table 1 that plasma Lp-PLA2 levels in the NC group (197.160 [32.61] ng/mL) were significantly lower than those in the MCI (0.1866 [0.2486] ng/mL) and MSCI (219.800 [32.83] ng/mL) groups. Therefore, through multiple analysis of three groups of plasma Lp-PLA2 differences (Figure 2), we found that the differences in plasma Lp-PLA2 between normal cognitive function and MCI and normal cognitive function and MSCI were statistically significant (p < 0.05). There was no significant difference in plasma Lp-PLA2 levels (0.2639 [0.3678] ng/mL) between MCI and MSCI. Multiple comparisons of positive measurement data are shown in Figure 2.
Correlation Between Plasma Lp-PLA2 Levels and MoCA Scores in Patients with CMBs
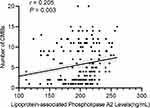
As shown in Figure 3, we explored the association between the plasma Lp-PLA2 and the MoCA score in patients with CMBs. The plasma Lp-PLA2 expression level was significantly negatively correlated with MoCA score in patients with CMBs (r =−0.389, p < 0.01). Moreover, there was a significantly positive correlation between the plasma Lp-PLA2 and the number of CMBs in patients with CMBs (r = 0.205, p < 0.01), as shown in Figure 4. To further test these relationships, we built the mediation model underlying the mediation pathway of Lp-PLA2, the number of CMBs, and MoCA scores by mediation analysis. We found that the number of CMBs has a significant mediation (IE=−0.017, p = 0.031) in the association between Lp-PLA2 and cognition, which indicates partial mediation rather than full mediation, with percent mediation (PM) of 28.5%. Mediation analysis diagrams are depicted in Figure S1.
Predictive Value of Lp-PLA2 in Patients with CMBs
Through multiple comparisons of plasma Lp-PLA2 and cognitive function levels in patients with CMBs (Figure 2), it was found that there was no significant difference between MCI and MSCI (p > 0.05). Therefore, we regrouped all patients with CMBs into the NC group and cognitive impairment group, which included the MCI and MSCI groups (n = 108). The results of the univariable regression analysis in investigating factors potentially influencing the cognitive impairment are detailed in Table S1.
According to a multivariate logistic regression analysis, the clinical risk factors for cognitive dysfunction in patients with CMBs included: Lp-PLA2 level (odds ratio [OR] = 1.013; 95% confidence interval [CI], 1.000–1.027; p = 0.048), number of CMBs (OR = 1.536; 95% CI, 1.320–1.788; p = 0.000), and location of CMBs (OR = 0.312, 95% CI, 0.163–0.599; p = 0.000) (Table 2). In the binary regression analysis after adjusting for the number of CMBs and the location of CMBs (Table 3), the high expression level of plasma Lp-PLA2 (model 1: p = 0.001, 95% CI 1.008–1.031; model 2: p = 0.000, 95% CI 1.010–1.032; model 3: p = 0.025, 95% CI 1.002–1.026) has an independent effect on cognitive dysfunction in patients with CMBs.
 |
Table 2 Multivariate Analysis of Possible Risk Factors for Cognitive Impairment of Patients with CMBs |
 |
Table 3 Risk Factors for CMBs Using Multiple Logistic Regression Analysis |
According to ROC curve (Figure 5), the optimal cut-off value of Lp-PLA2 for predicting cognitive impairment in patients with CMBs was 209.9 ng/mL, the Youden index was 0.3648, the sensitivity was 0.565, the specificity was 0.800, and the area under curve was 0.693 (95% CI, 0.622–0.764; p < 0.0001).
Discussion
With the wide application of magnetic SWI technology, the rate of detecting CMBs is increasing. Multiple studies have demonstrated that the progression of CMBs has a negative correlation with executive function, fluency, and overall cognitive function in patients with cognitive impairment.19,20 CMBs can cause trauma to normal brain tissue, disrupting neural functional networks, and ultimately leading to the development of cognitive impairment.21 CAA is the dominant cause of lobar CMBs. As well as causing strokes and cognitive impairment in a significant proportion of older patients, it is a key component of senile plaques found in patients with Alzheimer’s disease (AD).22 Based on extensive research models, advanced age and the presence of an APOE4 allele were associated with greater amyloid load and higher risk of the lobar CMBs.5,23 Among preventable factors, hypertension has the strongest association with CMBs, especially deep/infratentorial CMBs.24 A single observational study using repeat MRI showed that intervention with long-term immunosuppressive therapy for microbleeds present at baseline significantly reduced the number of cortical microbleeds, suggesting a link between CMBs and inflammation.25 Therefore, there is a need to further consider that the involvement of inflammatory factors such as Lp-PLA2 may promote the occurrence and development of cognitive impairment after CMBs. For the first time, we explored the relationship between Lp-PLA2 and cognitive decline in patients with SVD, specifically CMBs.
Lp-PLA2 is a calcium-independent serine lipase member of the PLA2 superfamily. It mediates vascular inflammation by regulating blood lipid metabolism, hydrolyzing oxidized phospholipids of oxidized low-density lipoprotein (ox-LDL) into lipid pro-inflammatory substances such as lysophosphatidylcholine (lysoPC) and oxidized non-esterified fatty acids (oxNEFAs). In this manner, Lp-PLA2 promotes oxidative stress and immune responses and induces the loss of pericytes in the central nervous system.26 Studies confirm that PLA2-mediated depletion of lipid metabolites in plasma affects neuronal membrane changes in the MCI stage, and changes in each of its subtypes may contribute to different aspects of neuropsychiatric disorders such as cognitive impairment.15,27 Nation et al28 believe that the inflammatory response or other risk factors associated with Lp-PLA2 can disrupt the blood–brain barrier of the highly specialized neurovascular system. A blood vessel can become more permeable and allow harmful substances to leak into the brain, thereby contributing to the decreased cognitive function. Likewise, Zhu et al29 supported the view that Lp-PLA2 is an independent risk factor for dementia in patients with SVD and suggested that Lp-PLA2 might help in rapidly assessing cognitive impairment in people with SVD. Therefore, lipid metabolites of the PLA2 family including Lp-PLA2 have been used to predict the conversion of MCI to dementia.30,31
We studied the correlation between Lp-PLA2 concentration and cognitive impairment in patients with CMBs. The results supported the view that a higher Lp-PLA2 level was associated with the occurrence and development of cognitive impairment, and this suggests a potential value of Lp-PLA2 in the prediction and treatment of cognitive decline in the field of CMBs. However, the results from previous studies on the relationship between Lp-PLA2 expression and cognitive decline in patients with cerebrovascular disease have been inconsistent. Savas et al32 found that although Lp-PLA2 level is significantly correlated with the decline of cognitive function, it was not related to cardiovascular disease and the change in inflammatory factors. Therefore, it was speculated that the change of Lp-PLA2 level was not related to inflammatory response. A cross-sectional study also found a suggested association between plasma Lp-PLA2 and the diagnosis of AD or amnestic mild cognitive impairment, but the authors noted that the results still need careful explanation.33
Our research suggests that there is a positive correlation between Lp-PLA2 and cognitive function in patients with CMBs. This correlation can be explained by the possibility that Lp-PLA2 can catalyze the production of inflammatory mediators by binding to LDL, which promotes atherosclerosis and further increase the risk of cerebrovascular disease and cognitive dysfunction.34 A case-control study from the Texas Alzheimer’s Research and Care Consortium Alliance also showed that high levels of Lp-PLA2 increased the risk of cognitive impairment by mediating vascular injury.35 The higher levels of Lp-PLA2 mass and activity increased the risk of dementia based on cardiovascular health studies, suggesting that a potential link between vascular disease and dementia may involve increased risk of CMBs and that Lp-PLA2 could be associated with cerebral atrophy and CMBs.36 The Framingham study held the opposing view that there is no link between Lp-PLA2 mass and all-cause dementia.37 In a meta-analysis, this result was also reported but there were statistically significant associations between elevated Lp-PLA2 activity and all-cause dementia.38 Most studies have only investigated the risk factors of cerebrovascular disease, a comprehensive disease including SVD. In patients with SVD, the mechanism of the effect of Lp-PLA2 on cognition needs to be explored further.
Additional finding from our study is a correlation between different locations of CMBs and cognitive impairment. Deep/infratentorial and mixed CMBs were associated with overall cognitive impairment. Similarly, some studies found a strongest association between the presence of deep CMBs and worse cognitive performance indeed.39,40 This may be because the internal white matter network is connected to the frontotemporal cortex and CMB-induced damage of the internal white matter affects the brain network related to cognition and affects cognitive functioning.41 Li et al20 held a different view in their longitudinal study, arguing that the decline of cognitive function is driven by strictly lobe CMBs, especially CMBs located in the temporal lobe and the underlying mechanisms of the pathological association between lobe CMBs and cognitive function are unknown. In different studies, the correlation between CMB location and cognitive decline differed, possibly due to underlying vasculopathy. Depending on location, CMBs are commonly related to two different SVD pathologies: hypertensive vasculopathy in deep regions and CAA in lobe regions. There is evidence that CAA is more prevalent in the temporal lobe.42 Therefore, a role for CAA in the pathogenesis may explain lobe CMB-related cognitive impairment.43 However, Barnaure et al44 found that the location and severity of CMBs were not related to cognitive function scores; this may be because that the participants in that study were older patients and the methods for measuring cognitive function were different from those in other studies. Moreover, Li et al6 hold that multiple microbleeds were shown to be associated with low cognitive scores and poor results in specific areas of cognitive assessment, including overall cognitive function, and these findings are similar to our own. This may be related to the local or extensive damage in the functional area of the brain.45 The higher the number of microbleeds, the greater the damage to the important cortical-subcortical or cortical-cortical connection pathways and the more serious the cognitive dysfunction.20,46 In addition, multiple microbleeds may not directly lead to neuronal injury but can promote neuronal death and neurological dysfunction through persistent inflammation associated with plasma infiltration in the brain.47 Considering the inconsistent results, further research is still required.
This study has the following limitations: First, because this is a cross-sectional study, a larger-scale longitudinal study is needed to explore the relationship between plasma Lp-PLA2 and the progressive changes of cognitive function in patients with CMBs, and further research is needed to explore the effects of Lp-PLA2 on various domains of cognitive function in patients with CMBs. These pursuits can better explore whether Lp-PLA2 can identify patients who may be at risk of cognitive impairment over time after suffering CMBs. Second, this study did not exclude the influence of CMBs combined with other confounding factors, such as cerebrovascular diseases, which may interfere with the research results and cause bias. Finally, whereas previous studies suggested that MoCA scores are consistent with cognitive assessments,48,49 our study did not confirm the specificity and sensitivity of MoCA scores in advance. We are aware that MoCA testing gives limited information about overall cognitive performance without about detailed neuropsychological syndromes. Therefore, it cannot be ruled out that depression and apathy or other neuropsychological syndromes related to CMBs may lead to the decrease of the MoCA score.
Conclusion
The plasma Lp-PLA2 is an independent risk factor for cognitive impairment. There is heterogeneity of plasma Lp-PLA2 in patients with cognitive impairment and the expression level of plasma Lp-PLA2 in patients with CMBs is correlated with cognitive impairment. Although plasma Lp-PLA2 seems unlikely to be specific for dementia, it may still play a role in predicting the onset of dementia in patients with CMBs. Our findings may support the early identification and prevention of cognitive impairment in patients with CMBs indicated by plasma Lp-PLA2.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82105004) and Huai’an Natural Science Research Plan (No. HAB202113).
Disclosure
The authors report no conflict of interest in this work.
References
1. Charidimou A, Krishnan A, Werring DJ, et al. Cerebral microbleeds: a guide to detection and clinical relevance in different disease settings. Neuroradiology. 2013;55(6):655–674. doi:10.1007/s00234-013-1175-4
2. Gyanwali B, Lui B, Tan CS, et al. Cerebral microbleeds and white matter hyperintensities are associated with cognitive decline in an asian memory clinic study. Curr Alzheimer Res. 2021;18(5):399–413. doi:10.2174/1567205018666210820125543
3. Li X, Yuan J, Yang L, et al. The significant effects of cerebral microbleeds on cognitive dysfunction: an updated meta-analysis. PLoS One. 2017;12(9):e185145. doi:10.1371/journal.pone.0185145
4. Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089–2097. doi:10.1212/WNL.0000000000003983
5. Lu D, Liu J, MacKinnon AD, et al. Prevalence and risk factors of cerebral microbleeds: an analysis from the UK biobank. Neurology. 2021;1:76.
6. Li X, Yuan J, Qin W, et al. Cerebral microbleeds are associated with impairments in executive function and processing speed. J Alzheimer’s Dis. 2021;81(1):255–262. doi:10.3233/JAD-201202
7. Jiang L, Cai X, Yao D, et al. Association of inflammatory markers with cerebral small vessel disease in community-based population. J Neuroinflamm. 2022;19(1):106. doi:10.1186/s12974-022-02468-0
8. Liu Y, Dong Y, Lyu P, et al. Hypertension-induced cerebral small vessel disease leading to cognitive impairment. Chin Med J. 2018;131(5):615–619. doi:10.4103/0366-6999.226069
9. Low A, Mak E, Rowe JB, et al. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. doi:10.1016/j.arr.2019.100916
10. Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–943. doi:10.1001/jamaneurol.2016.1017
11. Reddy KJ, Singh M, Bangit JR, et al. The role of lipoprotein-associated phospholipase a2 on cardiovascular disease risk assessment and plaque rupture: a clinical review. J Clin Lipidol. 2009;3(2):85–93. doi:10.1016/j.jacl.2009.01.004
12. Lerman A, McConnell JP. Lipoprotein-associated phospholipase a2: a risk marker or a risk factor? Am J Cardiol. 2008;101(12A):11F–22F. doi:10.1016/j.amjcard.2008.04.014
13. Zhang X, Liu L, Jiang N, et al. Correlation of lipoprotein-associated phospholipase a2 and cerebral microbleeds in patients with acute ischaemic stroke. Bmc Neurol. 2022;22(1):482. doi:10.1186/s12883-022-03000-w
14. Lerman AMJP. Lipoprotein-associated phospholipase a2: a risk marker or a risk factor? Am J Cardiol. 2008;101(12):S11–22. doi:10.1016/j.amjcard.2008.04.014
15. Duro MV, Ebright B, Yassine HN. Lipids and brain inflammation in apoe4-associated dementia. Curr Opin Lipidology. 2022;33(1):16–24. doi:10.1097/MOL.0000000000000801
16. Gregoire SM, Chaudhary UJ, Brown MM, et al. The microbleed anatomical rating scale (Mars): reliability of a tool to map brain microbleeds. Neurology. 2009;73(21):1759–1766. doi:10.1212/WNL.0b013e3181c34a7d
17. Mahammedi A, Wang LL, Williamson BJ, et al. Small vessel disease, a marker of brain health: what the radiologist needs to know. AJNR Am J Neuroradiol. 2022;43(5):650–660. doi:10.3174/ajnr.A7302
18. Fazekas F, Chawluk JB, Alavi A, et al. Mr signal abnormalities at 1.5 t in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356.
19. Igarashi S, Ando T, Takahashi T, et al. Development of cerebral microbleeds in patients with cerebral hyperperfusion following carotid endarterectomy and its relation to postoperative cognitive decline. J Neurosurg. 2021:1–7. doi:10.3171/2020.7.JNS202353
20. Li L, Wu D, Li H, et al. Association of cerebral microbleeds with cognitive decline: a longitudinal study. J Alzheimer’s Dis. 2020;75(2):571–579. doi:10.3233/JAD-191257
21. Taylor EN, Huang N, Wisco J, et al. The brains of aged mice are characterized by altered tissue diffusion properties and cerebral microbleeds. J Transl Med. 2020;18(1):277. doi:10.1186/s12967-020-02441-6
22. Yakushiji Y. Cerebral microbleeds: detection, associations and clinical implications. Front Neurol Neurosci. 2015;37:78–92. doi:10.1159/000437115
23. Graff-Radford J, Lesnick T, Rabinstein AA, et al. Cerebral microbleed incidence, relationship to amyloid burden: the mayo clinic study of aging. Neurology. 2020;94(2):e190–9. doi:10.1212/WNL.0000000000008735
24. Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(Pt 8):1988–2003.
25. Traschütz A, Tzaridis T, Penner A, et al. Reduction of microbleeds by immunosuppression in a patient with aβ-related vascular inflammation. Neurology Neuroimmunol Neuroinflammation. 2015;2(6):e165. doi:10.1212/NXI.0000000000000165
26. Geng J, Wang L, Zhang L, et al. Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front Aging Neurosci. 2018;10:129. doi:10.3389/fnagi.2018.00129
27. Costa AC, Joaquim HPG, Forlenza O, et al. Plasma lipids metabolism in mild cognitive impairment and Alzheimer’s disease. World J Biol Psychiatry. 2019;20(3):190–196. doi:10.1080/15622975.2017.1369566
28. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi:10.1038/s41591-018-0297-y
29. Zhu S, Wei X, Yang X, et al. Plasma lipoprotein-associated phospholipase a2 and superoxide dismutase are independent predicators of cognitive impairment in cerebral small vessel disease patients: diagnosis and assessment. Aging Dis. 2019;10(4):834–846. doi:10.14336/AD.2019.0304
30. Llano DA, Devanarayan V. Serum phosphatidylethanolamine and lysophosphatidylethanolamine levels differentiate Alzheimer’s disease from controls and predict progression from mild cognitive impairment. J Alzheimer’s Dis. 2021;80(1):311–319. doi:10.3233/JAD-201420
31. Paris D, Town T, Parker T, et al. A beta vasoactivity: an inflammatory reaction. Ann N Y Acad Sci. 2000;903:97–109.
32. Savas S, Kabaroglu C, Alpman A, et al. No relationship between lipoprotein-associated phospholipase a2, proinflammatory cytokines, and neopterin in Alzheimer’s disease. Exp Gerontol. 2016;77:1–6. doi:10.1016/j.exger.2016.01.014
33. Davidson JE, Lockhart A, Amos L, et al. Plasma lipoprotein-associated phospholipase a2 activity in Alzheimer’s disease, amnestic mild cognitive impairment, and cognitively healthy elderly subjects: a cross-sectional study. Alzheimer’s Res Therapy. 2012;4(6):51. doi:10.1186/alzrt154
34. Wu C, Zhou T, Zhou Y, et al. Association of serum lipoprotein-associated phospholipase a2 and a379v gene polymorphisms with carotid plaques. Genet Test Mol Biomark. 2020;24(3):131–137. doi:10.1089/gtmb.2019.0162
35. Doody RS, Demirovic J, Ballantyne CM, et al. Lipoprotein-associated phospholipase a2, homocysteine, and Alzheimer’s disease. Alzheimer’s Dementia. 2015;1(4):464–471. doi:10.1016/j.dadm.2015.08.001
36. Fitzpatrick AL, Irizarry MC, Cushman M, et al. Lipoprotein-associated phospholipase a2 and risk of dementia in the cardiovascular health study. Atherosclerosis. 2014;235(2):384–391. doi:10.1016/j.atherosclerosis.2014.04.032
37. van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: results from the Framingham heart study. Arch Neurol. 2012;69(5):594–600.
38. Darweesh SKL, Wolters FJ, Ikram MA, et al. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimer’s Dementia. 2018;14(11):1450–1459. doi:10.1016/j.jalz.2018.02.014
39. Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089–2097. doi:10.1212/WNL.0000000000003983
40. Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the ages-Reykjavik study. Neurology. 2010;75(24):2221–2228. doi:10.1212/WNL.0b013e3182020349
41. Wang Y, Jiang Y, Suo C, et al. Deep/mixed cerebral microbleeds are associated with cognitive dysfunction through thalamocortical connectivity disruption: the Taizhou imaging study. NeuroImage Clin. 2019;22:101749. doi:10.1016/j.nicl.2019.101749
42. van Norden AGW, van den Berg HAC, de Laat KF, et al. Frontal and temporal microbleeds are related to cognitive function: the Radboud university Nijmegen diffusion tensor and magnetic resonance cohort (run dmc) study. Stroke. 2011;42(12):3382–3386. doi:10.1161/STROKEAHA.111.629634
43. Poels MMF, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam scan study. Neurology. 2012;78(5):326–333. doi:10.1212/WNL.0b013e3182452928
44. Barnaure I, Montandon M, Rodriguez C, et al. Clinicoradiologic correlations of cerebral microbleeds in advanced age. AJNR Am J Neuroradiol. 2017;38(1):39–45. doi:10.3174/ajnr.A4956
45. Litak J, Mazurek M, Kulesza B, et al. Cerebral small vessel disease. Int J Mol Sci. 2020;21:24. doi:10.3390/ijms21249729
46. Zhang M, Chen M, Wang Q, et al. Relationship between cerebral microbleeds and cognitive function in lacunar infarct. J Int Med Res. 2013;41(2):347–355. doi:10.1177/0300060513476448
47. Rosidi NL, Zhou J, Pattanaik S, et al. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6(10):e26612. doi:10.1371/journal.pone.0026612
48. Ramírez-Moreno JM, Bartolomé Alberca S, Muñoz Vega P, et al. Screening for cognitive impairment with the Montreal cognitive assessment in Spanish patients with minor stroke or transient ischaemic attack. Neurologia. 2022;37(1):38–44. doi:10.1016/j.nrleng.2018.11.008
49. Zaidi K, Rich JB, Sunderland KM, et al. Methods for improving screening for vascular cognitive impairment using the Montreal cognitive assessment. Canadian J Neurolo Sci. 2020;47(6):756–763. doi:10.1017/cjn.2020.121
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.