Back to Journals » Cancer Management and Research » Volume 13
PKCα is a Potentially Useful Marker for Planning Individualized Radiotherapy for Nasopharyngeal Carcinoma
Authors Zhang J, Zhang L, Xie B, Duan Y, Wang Y , Shen L
Received 2 November 2020
Accepted for publication 3 March 2021
Published 17 March 2021 Volume 2021:13 Pages 2557—2566
DOI https://doi.org/10.2147/CMAR.S289421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Jing Zhang,1 Lu Zhang,2 Bowen Xie,2 Yumei Duan,3 Ying Wang,4 Liangfang Shen1
1Department of Oncology, Xiangya Hospital, Central South University (CSU), Changsha, 410008, People’s Republic of China; 2Key Laboratory of Molecular Radiation Oncology, Changsha, Hunan Province, 410008, People’s Republic of China; 3Department of Pathology, Xiangya Hospital, CSU, Changsha, 410008, People’s Republic of China; 4Department of Radiology, Xiangya Hospital, CSU, Changsha, 410008, People’s Republic of China
Correspondence: Liangfang Shen
Department of Oncology, Xiangya Hospital, Central South University (CSU), No. 87 Xiangya Road, Changsha, 410008, Hunan, People’s Republic of China
Email [email protected]
Purpose: To examine the expression of protein kinase C alpha (PKCα) in nasopharyngeal carcinoma (NPC) and determine its relationship to the radio-sensitivity of NPC in order to evaluate its potential as a molecular marker for the guidance of individualized radiation therapy for NPC.
Materials and Methods: PKCα expression levels were detected in tumor samples from patients and in NPC cell lines with varying degrees of radio-sensitivity. A survival analysis was performed to analyze the association of PKCα expression with the 5-year overall survival (OS), progression-free survival (PFS), locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) in patients. In vitro and in vivo experiments using NPC cell lines were performed to study the effects of down-regulation of PKCα by short hairpin RNA treatment on the radio-sensitivity of NPC.
Results: PKCα expression was up-regulated in the well-differentiated NPC tissues of patients and in the more radio-resistant NPC cell lines. Moreover, high PKCα expression was associated with a worse 5-year PFS and LRFS of patients. shRNA-mediated knockdown of PKCα led to an increase in the sensitivity of NPC cells to radiation therapy, both in vitro as cultured cells and in vivo as tumor xenografts.
Conclusion: The elevated expression of PKCα in NPC and its association with patient PFS indicates that PKCα is a potential molecular marker for guiding precision radiotherapy in NPC patients. Also, the increased radiosensitivity of NPC cells after loss of PKCα identifies PKCα as a promising therapeutic target for enhancing the radio-sensitivity of NPC.
Keywords: PKCα, marker, individualized radiotherapy, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy in the mucous epithelium of the nasopharyngeal tissues, and its prevalence shows a distinct geographic distribution. It is primarily endemic in Southeast Asia and the southern provinces of China, with a much higher incidence in these areas than in any other country or region in the world.1 Radiation therapy has been the first-line standard treatment for NPC,2 with some patients receiving intensity modulated radiotherapy (IMRT) and others receiving chemoradiotherapy. Although the overall survival (OS) of NPC patients has improved with advances in these combination radiotherapy treatments, for patients in whom these treatments fail to achieve local control, retreatment is often ineffective and causes serious complications. Because it is currently impossible to accurately predict the efficacy of radiation therapy for individuals, oncologists do not have a clear strategy for personalizing the treatment approach for individual NPC patients and, thus, are left to apply similar treatment regimens in all NPC cases. Therefore, research is urgently needed to identify markers that indicate which cases are likely to be more resistant to radiation therapy, as this information would allow for the planning of more individualized treatments.3
Protein kinase C (PKC) is a kinase that is widely expressed in human tissues, and PKC-alpha (PKCα), a subtype of PKC, has been closely associated with cancer cell proliferation, differentiation, and apoptosis.4 We previously explored the relationship between PKCα and local invasion of NPC and found that the PKCα expression level correlates with the 2-year progression-free survival (PFS) and overall survival (OS) of NPC patients.5 In the present study, we extended this research in NPC patients, investigating PKCα expression in NPC specimens and analyzing the correlation of PKCα expression with the 5-year survival of NPC patients. To better understand the observed correlation, we evaluated the expression of PKCα in NPC cell lines with varying degrees of radio-resistance and studying the effects of PKCα knockout on their response to radiation treatment both as cultured cells in vitro and as tumor xenografts in vivo. Together, the results from our analysis of patient specimens and our in vitro and in vivo experiments provide the evidence that PKCα represents a potential marker of NPC radio-sensitivity as well as a potential target for increasing the radio-sensitivity of NPC.
Materials and Methods
NPC Clinical Samples
NPC samples were collected from a total of 97 patients at initial diagnosis in the Oncology Department of Xiangya Hospital of Central South University between 2010 and 2015. The ethical committee of Xiangya Hospital approved this study (No: 201703513). Use of tissue samples, data collection and review of medical records were performed in accordance with the ethical standards of the committee and the 1964 Declaration of Helsinki and its later amendments. Because of the retrospective nature of our study and lack of patient identification, informed consent was waived for all participants involved in this study. The inclusion criteria included nasopharyngeal carcinoma diagnosed for the first time by biopsy and treated with radical intensity-modulated conformal radiotherapy. Patients were excluded according to the following criteria: performance status ≥2 on the Eastern Cooperative Oncology Group (ECOG) scale, distant metastasis (stage IVb), previous malignancy or other type of tumor; unstable cardiac disease; and current lactation or pregnancy. The nasopharyngeal tumors were paraffin-embedded and formalin-fixed. The samples were collected from untreated patients along with clinical follow-up data on the initial diagnosis. The histological classifications followed the World Health Organization (WHO) classification system: type I, keratinizing carcinomas; type II, differentiated subtype of non-keratinizing carcinoma; and type III, undifferentiated subtype of non-keratinizing carcinoma.
Clinical Staging Method, Image Assessment, and Treatment
Patients’ computed tomography (CT) and magnetic resonance imaging (MRI) scans were independently reviewed with the Varian Eclipse Plan System by two radiation oncologists. Stage was determined by comprehensively evaluating the patients’ clinical manifestations, physical examination results, and CT or MRI scans according to Seventh Edition of the American Joint Committee on Cancer (AJCC) clinical staging standard. Measurable disease was defined by the RECIST criteria. According to the No. 50 and 62 documents of the ICRU, all patients were treated by IMRT, with prescribed doses as follows: primary tumor, 70.62–73.92 Gy (2.14–2.24 Gy/33 fractions); high-risk lymph nodes, 69.96 Gy (2.12 Gy/33 fractions); intermediate-risk sites, 59.4–61.05 Gy (1.8–1.85 Gy/33 fractions), and low-risk sites, 50.4–51.8 Gy (1.8–1.85 Gy/28 fractions). The radiation was delivered by a linear accelerator (Varian, USA) with five daily fractions per week. Paclitaxel/docetaxel and/or platinum were used as neo-adjuvant, concurrent, and adjuvant chemotherapy. OS was defined as the duration from the first day of initial diagnosis to the date of death from any cause or the last follow-up on August 31, 2020. Progression-free survival (PFS) and locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) were defined as the interval from the first day of initial diagnosis to confirmed disease progression, local recurrence, and distant metastasis, respectively. Progressive disease was defined as at least a 20% increase in the sum of the diameters of target lesions (an absolute increase of at least 5 mm) or the appearance of one or more new lesions. Stable disease was defined as neither sufficient shrinkage to qualify as partial response nor sufficient growth to qualify as progressive disease.6
Immunohistochemical Staining
All xenograft samples and NPC tissue blocks were fixed and embedded in paraffin according to standard procedures. Following de-paraffinization, sections were subjected to heat-mediated antigen retrieval in 0.01 mol/L citrate buffer (pH 6.0). After cooling to room temperature, the samples were treated with 1% methanol/30% H2O2 to block endogenous peroxidase activity. Nonspecific protein binding was blocked using 5% bovine serum albumin (BSA) solution. Next, sections were rinsed with phosphate-buffered saline (PBS) and incubated with anti-PKCα (Santa Cruz Biotechnology, 1:40) overnight at 4°C. The next day, the sections were rinsed with PBS at room temperature and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, UK) for 30 min. Then, after 3–5 min of washing, the sections were incubated with 3,3ˊ-diaminobenzidine (DAB) for visualization. Finally, the samples were rinsed with distilled H2O and counterstained with hematoxylin.
The scores for PKCα-reactive NPC cells in patient samples were determined by evaluating the staining degree and percentage of positive cells. For cases of positive cytoplasmic staining, PKCα scores were assigned as follows: 0, absence of expression or expression in <10% of NPC-positive cells; 1, staining in 10–49% of NPC-positive cells; and 2, staining in ≥50% of NPC-positive cells. The immunohistochemical staining results were independently evaluated by two pathologists.
Cell Culture and Cell Line Generation
The NPC cell lines CNE1, CNE2, and HK1 were donated by the Cancer Research Institute of Central South University.7–9 The use of the cell lines was approved by the ethics committee of Xiangya Hospital, and the CNE1 and CNE2 cells were authenticated by STR profile before the study. A radio-resistant cell line, denoted as CNE2-resistant (CR) cells, was established via exposure of parental CNE2 cells to gradually increasing doses of irradiation.10,11 The final surviving CNE2 cells were verified with significant radiation-resistant characteristics and defined as radioresistant CNE2 cells (CR).10 The radiation was delivered with a Varian linear accelerator (2100EX, San Francisco, CA, USA). The cell culture flask was covered with a 1.5-cm layer of compensation glue during irradiation. In addition, a 6-MeV electron beam was chosen, and the radiation dose rate was 300 cGy/min. All cell lines were cultured at 37°C in 5% CO2 and grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, USA) with 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin/streptomycin (Invitrogen, USA). Cells in the exponential growth phase were used in the experiments.
Plasmid Construction and Transfection
To knockdown PKCα, two target sequences were selected: the human PKCα target 1 (5-GGGATCGAACAACAAGGAATG-3) and target 2 (5-GCGTCCTGTTGTATGAAATGC-3).12 To generate PKCα short hairpin (sh)RNA, the coding sequences for the shRNAs were cloned using the Lenti-X™ shRNA Expression System (Clontech). Then 293T cells were co-transfected with lentiviral vectors, envelope plasmids, and packaging plasmids for 24 h before collection of the lentiviral supernatant. The CR and CNE1 cells were transduced with control or shPKCα lentiviral particles for 48 h and then cultured for 72 h with 2 μg/mL puromycin for selection. The efficacy of transfection was verified by Western blot analysis.
Colony Formation and Radio-Sensitivity Assays
Cells were seeded in six-well plates and subjected to irradiation (IR) treatment for 24 h with a dose range 0–6 Gy delivered with the Varian linear accelerator (2100EX, San Francisco, California, USA). Our preliminary experiment showed that 6 Gy is an appropriate dose for further experiments. The medium was changed every 5 days after radiation, and after 10–12 days, the cells were washed three times at room temperature, fixed in paraformaldehyde for 15 min, and stained with crystal violet for 10 min. After washing with de-ionised water, the plates were left to air dry. For calculation of colony formation, clones with more than 50 cells were counted as colonies. Analysis was performed using the linear quadratic model of GraphPad software.
Flow Cytometry
Cells were seeded in six-well plates, irradiated with 2Gy 16h after seeding, and harvested at 48h. Then 1 x 104 cells were treated by Annexin V-FITC Apoptosis Detection Kit (Beyotime, China), and apoptosis was analyzed by flow cytometry (Guava easyCyte, Millipore, Germany).
Preparation of Proteins and Western Blotting
For protein extraction, cells were lysed with radioimmunoprecipitation assay (RIPA) buffer for 30 min on ice. Protein concentrations were then measured with BCA protein assay kits (Thermo-Scientific, USA). Protein samples (40–50 μg) were prepared and separated by a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto 0.22- and 0.45-μm polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were immersed in Tris-buffered saline with Tween 20 (TBST) containing 5% non-fat dry milk, shaken at room temperature for 1 h, and then incubated for 1 h at room temperature in solutions of the following primary antibodies at 4°C for 16–18 h: anti-PKCα (1:500, Santa Cruz Biotechnology, USA) and anti-tubulin (1:1000, Santa Cruz Biotechnology). After washing with TBST, the blots were incubated with the secondary antibodies (1:1000 Santa Cruz Biotechnology) for 1 h at room temperature. Finally, the protein bands were visualised with a Thermo Scientific-Pierce Enhanced Chemiluminescence (ELC) substrate detection system.
NPC Xenograft Model
The animal experiments were conducted according to the Guidelines of the Animal Handling and Care in Medical Research Committee of Central South University and were approved by the Animal Ethics Committee of Central South University (No: 201703512). Six- to eight-week-old female nude mice obtained from Vital River Laboratories (Beijing, the original breeding pairs were purchased from Charles River) were used to establish the human NPC xenograft model. After stable transduction with PKCα shRNA or shControl lentivirus, 5×106 CR cells were inoculated into the left flanks of the mice to form the NPC xenografts. Tumor volume was measured every 2 days from the third day after subcutaneous inoculation. Irradiation treatment was applied once the tumor volume reached 60–80 mm3. Radiation was delivered at 2 Gy per fraction every other day for three fractions. When the tumor volume reached a maximum of 1500 mm3, the mice were euthanized, and the xenografts were harvested for further analyses.13
Apoptosis in xenografts was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining using the ApopTag® Fluorescein In-Situ Apoptosis Detection Kit (Merck Millipore, Germany) after preparation of sections for staining as described above for human NPC specimens. The fluorescence intensity was analysed by ImageJ (National Institutes of Health, USA).
Statistical Analysis
All in vitro and in vivo experiments were performed at least three times. The statistical analyses were conducted using SPSS 23.0 software (SPSS, Inc., USA). For comparison of data between two groups, Student’s t test or chi-square test was used for in vitro data. Spearman rank correlation coefficients were used to determine the correlations of PKCα and histological classification. Survival was estimated using the Kaplan–Meier method, and the results were compared with the Log rank test. P values <0.05 were considered statistically significant. All statistical tests were two-sided.
Results
Expression of PKCα is Up-Regulated in Well-Differentiated NPC
Tumor samples were obtained from 97 patients with histologically diagnosed NPC. The patients ranged in age from 22–72 years and had WHO types I, II, or III NPC (stages II to Iva; Table 1). All patients exhibited a performance status of less than 2 on the Eastern Cooperative Oncology Group (ECOG) scale.
 |
Table 1 Characteristics of NPC Patients Whose Samples Were Used for Immunohistochemical Staining of PKCα |
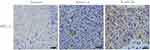
It is generally accepted in radiation oncology that poorly differentiated malignant tumours tend to be more sensitive to radiation therapy. As WHO type I is rare in NPC, we detected and analyzed PKCα expression in NPC specimens including WHO type II and III, considering WHO type II NPC to be well differentiated and WHO type III NPC to be poorly differentiated. Based on the expression level score derived from immunohistochemical staining analysis of PKCα (Figure 1A), a rank correlation analysis revealed that PKCa was differentially expressed between the WHO II and WHO III groups (Spearman correlation coefficient 0.290; p=0.005; Table 2).
 |
Table 2 Correlation Between the Intensity of PKCa Expression in Nasopharyngeal Carcinoma (NPC) and Histological Classification |
PKCα Expression is Associated with 5-Year Progression-Free Survival and LRFS in NPC Patients
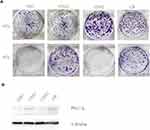
To further confirm whether PKCα expression is related to the survival outcomes of radiation therapy for NPC, we divided the NPC cases into a PKCα expression-positive group and PKC expression-negative group, according to pathological scores for PKCα staining (0, negative; and score 1 and 2, positive). From a survival analysis based on the 5-year follow-up, the OS rates of the PKCα-negative and PKCα-positive groups were 97.2% and 86.5%, respectively (p=0.084; Figure 2A). The PFS rate of the PKCα-negative group was significantly higher than that of the of the PKCα-positive group (91.7% vs 77.0%, p=0.045; Figure 2B). The LRFS rate of the PKCα-negative was also significantly higher than that of the PKCα-positive group (94.4% vs 80.3%, p=0.043; Figure 2C). Finally, the DMFS rates of the PKCα-negative and PKCα-positive groups did not differ significantly (94.4% vs 85.2%, p=0.139; Figure 2D).
PKCα Expression is Up-Regulated in Radiation-Insensitive NPC Cell Lines
Given that radiation therapy is the primary treatment for NPC and our results above suggest that PKCα expression is related to patients’ PFS, we examined the level of PKCα expression in NPC cell lines that exhibit varying degrees of radio-sensitivity. First, to test radio-sensitivity, colony formation by NPC cell lines was assessed after irradiation at 0 or 6 Gy (Figure 3A). Then PKCα expression in these cell lines was detected by Western blotting. CR cells, which are considered largely radio-resistant (Figure S1A and B), showed significantly greater PKCα expression compared with CNE2 cells. Also CNE1 cells, a cell line with rather poor response to radiation, also showed elevated PKCα expression. In sharp contrast, in the radiation-sensitive cell line HK1, PKCα expression was extremely low (Figure 3B).
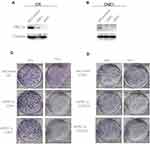
Reduced PKCα Expression Increases the Radio-Sensitivity of NPC Cells in vitro
To investigate whether PKCα regulates the radio-sensitivity of NPC cells, we performed loss-of-function assays using two independent shRNAs targeting PKCα. As shown in Figure 4A and B, shRNA treatment using two target sequences sh#1 and sh#2 resulted in successful down-regulation of PKCα in both the CR and CNE1 cell lines. We then examined the survival fractions of CR and CNE1 cells after 6 Gy of irradiation with or without PKCα knockdown. As shown in Figure 4C and D, knockdown of PKCα significantly increased the radio-sensitivity of CR and CNE1 cells compared with control cells.
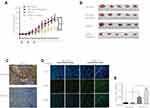
Reduced PKCα Expression Increases the Radio-Sensitivity of CR Xenografts in vivo
Next, we established a mouse NPC xenograft model using CR cells to investigate whether knockdown of PKCα could enhance NPC sensitivity to radiation in vivo. After subcutaneous inoculation of shPKCα- or scramble control-treated CR cells into nude mice, the two types of NPC cells formed tumors well. From day 7 to day 11 (5 days), we treated mice three times, once every other day, with 2-Gy fractions. Consistent with the results in vitro, tumor growth in the shPKCα group was significantly inhibited in response to radiation treatment, compared with that in the control groups (Figures 5A, B and S2). Immunohistochemical staining of harvested xenografts confirmed down-regulation of PKCα in the shPKCα-treated group (Figure 5C). Additionally, more apoptotic tumor cells were observed in the xenografts from the shPKCα group after radiation treatment (Figure 5D and E). Together, these results indicate that down-regulation of PKCα can increase the sensitivity of CR cells within NPC xenografts to radiation therapy.
Discussion
It is generally accepted that PKC functions as a carcinogenic promoter;14 however, decreased activity and expression levels of several subtypes of PKC also have been found in many malignant tumors.15 These seemingly conflicting findings reveal the complexity of the cellular functions of PKC and its subtypes in cancer development.16
Studies have also reported that PKC expression is related to therapeutic resistance in cancer cases,17 and PKCα expression has been suggested to be a predictor of tumor recurrence after treatment.18,19 In primary human fibroblasts, PKC signaling was shown to prevent irradiation-induced apoptosis.20 Furthermore, in 2017 Scott et al proposed a gene expression-based radiation-sensitivity index that includes PKCβ expression for planning an individualized radiation dose for NPC.21 These studies indicate that the PKC signaling pathway is involved in the regulation of resistance to therapy and, specifically, radio-resistance. Therefore, the results of our present study confirm the link between PKCα expression and radio-resistance and provide evidence for the potential value of PKCα as a molecular marker for guiding individualized radiation therapy for NPC. As a marker in NPC, high PKCα expression would indicate the need for a relatively high dose of radiation therapy to improve the likelihood of local disease control and thereby improve patients’ survival. It is foreseeable that under the guidance of individualized PKCα levels, radiation oncologists will be able to make radiation treatment plans more individualized. At the same time, comprehensive treatment plans including chemotherapy could be optimized to avoid excessive treatment. In addition, in view of the results of the present study, the significance of PKCα expression in the WHO classification deserves further exploration.
The main types of NPC are WHO type II and III, and WHO type I NPC, which is also defined as keratinizing carcinomas, is extremely rare. In our study, PKCα expression in WHO type II cases was higher than that in type III cases, which is consistent with previous conclusion that PKC is related to the differentiation of normal cells and malignant tumors.22–24 However, the NPC-specific mechanism of PKCα in the regulation of cancer cell differentiation requires further investigation.
A limitation of this study was the inability to determine the relationship between the expression of PKCα and the immunohistochemical markers of differentiation in order to understand more clearly whether PKCα is related to the differentiation of nasopharyngeal carcinoma. In addition, the apoptosis inhibition pathway related to PKCα has not been clarified and is worthy of further exploration.
Conclusions
PKCα expression in increased in NPC tissues as well as in radio-resistant NPC cell lines. Moreover, a significant association was found between PKCα expression and the 5-year PFS and LRFS of NPC patients. Together, the findings of this study indicate that PKCα is a potential molecular marker of radio-resistance in NPC, and thus, measurement of its expression level may aid in treatment planning for NPC patients. Moreover, our findings that loss of PKCα expression rendered NPC cells more resistant to radiation therapy both in vitro and in vivo suggest that PKCα is also a potential target for enhancing the radio-sensitivity of NPC.
Acknowledgment
We thank Yang XH for his help with the experiments.
Funding
This work was supported by grants from the from the National Natural Science Foundation of China (81530084).
Disclosure
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the paper.
References
1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi:10.1016/S0140-6736(15)00055-0
2. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi:10.1016/S0140-6736(05)66698-6
3. Tan LLY, Chua MLK. Discovering biomarkers of radioresistance in a radiosensitive cancer: a clinical paradox in nasopharyngeal carcinoma. Ann Transl Med. 2020;8:1284. doi:10.21037/atm-20-5405
4. Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi:10.1038/nrm2847
5. Zhang J, Wang Y, Duan Y, et al. PKCα promotes local advancement via its dual roles in nasopharyngeal carcinoma. Acta Otolaryngol. 2017;137:662–667. doi:10.1080/00016489.2016.1269195
6. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi:10.1016/S1470-2045(16)30410-7
7. Lu J, Tang M, Li H, et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett. 2016;380:191–200.
8. Hou Y, Zhu Q, Li Z, et al. The FOXM1-ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis. 2017;8:e2659. doi:10.1038/cddis.2017.53
9. He JJ, Li Z, Rong ZX, et al. m(6)A Reader YTHDC2 promotes radiotherapy resistance of nasopharyngeal carcinoma via activating IGF1R/AKT/S6 signaling axis. Front Oncol. 2020;10:1166. doi:10.3389/fonc.2020.01166
10. Li G, Liu Y, Su Z, et al. MicroRNA-324-3p regulates nasopharyngeal carcinoma radioresistance by directly targeting WNT2B. Eur J Cancer. 2013;49:2596–2607. doi:10.1016/j.ejca.2013.03.001
11. Fu S, Li Z, Xiao L, et al. Glutamine synthetase promotes radiation resistance via facilitating nucleotide metabolism and subsequent DNA damage repair. Cell Rep. 2019;28:1136–1143.e1134. doi:10.1016/j.celrep.2019.07.002
12. Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB. Activated PKC{delta} and PKC{epsilon} inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl- cotransporter NKCC1. J Biol Chem. 2010;285:34072–34085. doi:10.1074/jbc.M110.137380
13. Zhang L, Xie B, Qiu Y, et al. Rab25-mediated EGFR recycling causes tumor acquired radioresistance. iScience. 2020;23:100997. doi:10.1016/j.isci.2020.100997
14. Rahimova N, Cooke M, Zhang S, Baker MJ, Kazanietz MG. The PKC universe keeps expanding: from cancer initiation to metastasis. Adv Biol Regul. 2020;78:100755. doi:10.1016/j.jbior.2020.100755
15. Antal CE, Hudson AM, Kang E, et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160:489–502. doi:10.1016/j.cell.2015.01.001
16. Parker PJ, Brown SJ, Calleja V, Chakravarty P, Cobbaut M. Equivocal, explicit and emergent actions of PKC isoforms in cancer. Nat Rev Cancer. 2021;21:51–63. doi:10.1038/s41568-020-00310-4
17. Wang Q, Zhang T, Chang X, et al. Targeting Opsin4/Melanopsin with a novel small molecule suppresses PKC/RAF/MEK/ERK signaling and inhibits lung adenocarcinoma progression. Mol Cancer Res. 2020;18:1028–1038. doi:10.1158/1541-7786.MCR-19-1120
18. Deka SJ, Trivedi V. Potentials of PKC in cancer progression and anticancer drug development. Curr Drug Discov Technol. 2019;16:135–147. doi:10.2174/1570163815666180219113614
19. Lau WM, Teng E, Huang KK, et al. Acquired resistance to FGFR inhibitor in diffuse-type gastric cancer through an AKT-independent PKC-mediated phosphorylation of GSK3β. Mol Cancer Ther. 2018;17:232–242. doi:10.1158/1535-7163.MCT-17-0367
20. Bluwstein A, Kumar N, Léger K, et al. PKC signaling prevents irradiation-induced apoptosis of primary human fibroblasts. Cell Death Dis. 2013;4:e498. doi:10.1038/cddis.2013.15
21. Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18:202–211. doi:10.1016/S1470-2045(16)30648-9
22. Domínguez-García S, Geribaldi-Doldán N, Gómez-Oliva R. A novel PKC activating molecule promotes neuroblast differentiation and delivery of newborn neurons in brain injuries. Cell Death Dis. 2020;11:262. doi:10.1038/s41419-020-2453-9
23. Zhao H, Li M, Ouyang Q, Lin G, Hu L. VEGF promotes endothelial cell differentiation from human embryonic stem cells mainly through PKC-ɛ/η pathway. Stem Cells Dev. 2020;29:90–99. doi:10.1089/scd.2019.0172
24. Nakura A, Higuchi C, Yoshida K, Yoshikawa H. PKCα suppresses osteoblastic differentiation. Bone. 2011;48:476–484. doi:10.1016/j.bone.2010.09.238
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.