Back to Journals » Journal of Pain Research » Volume 17
Pharmacopuncture Therapy as an Adjunctive Treatment for Patients with Lumbar Spinal Stenosis: A Pragmatic Randomized Controlled Pilot Trial
Authors Oh Y , Han CH , Kim Y , Kim J , Yang C , Choi YE, Kang BK, Kim KH, Yang GY, Lee BR, Kim E
Received 6 October 2023
Accepted for publication 28 January 2024
Published 6 March 2024 Volume 2024:17 Pages 837—849
DOI https://doi.org/10.2147/JPR.S438219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Yoona Oh,1,2,* Chang-Hyun Han,3,4,* Yeonhak Kim,1 Jihun Kim,1 Changsop Yang,3 Young Eun Choi,5 Byoung-Kab Kang,3 Kun Hyung Kim,1,2 Gi Young Yang,1,2 Byung Ryul Lee,1,2 Eunseok Kim1,2
1Department of Acupuncture and Moxibustion Medicine, Pusan National University Korean Medicine Hospital, Yangsan, Republic of Korea; 2Division of Clinical Medicine, School of Korean Medicine, Pusan National University, Yangsan, Republic of Korea; 3KM Science Research Division, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea; 4Korean Convergence Medicine, University of Science & Technology (UST), Campus of Korea Institute of Oriental Medicine, Daejeon, Republic of Korea; 5Clinical Research Coordinating Team, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea
*These authors contributed equally to this work
Correspondence: Eunseok Kim, Division of Clinical Medicine, School of Korean Medicine, Pusan National University, 49, Busandaehak-ro, Mulgeum-eup, Yangsan-si, Gyeongnam, 50612, Republic of Korea, Tel +82 55 360 5950, Fax +82 55 360 5890, Email [email protected]
Purpose: Pharmacopuncture therapy (PPT) combines medicinal extracts with acupuncture and is widely used as an adjunct in clinical practice. This study assessed the safety and feasibility of PPT in addition to conventional Korean Medicine treatment (CKMT), including electroacupuncture, cupping and infra-red, for lumbar spinal stenosis (LSS).
Patients and Methods: Forty patients diagnosed with LSS were randomly assigned to undergo PPT with CKMT (experimental group) or CKMT alone (control group) at a 1:1 ratio, receiving 10 sessions of each intervention over five weeks. The primary clinical outcome was measured using the 100-mm Visual Analog Scale (VAS) for buttock and leg pain five weeks post-treatment. Secondary outcomes included clinically important difference (CID), Zurich Claudication Questionnaire, self-reported walking capacity, Modified–Modified Schober test, EuroQol 5-dimension 5-level questionnaire, and the patient’s global impression of change. The adverse events were assessed at each visit. The analysis of covariance was conducted to compare between two groups.
Results: Intervention completion rates were 95% and 100% in the experimental and control groups, respectively. No statistically significant differences were found between groups regarding the primary outcome (adjusted mean difference: 8.0; 95% confidence interval: − 1.4– 17.4). The mean difference in the 100-mm VAS for low back pain at week 5 (adjusted mean difference: 12.9; 95% confidence interval: 2.4– 23.4) and the proportion of patients who reached the minimum CID was higher in the experimental group than in the control group. However, no significant differences were observed with other secondary outcomes. One patient in the experimental group experienced a systemic skin rash that resolved the same day, whereas the adverse events in the other group were mild and transient.
Conclusion: This trial demonstrated the feasibility of add-on effects and the safety of pharmacopuncture in patients with LSS. Further studies are warranted to evaluate the add-on effects of PPT in treating LSS.
Trial Registration: Clinical Research Information Service (CRIS), KCT0007229; registered on April 26, 2022.
Keywords: lumbar spinal stenosis, pharmacopuncture therapy, add-on effect, conventional Korean medicine treatment, pragmatic randomized pilot trial
Introduction
Lumbar spinal stenosis (LSS) is a degenerative condition of the lumbar spine that is prevalent among geriatrics.1 As the population ages and life expectancy increases, the incidence and burden of LSS rise. According to the Health Insurance Review and Assessment Service of Korea, the number of patients with LSS is 1.7 million, and the associated medical expenditure was 700 billion won in 2021.2 It narrows the spinal canal, compressing the neural elements within the lumbar region. This chronic and progressive condition poses significant challenges, such as discomfort in the back and lower extremities, resulting in gait disturbances.3,4
Surgical intervention for LSS is carefully considered and individualized, except for cauda equina syndrome and severe nerve deficits, owing to the potential risks of increased instability of adjacent vertebrae and persistent pain, even postoperatively.5 Various conservative therapies are used for LSS management; however, recent studies revealed that acupuncture has better analgesic effects than nonsteroidal anti-inflammatory drugs or physical therapy.6,7 Pharmacopuncture therapy (PPT) is a combined technique that involves the infusion of medicinal extracts and acupuncture to achieve a synergistic effect.8,9 In Korean clinical practice, PPT is selectively incorporated into conventional Korean Medicine treatment (CKMT), including acupuncture, electroacupuncture, and cupping for treating musculoskeletal disorders. Common PPTs include bee venom, blood stasis, Hwangryunhaedok-tang, Jinseng, and Hominis placenta.10 Several studies have examined the effectiveness and safety of individual types of pharmacopuncture;11–14 however, no pragmatic clinical trial has evaluated the PPT modality reflecting clinical practice to the best of our knowledge.
This pilot study aimed to assess the feasibility and safety of incorporating PPT with CKMT before conducting a confirmatory clinical trial to validate the add-on effect of PPT in LSS treatment. This study was conducted to determine the possibility of evaluating the addition of PPT to CKMT compared to CKMT alone in improving pain and functional impairment from LSS.
Materials and Methods
Study Design and Participants
Patients diagnosed with LSS identified by the International Classification of Diseases (M48.06) were recruited from the Spine and Joint Center of Pusan National University Korean Medicine Hospital (PNUKH) between April and December 2022. Using a published article’s protocol, the study was designed as a pragmatic, randomized, two-parallel, and sex-stratified pilot clinical trial with a 1:1 allocation ratio.15 This study was approved by the Institutional Review Board of PNUKH (PNUKHIRB 2022-01-002-003) and registered with the Clinical Research Information Service (KCT0007229). We conducted the study in accordance with the Declaration of Helsinki.
Participants were aged 40–80, with LSS signs such as neurogenic claudication and posture-related complaints. The diagnosis was based on a physical examination conducted by certified Korean Medicine doctors, medical history assessment, and imaging evaluation performed by certified radiologists. Individuals with spinal fusion or laminectomy history and those with severe spinal canal defects such as cauda equina syndrome or paralysis were excluded. Before starting the study, all participants voluntarily agreed to the research goals and procedures and signed the informed consent form.
Sample Size Calculation
To ensure robustness in our study design, we conducted a sample size calculation following established guidelines.16 The recommended pilot trial sample sizes per treatment arm range from 75 to 10, depending on the standardized effect size. Specifically, suggested sample sizes for effect sizes categorized as extra small (≤0.1), small (0.2), medium (0.5), or large (0.8) are 75, 25, 15, and 10, respectively.16 Considering our expectation that the effect size ranges between small and medium, we determined a sample size of 20 per group.
Randomization and Blinding
Patients were randomized to the control or experimental group. The randomization sequence was generated by an independent statistician who did not participate in the clinical trial interventions or assessments using SAS® version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA). Sex-stratified randomization was used with a block size of four. The block size was concealed from all participants except the statistician until the study ended. The generated randomization table was securely stored in a locked cabinet by the independent statistician.
Considering that PPT was administered exclusively to the experimental group, the researcher performing PPT, and the patients could not be blinded from its allocation information. Therefore, the researchers conducting the CKMT (a common intervention for both groups) remained blinded to the group information to minimize bias favoring the experimental group. Additionally, an independent researcher performed the PPT. The evaluators were unaware of patient group allocation.
Interventions
Participants assigned to the experimental and control groups were administered PPT in addition to CKMT and CKMT alone, respectively, twice weekly for five weeks.
A professional Korean Medicine doctor with over 10 years of experience administered the PPT (bee venom, Hominis placenta, or bamboo salt) based on clinical features such as spine impairment extent, confirmed via imaging and the pattern of radiating pain in the lower extremities. Each session involved a single pharmacopuncture dose. Pharmacopuncture using 10% sweet bee venom (Kirin Herbal Dispensary, Wonju, Republic of Korea), from which macromolecular substances acting as antigens have been removed, was applied solely to the stenotic lesion level (EX-B2). In cases where participants had hypersensitivity to bee venom, an alternative, such as bamboo salt (Kirin Herbal Dispensary, Wonju, Republic of Korea) or Hominis placenta (Kirin Herbal Dispensary, Wonju, Republic of Korea), was selectively applied. Additional acupoints used in the PPT based on their relevance to symptom management included the GV3, BL23, BL51, BL52, BL32, BL54, GB30, BL39, GB34, BL57, GB39, and Ashi points (Figure 1). The sizes of the pharmacopuncture needles used in the study were: 30 gauge x 12.7 mm, 27 gauge x 38 mm, and 27 gauge x 60 mm. The needle size was selected based on the location of the acupoints. The maximum dose administered per session was 2 mL, which is often used in clinical practice.8
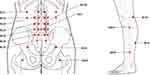 |
Figure 1 Acupoints Used in Pharmacopuncture Therapy. Notes: Essential acupoints depending on individual stenotic level; Additional acupoints depending 470 on individual’s symptom. |
CKMT comprised cupping, acupuncture, electroacupuncture, and infrared irradiation. Cupping was applied to the back for 5 min, followed by acupuncture using 0.25 mm x 40 mm or 0.35 mm x 60 mm needles (Dongbang Acupuncture, Dongbang Medical, Seongnam, Republic of Korea). The essential acupoints used was EX-B2 (lumbar region) and other selective acupoints included the GV3, BL23, BL25, BL51, BL52, BL32, BL54, GB30, BL40, BL57, BL39, BL60, SP9, GB34, GB39, and Ashi points. Subsequently, electroacupuncture was applied to EX-B2 using a low-frequency stimulator (ES-160, ITO Co., LTD, Tokyo, Japan) at an alternating frequency of 2–100 Hz for 20 min. An infrared device (Omega-302, ENS Tech., Gwangju, Republic of Korea) was applied during the treatment retention time. Both groups were allowed to take rescue medication (acetaminophen) if necessary.
Outcome Measurements
Feasibility outcomes included intervention completion, follow-up completion, and clinical outcome measurement completion rates for each group. The primary clinical outcome was the mean change in the 100-mm visual analog scale (VAS)17 score for buttock and leg pain at the primary endpoint (week 5). Secondary outcomes were the clinical relevance,18 Zurich Claudication Questionnaire (ZCQ),19,20 self-reported walking capacity,15 Modified–Modified Schober test,21 EuroQol 5-dimension 5-level questionnaire (EQ-5D-5L),22,23 and patients’ global impression of change (PGIC).24 All outcomes except PGIC were measured at weeks 1, 5, 7, and 13, whereas adverse events (AEs) were documented per visit.
Statistical Methods
An independent statistician conducted the statistical analyses using Statistical Analysis Software version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). The significance level was set at p < 0.05 for two-tailed tests. The full analysis set (FAS) and per-protocol (PP) were defined as the analysis sets. The FAS included all study participants evaluated at least once after randomization, whereas the PP included those who completed the study without major protocol violations and underwent eight treatment episodes. Missing data in the main outcome analysis were imputed using the multiple imputation method.25 For confirmatory analysis of the outcomes, analysis of covariance was performed using the baseline values and sex as the covariates. Repeated-measures analysis of variance was conducted to assess trends over time in both groups. Descriptive statistics was used to present the safety analysis, including all study participants who underwent at least one treatment.
Results
Recruitment and Baseline Characteristics
Forty-nine individuals were assessed for eligibility between April and October 2022. Eight patients did not meet the selection criteria, and one patient withdrew consent immediately before the clinical study owing to uncontrolled wrist pain. Ultimately, 40 patients were randomly assigned to the control (n = 20) and experimental groups (n = 20). Figure 2 illustrates the study progression.
 |
Figure 2 Flow diagram of the study. Abbreviations: CKMT, conventional Korean Medicine treatment; FAS, full analysis set; PP, per protocol; PPT, pharmacopuncture therapy. |
Table 1 summarizes the 40 participants’ demographic and clinical characteristics. A higher proportion of the study participants were female (75%), with a mean age of 64.7 years (standard deviation (SD): 8.41). The average duration of buttock/leg and low back pain was 60.5 months (SD: 57.19) and 89.7 months (SD: 97.11), respectively. The experimental group had longer durations of buttock/leg and lower back pain than the control group; however, these differences were not significant. No significant differences were observed in other demographic or clinical characteristics between both groups.
 |
Table 1 Baseline Demographics and Clinical Characteristics |
Intervention
During the 193 PPT sessions, 59.1% (SD: 2.36), 22.2% (SD: 2.22), and 14.0% (SD: 2.08) of the patients were administered bee venom, Hominis placenta, and bamboo salt, respectively. In the first session, all participants except one with hypersensitivity reactions in the bee venom skin test were administered bee venom pharmacopuncture (Table 2). The initial dose was 0.8–1.0 mL, which was increased to 2.0 mL based on the participant’s response. The dose for each bamboo salt and Hominis placenta session was 2.0 mL. The injections were administered intramuscularly in the EX-B2, except for two sessions where subcutaneous injections as weak stimuli were used because of the participant’s poor condition. Among intramuscular injections, 93%, 4%, and 3% used 27 gauge x 38 mm, 27 gauge x 60 mm, and 30 gauge x 12.7 mm needles, respectively.
 |
Table 2 Pharmacopuncture Therapy Status in the Experimental Group |
Feasibility Outcomes
Intervention completion rates were 95% and 100% in the experimental and control groups, respectively. Among the three participants who did not complete the follow-up, one withdrew consent during the follow-up, and the remaining participants (one each from the control and experimental groups) were excluded because they underwent invasive treatment to relieve LSS symptoms. One participant in the control group violated the study protocol after the last evaluation owing to treatment disclosure during the follow-up. Therefore, 92.5% and 90% were the clinical outcome measurement and the follow-up completion rates, respectively.
Primary Outcomes
At week 5, the observed mean change in buttock/leg pain, measured using the 100-mm VAS, was −31.5 mm (95% confidence interval (CI): −38.2–-16.4) and −24.3 mm (95% CI: −32.2–-16.4) in the experimental and control groups, respectively. The adjusted mean difference between both groups was 8.0 (95% CI: −1.4–17.4), which was not significant (p =0.094) (Table 3).
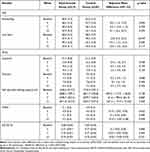 |
Table 3 Observed Outcomes and Adjusted Group Differences |
Secondary Outcomes
The mean difference in the 100-mm VAS for low back pain at week 5 (adjusted mean difference: 12.9, 95% CI: 2.4–23.4) revealed a significant difference (Table 3). In addition, the proportion of patients who satisfied the minimum clinically important difference (CID) (Figure 3) was significantly higher in the experimental group (90%) than in the control group (60%). However, no significant differences existed in other secondary outcomes, including ZCQ, self-reported walking capacity, Modified–Modified Schober test, EQ-5D-5L and PGIC (Table 3 and Figure 4). Furthermore, no significant group-time interaction effects on the measured outcomes were observed (Figure 5).
 |
Figure 3 Clinical relevance. Abbreviation: CID, clinically important difference. Note: *Significant difference (p < 0.05, chi-square test). |
 |
Figure 4 Patient global impression of change. |
Adverse Events
Table 4 describes AEs that occurred during the trial. During the 193 PPT sessions, five (2.6%) reported AEs due to bee venom pharmacopuncture. Four patients experienced four minor and one moderate AE in 114 bee venom sessions. The symptoms included itching and pain at the injection site, fatigue, and myalgia, classified as mild AEs that resolved within 10 days. One patient experienced a systemic skin rash within 30 min of the first bee venom pharmacopuncture administration. Ice packs were applied to the affected areas, and the vital signs were continuously monitored to detect abnormalities. After two hours of observation, the rash pattern was alleviated, and the patient reported no symptoms. Out of 200 sessions in the control group, AEs were reported in four (2.0%), including three mild and one moderate. The mild AEs were myalgia, foot numbness, and calf pain, while the moderate AE was fatigue. All reported AEs resolved without complications or additional interventions. Furthermore, no abnormalities were identified when blood tests were compared before and after the intervention.
 |
Table 4 Summary of Intervention-Related Adverse Events |
Discussion
This study determined the feasibility of investigating the add-on effects of PPT on CKMT in patients with LSS. The intervention completion rates, which measure feasibility, were 95% and 100% in the experimental and control groups, respectively. The follow-up and clinical outcome measurement completion rates were 90% and 92.5%, respectively, indicating that the study was feasible. In the experimental group, the rates of improvement in back pain and satisfaction with the minimum CID at the end of treatment were significantly different from those of the control group. However, no significant differences existed between both groups in terms of other outcomes.
The difference in the mean change of buttock/leg pain between the groups at five weeks revealed a wide SD range (95% CI: −1.4–17.4), which might necessitate a larger sample size for future studies.26 This suggests that the treatment responses may vary significantly among patients. Unmeasured confounding variables such as previous experience with CKMT and expectations of related treatments could influence responses.
In clinical practice, pharmacopuncture is performed based on the patient’s symptoms and disease characteristics. Existing studies have primarily assessed the effects of each pharmacopuncture type rather than evaluating them as a comprehensive LSS treatment.11–14 A randomized controlled study that investigated the effects of bee venom pharmacopuncture on chronic back pain11 revealed that participants administered twice weekly for three weeks experienced significantly improved pain intensity compared to the sham control group at the end of treatment (mean difference: −0.95, 95% CI: −1.89–-0.01). Another clinical study reported a similar outcome as measured by the VAS in participants who received treatment twice weekly for four weeks compared to the sham control group at the end of treatment (mean difference: −13, 95% CI: −22.81–-3.19).12 However, no significant differences were observed in pain intensity between the experimental and control groups during the follow-up period in both studies. Here, we applied 10 treatment sessions and observed no significant differences at two and eight weeks, consistent with findings from previous studies. Nevertheless, the differences between both groups increased over time in the ZCQ, EQ-5D-5L, and self-reported walking capacity (Table 3). Considering that LSS treatment duration is longer than that for chronic back pain; therefore, factors such as Hominis placenta and bamboo salt, in addition to bee venom pharmacopuncture, or an increased number of treatments may have influenced the changes in symptoms and function.
Bee venom, Hominis placenta and bamboo salt pharmacopuncture are commonly used for pain relief and functional recovery in patients with musculoskeletal disorders.9 Bee venom pharmacopuncture is obtained by processing venom extracted from the venom sac of honeybees (Apis mellifera). Melittin, a key component of bee venom, has been reported in previous studies to inhibit signaling pathways such as toll-like receptor (TLR) 2, TLR 4, and cluster of differentiation 14, reducing the expression of inflammatory mediators. Additionally, research has suggested that bee venom, through the regulation of iron metabolism, inhibits M1 (pro-inflammatory) differentiation of macrophages while promoting M2 (anti-inflammatory) differentiation, thus suppressing pain induced by LSS.27,28 Hominis placenta pharmacopuncture has been shown to regulate inflammatory cytokines and reduce the infiltration of inflammatory cells.29,30 It has also been reported to contribute to the regeneration of damaged nerve cells by regulating protein synthesis.31 The study on bamboo salt pharmacopuncture is not as extensive compared to studies on bee venom pharmacopuncture and Hominis placenta pharmacopuncture. However, a study has been reported suggesting that when injecting hypertonic saline into the peripheral coccygeal area in chronic low back pain patients, it may affect the decrease in intradiscal pressure within the peripheral coccygeal area, influencing the relief of nerve entrapment.32 There is also research indicating that injecting saline solution with concentrations ranging from 1.5% to 7.0% could block C-fibers mediating pain.33 Although the bamboo salt pharmacopuncture used in this study is at a concentration of 2% NaCl, which might be expected to have similar effects, directly applying the results from preclinical studies to clinical research has limitations. Therefore, further clinical studies are necessary to verify its effects.
Intervention-related AEs were reported in 2.6% and 2.0% of the sessions in the experimental and control groups, respectively. The collection of AEs in this study utilized open-ended questions instead of a standardized checklist, which may have introduced limitations in the completeness of AE reports.34 However, independent evaluators exhaustively inquired concerning AEs at every visit to ensure a thorough safety assessment. Furthermore, they actively monitored the participants’ progress and provided additional treatments to address their symptoms, further enhancing the safety measures implemented in the study. Despite the negative skin tests, one case of moderate systemic skin rash caused by bee venom was reported. Retrospective chart analysis revealed that the incidence of bee venom-related systemic immune responses is 0.02–0.234% in clinical practice.35,36 A systematic literature review indicated that bee venom pharmacopuncture increased the relative risk of AEs by 2.6 times compared to normal saline injection.37 Furthermore, this study reaffirmed the potential risk of a systemic hypersensitive immune response despite using sweet bee venom and its application.
Here, stratification by sex was implemented, with the male-to-female ratio being equally assigned based on previous research indicating that females exhibit higher sensitivity to back and lower extremity pain when adjusted for age and intervertebral disc degeneration.38 Our study revealed no significant differences in the location, severity, and type of stenosis between both groups. However, the correlation between the degree of stenosis observed in magnetic resonance imaging scans and patient-reported symptoms remains debatable.39 Underlying factors, such as diabetes, low bone density, and high body mass index,40,41 associated with LSS symptom severity, did not differ significantly between the groups. In cases of LSS accompanied by scoliosis, conservative treatment yielded a limited response and increased complaints.42 Therefore, imaging tests were used to identify spinal diseases other than LSS, and no significant differences existed between both groups.
This study had several limitations. First, owing to the characteristics of the pharmacopuncture intervention, blinding the patients was impossible, resulting in a bias in favor of the experimental group that underwent additional treatment. Measures were taken to minimize bias, and the evaluator of the study outcomes was unaware of the AE evaluations, further minimizing the potential impact on the study results to mitigate these issues. Secondly, this study adopted a pragmatic approach and did not select a sham control group for the intervention. Moreover, the intervention method might have been influenced by operator preference, resulting in variations in acupoints, insertion method, and pharmacopuncture type selection. Third, the sample size was small, and the follow-up period was short to comprehensively evaluate our findings.
Despite these limitations, this study is profound, as it is the first preliminary investigation to examine the feasibility of using PPT as an additive treatment for patients with LSS. Furthermore, it demonstrated its feasibility by exhibiting a high intervention and completion rate for clinical outcome measurements with low AE incidence.
Conclusions
This randomized, controlled pilot trial demonstrated that PPT could be incorporated as an add-on treatment in patients with LSS. This study provides insights for future investigations to enhance the efficacy and safety of PPT for LSS treatment. Future research with adequate sample sizes, optimal intervention and follow-up periods, well-designed eligibility criteria, and systematic adverse event response protocols are crucial to evaluate the add-on effects of PPT on LSS.
Abbreviations
AE, adverse event; CI, confidence interval; CKMT, conventional Korean medicine treatment; CT, computed tomography; EQ-5D-5L, EuroQol 5-dimension 5-level questionnaire; FAS, full analysis set; LSS, lumbar spinal stenosis; MMST, Modified-Modified Schober test; PNUKH, Pusan National University Korean Medicine Hospital; PP, per-protocol; PPT, pharmacopuncture therapy; SD, standard deviation; VAS, visual analog scale; ZCQ, Zurich Claudication Questionnaire.
Data Sharing Statement
The datasets are available from the corresponding author on reasonable request.
Acknowledgments
This study was supported by grants from the project of the Korea Institute of Oriental Medicine (KSN1823211).
Author Contributions
All authors made a significant contribution to the current manuscript, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Covaro A, Vila-Canet G, de Frutos AG, et al. Management of degenerative lumbar spinal stenosis: an evidence-based review. EFORT Open Rev. 2016;1(7):267–274.
2. Service HirA. Healthcare bigdata hub of Korea; 2021. Available from: https://opendata.hira.or.kr/.
3. Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358(8):818–825. doi:10.1056/NEJMcp0708097
4. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi:10.1136/bmj.h6234
5. Paul P, Hugh JG, Vishal CG, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. SPINE. 2004;29(17):1938–1944. doi:10.1097/01.brs.0000137069.88904.03
6. Alec C, Lucke-Wold BP. Acupuncture and spinal stenosis: considerations for treatment. Future Integr Med. 2022;1(1):23–31. doi:10.14218/fim.2022.00010
7. Oka H, Matsudaira K, Takano Y, et al. A comparative study of three conservative treatments in patients with lumbar spinal stenosis: lumbar spinal stenosis with acupuncture and physical therapy study (LAP study). BMC Complement Altern Med. 2018;18(1):19. doi:10.1186/s12906-018-2087-y
8. Park J, Lee H, Shin BC, et al. Pharmacopuncture in Korea: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2016;2016:4683121. doi:10.1155/2016/4683121
9. Lee YJ, Shin JS, Lee J, et al. Usage report of pharmacopuncture in musculoskeletal patients visiting Korean medicine hospitals and clinics in Korea. BMC Complement Altern Med. 2016;16(1):292. doi:10.1186/s12906-016-1288-5
10. Park JE, Kim KH, Kang SH, et al. Usage status and satisfaction with pharmacopuncture in Korea: a survey among Korean medicine doctors. Eur J Integr Med. 2019;27(2019):121–130.
11. Seo BK, Han K, Kwon O, et al. Efficacy of bee venom acupuncture for chronic low back pain: a randomized, double-blinded, sham-controlled trial. Toxins. 2017;9(11):361. doi:10.3390/toxins9110361
12. Shin BC, Kong JC, Park TY, et al. Bee venom acupuncture for chronic low back pain: a randomised, sham-controlled, triple-blind clinical trial. Eur J Integr Med. 2012;4(3):e271–e280.
13. Lee YH, Kim JH, Jeong JY, et al. Clinical review of the effects of Chukyu (spinehealing) pharmacopuncture in the treatment of lumbago and skelalgia Patients. J Pharmacopuncture. 2012;15(3):1–6.
14. Song HG, Choi JY, Kang JH, et al. The effect of the acupuncture therapy in combination with Soyeom pharmacopuncture therapy on the improvement of the symptoms of the patients with herniated intervertebral disk of L-spine in his initial stage of hospitalization. J Pharmacopuncture. 2009;12(4):111–118.
15. Oh YN, Han CH, Kim YH, et al. Add-on effect and safety of pharmacopuncture therapy in the treatment of patients with lumbar spinal stenosis. J Acupunct Meridian Stud. 2023;16(1):40–48.
16. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
17. Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(11):1.
18. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
19. Stucki G, Daltroy L, Liang MH, et al. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21(7):796–803. doi:10.1097/00007632-199604010-00004
20. Kim HJ, Lee YK, Kim DO, et al. Validation and cross-cultural adaptation of the Korean version of the Zurich Claudication Questionnaire in patients with lumbar spinal stenosis. Spine. 2018;43(2):E105–E110. doi:10.1097/BRS.0000000000002241
21. Tousignant M, Poulin L, Marchand S, et al. The modified-modified Schober test for range of motion assessment of lumbar flexion in patients with low back pain: a study of criterion validity, intra- and inter-rater reliability, and minimum metrically detectable change. Disabil Rehabil. 2005;27(10):553–559. doi:10.1080/09638280400018411
22. Kim MH, Cho YS, Uhm WS, et al. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res. 2005;14(5):1401–1406. doi:10.1007/s11136-004-5681-z
23. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi:10.3109/07853890109002087
24. Farrar JT, Young JP
25. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi:10.1136/bmj.b2393
26. Karlsson J, Engebretsen L, Dainty K. Considerations on sample size and power calculations in randomized clinical trials. Arthroscopy. 2003;19(9):997–999. doi:10.1016/j.arthro.2003.09.022
27. Lee G, Bae H. Anti-inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules. 2016;21(5):616. doi:10.3390/molecules21050616
28. Kim H, Hong JY, Jeon WJ, et al. Melittin regulates iron homeostasis and mediates macrophage polarization in rats with lumbar spinal stenosis. Biomed Pharmacother. 2022;156:113776. doi:10.1016/j.biopha.2022.113776
29. Yeom MJ, Lee HC, Kim GH, et al. Therapeutic effects of Hominis placenta injection into an acupuncture point on the inflammatory responses in subchondral bone region of adjuvant-induced polyarthritic rat. Biol Pharm Bull. 2003;26(10):1472–1477. doi:10.1248/bpb.26.1472
30. Park JY, Lee J, Jeong M, et al. Effect of Hominis Placenta on cutaneous wound healing in normal and diabetic mice. Nutr Res Pract. 2014;8(4):404–409. doi:10.4162/nrp.2014.8.4.404
31. Seo TB, Han IS, Yoon JH, et al. Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve. Acta Pharmacol Sin. 2006;27(1):50–58. doi:10.1111/j.1745-7254.2006.00252.x
32. Manchikanti L, Rivera JJ, Pampati V, et al. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: a randomized, double-blind trial. Pain Physician. 2004;7(2):177–186.
33. King JS, Jewett DL, Sundberg HR. Differential blockade of cat dorsal root C fibers by various chloride solutions. J Neurosurg. 1972;36(5):569–583. doi:10.3171/jns.1972.36.5.0569
34. Chung KF, Yeung WF, Yu YM, et al. Adverse events related to acupuncture: development and testing of a rating scale. Clin J Pain. 2015;31(10):922–928. doi:10.1097/AJP.0000000000000189
35. Bae IH, Jung WS, Kwon S, et al. Investigation of the adverse events associated with bee venom pharmacopuncture in patients hospitalized in a Korean hospital: a retrospective chart review study. Toxins. 2022;14(10):662.
36. Kim MR, Shin JS, Lee J, et al. Safety of acupuncture and pharmacopuncture in 80,523 musculoskeletal disorder patients: a retrospective review of internal safety inspection and electronic medical records. Medicine. 2016;95(18):e3635.
37. Park JH, Yim BK, Lee JH, et al. Risk associated with bee venom therapy: a systematic review and meta-analysis. PLoS One. 2015;10(5):e0126971. doi:10.1371/journal.pone.0126971
38. Kim HJ, Suh BG, Lee DB, et al. Gender difference of symptom severity in lumbar spinal stenosis: role of pain sensitivity. Pain Physician. 2013;16(6):E715–E723.
39. Burgstaller JM, Schuffler PJ, Buhmann JM, et al. Is there an association between pain and magnetic resonance imaging parameters in patients with lumbar spinal stenosis? Spine. 2016;41(17):E1053–E1062. doi:10.1097/BRS.0000000000001544
40. Lee CK, Choi SK, Shin DA, et al. Influence of diabetes mellitus on patients with lumbar spinal stenosis: a nationwide population-based study. PLoS One. 2019;14(3):e0213858. doi:10.1371/journal.pone.0213858
41. Maeda T, Hashizume H, Yoshimura N, et al. Factors associated with lumbar spinal stenosis in a large-scale, population-based cohort: the Wakayama Spine Study. PLoS One. 2018;13(7):e0200208. doi:10.1371/journal.pone.0200208
42. Wang C, Chang H, Gao X, et al. Risk factors of degenerative lumbar scoliosis in patients with lumbar spinal canal stenosis. Medicine. 2019;98(38):e17177. doi:10.1097/MD.0000000000017177
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

