Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Physical Activity, Exercise Capacity and Sedentary Behavior in People with Alpha-1 Antitrypsin Deficiency: A Scoping Review
Authors O'Shea O , Casey S, Giblin C, Stephenson A, Carroll TP, McElvaney NG, McDonough SM
Received 15 September 2022
Accepted for publication 7 March 2023
Published 16 June 2023 Volume 2023:18 Pages 1231—1250
DOI https://doi.org/10.2147/COPD.S389001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Orlagh O’Shea,1 Saidhbhe Casey,1 Ciaran Giblin,1 Aoife Stephenson,1,2 Tomás P Carroll,3 Noel G McElvaney,3 Suzanne M McDonough1,2,4,5
1School of Physiotherapy, Royal College of Surgeons in Ireland University of Medicine and Health Sciences, Dublin 2, Ireland; 2School of Health Sciences, University of Southampton, Southampton, UK; 3Irish Centre for Genetic Lung Disease, Department of Medicine, Royal College of Surgeons in Ireland University of Medicine and Health Sciences, Beaumont Hospital, Dublin 9, Ireland; 4Centre for Health and Rehabilitation Technologies, School of Health Sciences, Ulster University, Newtownabbey, BT37 0QB, UK; 5School of Physiotherapy, University of Otago, Dunedin, New Zealand
Correspondence: Orlagh O’Shea, School of Physiotherapy, Royal College of Surgeons in Ireland University of Medicine and Health Sciences, 123 St Stephen’s Green, Dublin 2, Ireland, Tel +353 1 402 8652, Email [email protected]
Abstract: Alpha-1 antitrypsin deficiency (AATD) is a hereditary disorder and a genetic risk factor for chronic obstructive pulmonary disease (COPD). Physical activity (PA) is important for the prevention and treatment of chronic disease. Little is known about PA in people with AATD. Therefore, we aimed to map the research undertaken to improve and/or measure PA, sedentary behaviour (SB) or exercise in people with AATD. Searches were conducted in CINAHL, Medline, EMBASE and clinical trial databases for studies published in 2021. Databases were searched for keywords (physical activity, AATD, exercise, sedentary behavior) as well as synonyms of these terms, which were connected using Boolean operators. The search yielded 360 records; 37 records were included for review. All included studies (n = 37) assessed exercise capacity; 22 studies reported the use of the six-minute walk test, the incremental shuttle walk test and cardiopulmonary exercise testing were reported in three studies each. Other objective measures of exercise capacity included a submaximal treadmill test, the Naughton protocol treadmill test, cycle ergometer maximal test, endurance shuttle walk test, constant cycle work rate test, a peak work rate test and the number of flights of stairs a participant was able to walk without stopping. A number of participant self-reported measures of exercise capacity were noted. Only one study aimed to analyze the effects of an intensive fitness intervention on daily PA. One further study reported on an exercise intervention and objectively measured PA at baseline. No studies measured SB. The assessment of PA and use of PA as an intervention in AATD is limited, and research into SB absent. Future research should measure PA and SB levels in people with AATD and explore interventions to enhance PA in this susceptible population.
Keywords: lung disease, AATD, COPD, physical activity measurement, sedentary behavior, exercise capacity
Introduction
Alpha-1 antitrypsin deficiency (AATD) is one of the most common hereditary disorders in adults of European descent1 and remains the most common, readily identifiable genetic risk factor for chronic obstructive pulmonary disease (COPD).2 The pulmonary manifestations of AATD include a spectrum of disorders associated with COPD3 including emphysema, chronic bronchitis, and bronchiectasis. The pathophysiology of AATD reflects the absence of alpha-1 antitrypsin (AAT) which normally protects the lung tissues from proteolytic destruction,4 and an excess of abnormally folded AAT within the liver.5 Extra-pulmonary associations with liver cirrhosis, hepatocellular cancer, vasculitis and panniculitis are well recognised in AATD.6,7
Physical activity (PA), defined as “any bodily movement produced by skeletal muscles that requires energy expenditure”,8 is fundamental for supporting physical and mental health and wellbeing across the life course9 for all age groups and for those living with long-term conditions.10 PA includes all activities done as part of daily living such as social and domestic activities, commuting, recreational and leisure activities and may or may not include exercise.11 Exercise is a subcategory of PA that is planned, structured, repetitive, with a specific purpose ie to increase fitness or improve strength.8 Exercise and PA are recognised as a key care feature and should be encouraged for all patients with chronic respiratory disease.12 It is well established that people with chronic respiratory disease demonstrate lower PA levels compared to their healthy counterparts.13–15 In addition, many patients with AATD develop a sedentary lifestyle,16 increasing the risk of comorbidities. Sedentary behavior (SB) is defined as any behavior undertaken during waking hours, in sitting or reclining posture, that requires energy expenditure ≤1.5 metabolic equivalent tasks.17 High levels of SB are associated with poorer cardiometabolic health, while high levels and intensity of PA are associated with improved cardiometabolic health.10 Moreover, given people with severe AATD-related COPD tend to be younger than usual COPD,18 it is imperative to support this high-risk population to engage in health enhancing PA and to reduce their SB.
It has been noted that updated guidance on lifestyle modifications in AATD is warranted.19 In particular, further guidance is required to facilitate the appropriate implementation of non-pharmacologic treatment measures such as PA and exercise.19 Certain medications including bronchodilator therapy have been demonstrated to increase exercise capacity in people with COPD.20 The only specific pharmacological treatment for severe AATD-related lung disease is intravenous infusion of plasma-purified alpha-1 antitrypsin, known as augmentation therapy.21 Given the recognised importance of PA in reducing hospitalisations, enhancing quality of life and improving life expectancy in people with COPD,13,22 interventions to enhance PA and exercise and reduce SB in those medically optimised may be important for improving health outcomes in AATD. Preliminary searches of the literature suggest that this area is largely unexplored, and research is limited. To our knowledge, to date, there are no reviews published that explore either PA levels, exercise capacity or SB in people with AATD or reviews of interventions targeting PA, exercise or SB in people with AATD.
Therefore, this scoping review aims to map the research undertaken in the area of PA, exercise and SB in people with AATD. This will allow us to bring together, identify and describe interventions that support people with AATD to improve their PA levels, exercise capacity, and to reduce SB.
This scoping review aims to answer the following question: What is the “extent (size), range (variety) and nature (characteristics) of the evidence around PA, exercise and SB for people with AATD?”
Materials and Methods
This review was conducted in accordance with guidance from the Preferred Reporting Items for Scoping Reviews (PRISMA-ScR).23
Eligibility Criteria
The inclusion and exclusion criteria were discussed and agreed by all authors. The inclusion criteria were: (a) research papers published from database inception to July 2021; (b) published in English; (c) quantitative, qualitative or mixed-method reports including abstracts, protocols, reviews and conference proceedings; (d) diagnosis with any form of AATD; (e) no age restriction; (f) Studies including any measure of exercise capacity, PA or SB (participant report or device measured). Restrictions on language were due to limited resources.
Search Strategy
The search strategy (Supplemental File 1) was developed alongside an academic librarian and members of the research team. The search strategy was made up of keywords (physical activity, AATD, exercise, pulmonary rehabilitation, sedentary behaviour) as well as synonyms of these terms, which were connected using Boolean operators.
Comprehensive searches were undertaken in Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE and Embase for all relevant studies from database inception to July 2021. Study/trial registries Australian New Zealand Clinical Trials Registry (ANZCTR), clinicaltrials.gov, The European Union Clinical Trials Register (EU CTR) and PROSPERO were searched to ensure that ongoing and recently completed studies were not missed. Reference lists of included studies were hand searched to identify additional studies that may have been missed in the original search.
The search results were imported into EndNote reference management software (version X9), where duplicates were removed.
Selection of Sources of Evidence
Five percent of the same titles and abstracts were assessed by two independent reviewers to confirm the eligibility criteria, consensus was agreed across these results and no changes were made to the eligibility criteria. The team determined the eligibility of articles using a two-stage screening process. First, the reviewers screened the titles and abstracts of all identified literature against the research question and inclusion criteria and categorised them as include, exclude or uncertain. Screening of titles/abstracts were divided amongst the review team. Doubts about the relevance of a study based on its abstract, resulted in retrieval of the full-text version. Uncertainties and disagreements were resolved through discussion and group consensus. Throughout the process, reviewers met regularly via an internet-based video conferencing platform and e-mail to further discuss the screening process. Full-text screening followed, using the same procedure.
Data Charting Process
Study details and data were extracted using a customized form, based on the objectives of the review. The charting form was available on an online portal allowing the team to see each other’s contribution and discuss any concerns. The following data were independently extracted from each article: authors, year, title, aim, study design, participant demographics, outcome measure used to assess PA/exercise capacity/SB and intervention/treatment received.
Collating and Summarizing Results
The results grouped together according to study design and tabulated for ease of reporting.
Results
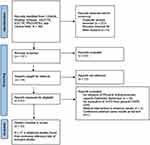
The PRISMA-ScR flow diagram of the literature search can be found in Figure 1. After the removal of duplicates, 137 titles and abstracts records were screened. Sixty-one full-text articles were retrieved. Thirteen records could not be retrieved, and attempts were made to contact the authors via the email. Thirty-three records fulfilled the inclusion criteria. A further four records were identified from screening the reference lists of records meeting the inclusion criteria. A total of 37 records were included in this review.
Study and Participant Characteristics
Study and participant characteristics are summarized in Table 1. A number of different study designs were identified: six cross-sectional studies,24–29 six prospective studies,30–35 three retrospective,36–38 three randomized controlled trials (RCT),16,39,40 and one non-randomized and non-concurrent trial.41 Ten included reports were conference abstracts of which there were three retrospective studies,42–44 two RCTs,45,46 two case studies,47,48 two cross-sectional49,50 and one prospective study.51 A further eight protocols52–59 were identified: one cross-sectional study,52 two prospective studies,53,54 four RCTs55–58 and one non-randomized parallel group study.59 All reports were published between 1998 and 2021.
 |
Table 1 Participant Demographics of Included Studies |
A total of 10,645 (range 1–3526) participants with AATD were included, and a further 3722 (25–3000) were planned to be recruited for protocol studies. Trials which reported on gender included 5617 males and 4960 females. The youngest average (standard deviation) age reported in a study was 46.1 (8.8) years,21 and the oldest average age cohort was 70.1 (9.2) years.40 The ZZ genotype was the most common AATD genotype included in the studies, other genotypes reported included ZZ, MZ, MS, MP, Null and other/unknown genotypes.
Interventions
The interventions are summarized in Table 2. For studies reporting an intervention or treatment, there were nine studies reporting on a surgical intervention or treatment: six on lung volume reduction surgery,29–32,36,51 two endobronchial valve42,44 and one lung transplant study,33 all of which assessed exercise capacity. There were 11 studies reporting on an exercise intervention.28,34,35,37,39,41,43,48,53,56 Only one of these results reported an aim related to improving daily PA. Choate et al 201743 (a conference abstract) reported aiming to identify whether a more intensive fitness intervention would improve physical activity and weight outcomes. A further three studies reported a treatment relating to a medicinal product, specifically oxygen therapy36 and an alpha-1 proteinase inhibitor (known as augmentation therapy).45,52 One study described an education self-management programme.38 No studies reported specifically on a PA intervention or an intervention to reduce SB.
 |
Table 2 The Study Design, Exercise or Physical Activity Outcome Measure, Intervention/Treatment Received and Control/Comparator and Aim of Included Studies |
Outcome Measures to Assess Exercise Capacity, Physical Activity and Sedentary Behavior
The outcome measures reported are in Table 2. Regardless of the intervention focus (which was broad), exercise capacity was the most commonly reported outcome, 22 studies reported the use of the six-minute walk test to measure exercise capacity,30–40,44,45,47,49–55,58 the incremental shuttle walk test and cardiopulmonary exercise testing were reported in three studies each.26,29,43,53,56,57 Other objective measures of exercise capacity included a submaximal treadmill test,24 the Naughton protocol treadmill test,29 cycle ergometer maximal test,39 endurance shuttle walk test, constant cycle work rate test,59 a peak work rate test42,51 and the number of flights of stairs a participant was able to walk without stopping.27 A number of participant self-reported measures of exercise capacity were noted. For example, participants reporting on the regularity and location of the exercise,25 individuals asked whether they exercised regularly, irregularly, or not at all,28 participants reporting the number of exercise minutes per week.16,46 Another study asked participants to categorise themselves as “does not exercise regularly, exercises regularly or has started to exercise”41 and finally a case study reported on the number of blocks the patient could walk pre- and post-treatment in an urban setting.48 There was one device-based measure of PA recorded in an exercise intervention.31 Nine AATD participants were included in this study of COPD patients with and without AATD. There were no measures of SB reported in the included studies.
Discussion
The aim of this study was achieved. There is a dearth in the assessment and reporting of PA in people with AATD. Only one study in this review objectively assessed PA in a small number of participants with AATD. Exercise capacity was the most commonly assessed outcome of interest.
Physical inactivity contributes to about five million deaths in the world each year from noncommunicable diseases.60 Specifically, regular PA has been associated with a reduction in the risk for premature mortality and is an established means of reducing the risks for more than 25 chronic medical conditions including COPD.61 PA has been established as the strongest predictor of mortality in people with COPD.62 The current gap in the literature for the measurement of PA and PA interventions in people with AATD is therefore surprising. Eleven of the included studies reported on an exercise intervention. One could argue that the limitation of exercise interventions is that only a moderate to weak relationship has been established between PA and exercise capacity in people with COPD and other conditions.63 Additionally, current evidence in the COPD population has demonstrated that improvements in exercise capacity do not automatically translate to enhanced PA levels, even when the improvements are gained through exercise training. Enhanced functional capacity and adaptive behavior change are necessary to achieve significant and lasting increases in daily PA in patients with COPD.64 An added benefit of PA interventions in AATD is that the risk of liver disease related to AATD may also be reduced.65 Future research should consider the impact of PA, exercise and SB on the entire clinical sequelae in people with AATD.
There has been increasing awareness around SB in recent years and the risks associated with premature mortality and morbidity for the general population.66,67 A recent systematic review and meta-analysis demonstrated that high levels of SB are associated with a higher risk of metabolic syndrome which enhances the risk of both cardiovascular diseases and type 2 diabetes68 which are common comorbidities observed in those with COPD.69 Furthermore, a cohort study demonstrated the negative health impacts of prolonged SB in people with COPD including mortality risk and the development of type 2 diabetes.70 There does not appear to be any published literature specifically exploring the impacts of SB in people with AATD. It is likely that intervention aimed at reducing SB by increasing participation in light-intensity PA is a realistic goal for people with AATD, which may be most applicable for those with marked functional impairment.71 Future research is required to explore behavior change interventions that could enhance PA and reduce SB in people with AATD.
There are a wide range of validated and reliable tools for assessing PA and SB, including self-reported tools such questionnaires and device-based measures such as accelerometry which are routinely used in COPD research.72–74 Given the availability of these tools, it is therefore unclear why researchers chose to use measurement methods which have not been validated or tested for reliability.
The use of these standardized tools is not only important to enhance the quality of the research in the AATD population but also to allow researchers to compare research studies and inform future research and practice. The use of validated and reliable outcome measures is recommended for future studies in this area. The inclusion of validated and reliable subjective measures of PA and SB as well as device-based measures could benefit future randomized controlled trials in AATD.
Strengths and Limitations
This scoping review covered a wide body of literature relating to PA in AATD. It has highlighted some important shortcomings in the AATD literature that if addressed, could improve the treatment of people with AATD. However, some relevant studies could have been excluded from this review as the authors did not distinguish the AATD population from other participants or they could have included people with underlying AATD that has not been diagnosed. Furthermore, some studies included in our review did not clarify if their AATD cohort was comprised of people with severe AATD, moderate AATD, or both. We have included three studies which are most likely duplicates due to reporting in different platforms eg conference and full-text publications with some differences in terms of number of participants and participant characteristics. Given the broad scoping nature of this review, they were included.
Conclusion
Exercise capacity is the most commonly tested intervention and outcome measure in people with AATD. The assessment and use of interventions to enhance PA and reduce SB in AATD is limited. There is not only a need to test these interventions, but future research should use validated and reliable measures of PA and SB in this population.
Disclosure
Professor Noel G McElvaney reports grants from Grifols, Csl Behring; advisory board for vertex and inhibrx, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α 1 -antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610. doi:10.1183/13993003.00610-2017
2. Franciosi AN, Hobbs BD, McElvaney OJ, et al. Clarifying the risk of lung disease in SZ alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2020;202(1):73–82. doi:10.1164/rccm.202002-0262OC
3. McElvaney NG, Stoller JK, Buist AS, et al.; Group1 taDRS. Baseline characteristics of enrollees in the national heart, lung and blood InstituteRegistry of a-,-antitrypsin deficiency*. Chest. 1997;11:395–403.
4. Janoff A, White R, Carp H, Harel S, Dearing R, Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979;97(1):111–136.
5. Lomas DA, Li-Evans D, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357(6379):605–607. doi:10.1038/357605a0
6. Sveger T. Liver disease in alpha 1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294(24):1316–1321. doi:10.1056/NEJM197606102942404
7. Franciosi AN, Carroll TP, McElvaney NG. Pitfalls and caveats in α1-antitrypsin deficiency testing: a guide for clinicians. Lancet Respir Med. 2019;7(12):1059–1067. doi:10.1016/S2213-2600(19)30141-9
8. Caspersen CJ, PowelL KE, Christenson GM. Physical activity, exercise and physical fitness: definitions and distinctions for health-related research. Public Helath Rep. 1985;100(2):154.
9. uk-chief-medical-officers-physical-activity-guidelines; 2019. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf.
10. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
11. Marley J, Tully MA, Porter-Armstrong A, et al. The effectiveness of interventions aimed at increasing physical activity in adults with persistent musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):482. doi:10.1186/s12891-017-1836-2
12. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax. 2013;68:ii1–ii30. doi:10.1136/thoraxjnl-2013-203808
13. Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC
14. Jose A, Ramos TM, de Castro RAS, et al. Reduced physical activity with bronchiectasis. Respir Care. 2018;63(12):1498–1505. doi:10.4187/respcare.05771
15. Nishiyama O, Yamazaki R, Sano H, et al. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir Investig. 2018;56(1):57–63. doi:10.1016/j.resinv.2017.09.004
16. Choate R, Mannino DM, Holm KE, Beiko T, Boyd B, Sandhaus RA. Home-based multicomponent intervention increases exercise activity and improves body mass index: results of a 5-year randomized trial among individuals with alpha-1 antitrypsin deficiency-associated lung disease. Chronic Obstr Pulm Dis. 2021;8(1):25.
17. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi:10.1186/s12966-017-0525-8
18. Green CE, Vayalapra S, Hampson JA, Mukherjee D, Stockley RA, Turner AM. PiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax. 2015;70(10):939–945. doi:10.1136/thoraxjnl-2015-206906
19. Chapman KR, Chorostowska-Wynimko J, Koczulla AR, Ferrarotti I, McElvaney NG. Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent developments and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:419–432. doi:10.2147/COPD.S149429
20. Carone M, Pennisi A, D’Amato M, et al. Physical functioning in patients with chronic obstructive pulmonary disease treated with tiotropium/olodaterol respimat in routine clinical practice in Italy. Pulmon Ther. 2020;6(2):261–274. doi:10.1007/s41030-020-00122-9
21. Wewers MD, Casolaro MA, Sellers SE, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055–1062. doi:10.1056/NEJM198704233161704
22. Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962–969. doi:10.1016/j.rmed.2012.03.016
23. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
24. Wencker M, Konietzko N. Blood gases at rest and during exercise in patients with alpha 1-Pi deficiency. Respir Med. 2000;94(12):1177–1183. doi:10.1053/rmed.2000.0947
25. Perkins JT, Choate R, Mannino DM, Browning SR, Sandhaus RA. Benefits among patients with alpha-1 antitrypsin deficiency enrolled in a disease management and prevention program. Chronic Obstr Pulm Dis. 2016;4(1):56–64. doi:10.15326/jcopdf.4.1.2016.0161
26. Olfert IM, Malek MH, Eagan TML, Wagner H, Wagner PD. Inflammatory cytokine response to exercise in alpha-1-antitrypsin deficient COPD patients ‘on’ or ‘off’ augmentation therapy. BMC Pulm Med. 2014;14(1):106. doi:10.1186/1471-2466-14-106
27. Kohnlein T, Janciauskiene S, Welte T. Diagnostic delay and clinical modifiers in alpha-1 antitrypsin deficiency. Ther Adv Respir Dis. 2010;4(5):279–287. doi:10.1177/1753465810376407
28. Holm KE, Mannino DM, Choate R, Sandhaus RA. Genotype is associated with smoking and other key health behaviors among individuals with alpha-1 antitrypsin deficiency-associated lung disease. Respir Med. 2018;143:48–55. doi:10.1016/j.rmed.2018.08.016
29. Dowson LJ, Newall C, Guest PJ, Hill SL, Stockley RA. Exercise capacity predicts health status in alpha 1-antitrypsin deficiency. Am J Respir Crit Care Med. 2001;163:936–941.
30. Thabut G, Mornex JF, Pison C, et al. Performance of the BODE index in patients with alpha 1-antitrypsin deficiency-related COPD. Eur Respir J. 2014;44(1):78–86. doi:10.1183/09031936.00168113
31. Jarosch I, Gehlert S, Jacko D, et al. Different training-induced skeletal muscle adaptations in COPD patients with and without alpha-1 antitrypsin deficiency. Respiration. 2016;92(5):339–347. doi:10.1159/000449509
32. Cassina PC, Teschler H, Konietzko N, Theegarten D, Stamatis G. Two-year results after lung volume reduction surgery in alpha 1-antitrypsin deficiency versus smoker’s emphysema. Eur Respir J. 1998;12(5):1028–1032. doi:10.1183/09031936.98.12051028
33. Tutic M, Bloch KE, Lardinois D, Brack T, Russi EW, Weder W. Long-term results after lung volume reduction surgery in patients with alpha 1-antitrypsin deficiency. J Thorac Cardiovasc Surg. 2004;128(3):408–413. doi:10.1016/j.jtcvs.2004.03.040
34. Tanash HA, Riise GC, Hansson L, Nilsson PM, Piitulainen E. Survival benefit of lung transplantation in individuals with severe alpha 1-anti-trypsin deficiency (PiZZ) and emphysema. J Heart Lung Transplant. 2011;30(12):1342–1347. doi:10.1016/j.healun.2011.07.003
35. Dauriat G, Mal H, Jebrak G, et al. Functional results of unilateral lung volume reduction surgery in alpha1-antitrypsin deficient patients (1). Int J COPD. 2006;1:201–207.
36. Tanash HA, Riise GC, Ekstrom MP, Hansson L, Piitulainen E. Survival benefit of lung transplantation for chronic obstructive pulmonary disease in Sweden. Ann Thorac Surg. 2014;98(6):1930–1935. doi:10.1016/j.athoracsur.2014.07.030
37. Jarosch I, Hitzl W, Koczulla AR, et al. Comparison of exercise training responses in COPD patients with and without Alpha-1 antitrypsin deficiency. Respir Med. 2017;130:98–101. doi:10.1016/j.rmed.2017.07.009
38. Kenn K, Gloeckl R, Soennichsen A, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–1077. doi:10.1097/TP.0000000000000472
39. Stoller JK, Gildea TR, Ries AL, Meli YM, Karafa MT; National Emphysema Treatment Trial Research Group. Lung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiency. Ann Thorac Surg. 2007;83(1):241–251. doi:10.1016/j.athoracsur.2006.07.080
40. Stoller JK, Aboussouan LS, Kanner RE, et al. Characteristics of alpha-1 antitrypsin-deficient individuals in the long-term oxygen treatment trial and comparison with other subjects with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(12):1796–1804. doi:10.1513/AnnalsATS.201506-389OC
41. Campos MA, Alazemi S, Zhang G, Wanner A, Sandhaus RA. Effects of a disease management program in individuals with alpha-1 antitrypsin deficiency. COPD. 2009;6(1):31–40. doi:10.1080/15412550802607410
42. Heinzelmann I, Gehlert S, Welte T, Bloch W, Janciauskiene S, Kenn K. Differences in training response between patients with alpha-1 antitrypsin deficiency and COPD patients. Eur Respir J. 2015;46(59):161.
43. Czech National Alpha-1 Antitrypsin Registry. Chlumsky (Praha 4, Czech Republic), K. Kusalova (Brno, Czech Republic). 2022:2440.
44. Balbi B, Vitacca M, Piero C, et al. Pulmonary rehabilitation (PR) in patients with COPD due to alpha-1 antitrypsin deficiency (AATD)- 15 years experience at the Mauge.
45. Delage A, Hogarth DK, Zgoda M, Reed M. Endobronchial valve treatment in patients with severe emphysema due to alpha-1 antitrypsin deficiency. Intervent Pulmonol. 2019;2019:1544.
46. Choate R, Holm K, Sandhaus RA. Increase in exercise activities in alpha-1 antitrypsin deficient patients- results of a randomized trial (1). Am J Respir Crit Care Med. 2017;2017:654.
47. Tseng S, Vick L, Sue R, Alalawi R. Exercise ventilation perfusion scan with single photon emission Ct to assess target lobe for endobronchial valve placement. Chest. 2019;156(4):A28. doi:10.1016/j.chest.2019.08.131
48. Menon S, Ahmad M, Khan F, Malik A. Alpha 1 antitrypsin deficiency with normal levels presenting as a diagnostic challenge. Am J Respir Crit Care Med. 2017;24:175.
49. Murray B, Carroll TP, Fee LT, et al. Examining Diffusing Capacity (DLCO) and functional impairment in a population of ZZ AATD individuals. Ir J Med Sci. 2014;183(Suppl 11):533. doi:10.1007/s11845-013-1044-5
50. Durkan E, Carroll T, Moyna NM, McElvaney NG. Exercise capacity may be more strongly associated with health status. Am J Respir Crit Care Med. 2019;2019:25.
51. Heinzelmann GS, Bloch W, Kenn K. Differences in response to pulmonary rehabilitation between COPD patients with and without alpha-1 A.
52. Strange C, Senior RM, Sciurba F, et al. Rationale and design of the genomic research in alpha-1 antitrypsin deficiency and sarcoidosis study. alpha-1 protocol. Ann Am Thorac Soc. 2015;12(10):1551–1560. doi:10.1513/AnnalsATS.201503-143OC
53. Greulich T, Altraja A, Barrecheguren M, et al. Protocol for the EARCO registry: a pan-European observational study in patients with α 1 -antitrypsin deficiency. ERJ Open Res. 2020;6(1):00181–2019. doi:10.1183/23120541.00181-2019
54. Endoscopic lung volume reduction in patients with advanced emphysema due to alpha 1 antitrypsin deficiency. Available from: https://clinicaltrials.gov/ct2/show/NCT01357460.
55. Evaluate efficacy and safety of “Kamada-AAT for Inhalation” in patients with AATD (InnovAATE). Available from: https://clinicaltrials.gov/ct2/show/NCT04204252.
56. Effects of different exercise training modalities in alpha-1 antitrypsin deficiency patients. Available from: https://clinicaltrials.gov/ct2/show/NCT03802357.
57. Zemaira in subjects with emphysema due to alpha 1-proteinase inhibitor deficiency. Available from: https://clinicaltrials.gov/ct2/show/NCT00261833.
58. Environment effect on six-minute walk test performance (6MWTAATD) Available from: https://clinicaltrials.gov/ct2/show/NCT02502201.
59. Effects of pulmonary rehabilitation on skeletal muscle in COPD patients. Available from: https://clinicaltrials.gov/ct2/show/NCT02915614.
60. Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. 29. doi:10.1016/S0140-6736(12)61031-9
61. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–556. doi:10.1097/HCO.0000000000000437
62. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
63. Zwerink M, van der Palen J, van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med. 2013;107(2):242–248. doi:10.1016/j.rmed.2012.09.018
64. Spruit MA, Pitta F, McAuley E, ZuWallack RL, Nici L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–933. doi:10.1164/rccm.201505-0929CI
65. Hakim A, Moll M, Qiao D, et al. Heterozygosity of the alpha 1-antitrypsin Pi*Z allele and risk of liver disease. Hepatol Commun. 2021;5(8):1348–1361. doi:10.1002/hep4.1718
66. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi:10.2337/dc11-1931
67. Dunstan DW, Howard B, Healy GN, Owen N. Too much sitting--a health hazard. Diabetes Res Clin Pract. 2012;97(3):368–376. doi:10.1016/j.diabres.2012.05.020
68. Wu J, Zhang H, Yang L, et al. Sedentary time and the risk of metabolic syndrome: a systematic review and dose-response meta-analysis. Obes Rev. 2022;23(12):e13510. doi:10.1111/obr.13510
69. Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20(8):1160–1171. doi:10.1111/resp.12642
70. McKeough Z, Cheng SWM, Alison J, Jenkins C, Hamer M, Stamatakis E. Low leisure-based sitting time and being physically active were associated with reduced odds of death and diabetes in people with chronic obstructive pulmonary disease: a cohort study. J Physiother. 2018;64(2):114–120. doi:10.1016/j.jphys.2018.02.007
71. Coll F, Cavalheri V, Gucciardi DF, Wulff S, Hill K. In people with COPD, there is limited evidence that exercise training reduces sedentary time, and behavior change techniques are poorly reported: systematic review and meta-analysis. Phys Ther. 2021;101(7). doi:10.1093/ptj/pzab097
72. Liao SY, Benzo R, Ries AL, Soler X. Physical activity monitoring in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2014;1(2):155–165. doi:10.15326/jcopdf.1.2.2014.0131
73. Andersson M, Stridsman C, Ronmark E, Lindberg A, Emtner M. Physical activity and fatigue in chronic obstructive pulmonary disease - a population based study. Respir Med. 2015;109(8):1048–1057. doi:10.1016/j.rmed.2015.05.007
74. Byrom B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials. 2016;47:172–184. doi:10.1016/j.cct.2016.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.