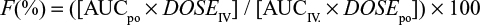Back to Journals » Veterinary Medicine: Research and Reports » Volume 7
Pharmacokinetics of esomeprazole following intravenous and oral administration in healthy dogs
Authors Cook E, Satake N, Sykes B, Bennett E, Mills P
Received 12 May 2016
Accepted for publication 3 June 2016
Published 31 August 2016 Volume 2016:7 Pages 123—131
DOI https://doi.org/10.2147/VMRR.S112643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Emily K Cook, Nana Satake, Ben W Sykes, Emma L Bennett, Paul C Mills
School of Veterinary Sciences, The University of Queensland, Gatton, Queensland, Australia
Abstract: Investigation into the pharmacokinetic profile of esomeprazole was conducted using eight healthy dogs after intravenous (IV) and oral (po) administration in a two-part randomized crossover study. The dogs were fasted for a minimum of 12 hours and then received esomeprazole either intravenously (dose range 0.93–1.48 mg/kg) or orally using an enteric-coated formulation (dose range 0.95–1.50 mg/kg). After a 1-week washout period, the dogs received an alternative treatment. Serial blood samples were collected at predetermined time points, and plasma esomeprazole concentrations were determined by using ultra-high-performance liquid chromatography–mass spectrometry. Noncompartmental pharmacokinetic analyses were performed. Then, the area under the plasma concentration/time curve (AUC) and maximal plasma concentration (Cmax) values were normalized to a 1.0 mg/kg dose of esomeprazole, that is, AUC/dose. Median (range) dose-normalized peak plasma concentration (Cmax) values for the IV and po formulations were 4.06 µg/mL (2.47–4.57 µg/mL) and 1.04 µg/mL (0.31–1.91 µg/mL), respectively. The median (range) time-to-peak concentration (Tmax) for the po formulation was 105 minutes (45–360 minutes). Median (range) plasma terminal half-life (t½) was 45.56 minutes (39.43–64.20 minutes) for the IV formulation and 63.97 minutes (44.02–109.94 minutes) for the enteric-coated po formulation. The median (range) po bioavailability was 63.33% (32.26%–79.77%). Clinically, both po and IV formulations were well tolerated with minimal side effects observed.
Keywords: proton pump inhibitors, gastric ulcers, and oesophagitis
Introduction
Proton pump inhibitors (PPIs) are widely used in the treatment of gastric ulceration and esophageal disease in a number of species, including humans. They are weak bases that accumulate in the parietal cell of the stomach and are rapidly activated in this acidic environment. Irreversible covalent bonding of PPIs to H+/K+-ATPase (the proton pump) inhibits the transportation of H+ ions into the gastric lumen.1–3 This is the final pathway of acid production. They have also been shown to be superior to histamine receptor antagonists in the treatment and prevention of gastric acid-related disorders.4–7 Suppression of acid production is imperative in the management of gastric disease. Augmentation of the normally acidic and proteolytic environment of the stomach prevents further injury and promotes tissue healing.3,4
In human gastric ulcer therapy and gastroesophageal reflux (GER) disease, esomeprazole, the S-enantiomer of omeprazole, is preferred over omeprazole due to its superior pharmacokinetic profile, longer duration of inhibition of acid secretion, and enhanced clinical efficacy.8,9 For example, esomeprazole attains a higher area under the plasma concentration/time curve (AUC) than omeprazole, which translates to higher intragastric pH.1,10 Furthermore and more importantly, esomeprazole has a higher aqueous solubility and stability than omeprazole, so it can readily be formulated as a parenteral solution for intravenous (IV) administration,11 which can be useful for patients who are unable to take medications orally. Effective acid control has been shown with both oral (po) and IV formulations in human studies.12
Proton pump inhibitors are recommended over H2-receptor antagonists in veterinary medicine for the treatment of acid-related disorders; indeed, PPI’s are considered a standard of care for gastrointestinal bleeding in dogs.7 Gastric or duodenal ulceration is well described in canine patients, with cases arising as complications of metabolic disease (eg, hepatic and renal disease, hypoadrenocorticism, and pancreatitis), neoplastic or paraneoplastic processes, inflammation, ischemia, hypotension, or a side effect of drug administration.13–17 Nonsteroidal anti-inflammatory drugs and corticosteroids are two commonly used drug classes that have been well documented to cause gastroduodenal ulceration in dogs.13 GER has been well documented in dogs and is most commonly seen as a complication from anesthesia,18,19 although it can also occur secondary to chronic vomiting, ingestion of caustic substances, and trauma associated with esophageal foreign body obstruction.20 A recent study demonstrated the likely presence of idiopathic pathologic GER in dogs, similar to what is described as GER disease in humans and which has not previously been reported in this species. In this study, 17.4% of dogs presenting with clinical signs of esophageal disease had no underlying cause identified and generally responded well to treatment with omeprazole.21
Omeprazole has been shown to be both well tolerated and clinically efficacious in dogs.4,6,22 Furthermore, successful treatment of Helicobacter spp. infections in dogs has been reported when omeprazole was used in conjunction with clarithromycin and amoxicillin.23 However, there are few studies investigating the use of esomeprazole in dogs although, anecdotally, it is increasingly used by clinicians based on its success in the treatment for human gastric acid-related diseases. Of the few studies on dogs, most have focused on the ability of esomeprazole to reduce acid production rather than on the pharmacokinetic profile of the medication. One study demonstrated successful stomach acid suppression after esomeprazole administration in dogs with gastric fistulas.2 A pH ≥4 was maintained for 59% of a 24-hour period after a single 1.6 mg/kg intraduodenal dose.2 A more recent study on clinical patients assessed the effect of esomeprazole on GER in dogs undergoing general anesthesia.24 It was observed that prior administration of esomeprazole (1 mg/kg, administered twice, 12–18 hours and 1–1.5 hours before general anesthesia) significantly reduced the acid content of GER, if it were to occur.24
To the authors’ knowledge, no previous pharmacokinetic studies of esomeprazole in dogs have been performed. Therefore, the primary objective of this study was to document the pharmacokinetics of IV and enteric-coated esomeprazole in fasted healthy dogs, using an ultra-high-performance liquid chromatography–mass spectrometry (UHPLC-MS) method to measure esomeprazole in canine plasma. A secondary objective was to evaluate the tolerability of each preparation when used clinically.
Materials and methods
Animals
Privately owned domestic mixed-breed dogs (n=8) aged between 1 and 5 years and 21.1–41.4 kg bodyweight were enrolled in this trial. There were five female and three male dogs; all are desexed and consisted of the following breeds: golden retriever (n=1), labrador (n=1), German shepherd (n=1), English setters (n=2), and crossbreed dogs (n=3). All the dogs underwent physical and biochemical (hematology, biochemistry, and urinalysis) examinations, within 1 month of study initiation to assess overall health. All animals were with no history of chronic gastrointestinal disease (eg, vomiting, diarrhea, and anorexia).
Dogs were housed within the University of Queensland Veterinary Teaching Hospital, with standard husbandry practice for bedding, diet (following owners’ instructions), and exercise, unless stated otherwise, and veterinary supervision was provided for the study duration. Ethical clearance was approved by the University of Queensland’s Animal Ethics Committee – approval number: SVS/147/15, and the National Health and Medical Research Council (NHMRC) guidelines followed regarding the animals welfare. Written informed consent was obtained from all owners at the time of enrollment into the study.
Medications
Commercial formulation of esomeprazole Nexium® IV (AstraZeneca Australia Pty Ltd, Sydney, NSW, Australia) containing the active constituent (S)-5-methoxy-2([{4-methoxy-3,5dimethyl-2-pyridinyl}-methyl]sulfinyl)-1 H-benzimidazole (esomeprazole) sodium and esomeprazole RBX (Ranbaxy Australia Pty Ltd, Sydney, NSW, Australia) enteric-coated tablets containing the active constituent esomeprazole magnesium salt was used. Nexium IV powder (40 mg) for IV injection was reconstituted with 5 mL of sterile water for an 8 mg/mL solution 30 minutes prior to administration.
Experimental design
Each dog was administered esomeprazole either intravenously (dose range 0.93–1.48 mg/kg) or orally (dose range 0.95–1.50 mg/kg) in a randomized, crossover study design. Animals were randomized to receive either the po or the IV preparation first. A washout period of at least 1 week was permitted between treatments. All dogs were fasted overnight prior to each drug administration, with access to ad lib water overnight and then again 1 hour after po tablet administration. Prior to each drug administration, each dog was weighed, and an 18G IV catheter was inserted into a cephalic vein for blood collection, whereas the second catheter was inserted into the alternate cephalic vein for IV drug administration.
To achieve a drug dose of approximately 1.0–1.5 mg/kg, each animal received a 20 mg or 40 mg po tablet depending on its bodyweight. The same po dose rate for each dog was used for the IV esomeprazole administered, which was via a bolus delivered over ~10 seconds, as has been described in similar pharmacokinetics studies on humans.12,25 Blood samples of ~2 mL were collected before (T=0) and then at 2 (IV only), 5, 10, 20, 30, 45, 60, 75, 90, 120, 150, 180, 240, 300, 360, 420 (po only), and 480 (po only) minutes after esomeprazole administration and placed in plastic tubes lined with lithium heparin (Vacutainer, BD, North Ryde, NSW, Australia). The samples were centrifuged at 4,000× g for 10 minutes following collection, and plasma was decanted and frozen (−20°C) within 2 hours of collection.
Each dog was monitored closely following drug administration for adverse events. The potential development of clinical signs, including changes in attitude or behavior, vomiting, signs of nausea, number of defecations, and fecal consistency, was recorded during the study in the hospital and also by the owners in the 24 hours following discharge.
Chemicals
Methanol, acetone, acetonitrile, and formic acid of high-performance liquid chromatography–grade reagents from the Optima® range were purchased from Thermo Fisher Scientific Australia Pty Ltd (Melbourne, VIC, Australia). High-performance liquid chromatography–grade water was obtained from Milli-Q water purification system (Advantage A10; Merck Millipore Corporation, Melbourne, VIC, Australia). All other analytical grade reagents were purchased from Sigma Aldrich Corporation (Sydney, NSW, Australia) and Thermo Fisher Scientific Australia Pty Ltd, unless otherwise stated.
Instrumentation
A Shimadzu Nexera™ UHPLC connected to a triple-quadrupole mass spectrometer (Shimadzu LCMS 8030; Shimadzu Corporation, Tokyo, Japan) was used to perform the liquid chromatography–tandem mass spectrometric analyses with the following configuration: Nexera UHPLC consisted of two LC-30AD chromatographic pumps, CTO-30A column oven, SIL-30AC autosampler, and DGU-20A5 degasser; LCMS 8030 was connected to medical grade argon gas (BOC Australia Ltd., Brisbane, QLD, Australia) into the collision cell and a nitrogen generator (NM32L, Peak Scientific Australia Pty Ltd, Melbourne, VIC, Australia) with an electrospray ion source. LabSolutions software (Version 5.6; Shimadzu Corporation, Tokyo, Japan) was used for data processing.
Analytical conditions
The chromatographic separation was performed on a reverse-phase C18 column (Kinetex 1.7 µm, C18, 100Å; size 50×9×2.1 mm; Phenomenex Inc., Lane Cove, NSW, Australia) with a SecurityGuard™ Ultra Guard Cartridge (Phenomenex Inc.). Mobile phases of 0.1% formic acid in Milli-Q water and acetonitrile were used for Pumps A and B, respectively. Analytical conditions were modified from Sykes et al.26 Briefly, the elution was conducted on a binary gradient from 5% of Mobile Phase B to 95% over 2.00 minutes; then, Mobile Phase B was held at 95% over 2.10-minute elapse time before rapidly reducing Mobile Phase B back to 5% at 2.11 minutes and maintained at 5% until pump pressures were returned to stable initial column pressure. The total chromatographic separation was carried out over 3 minutes. Total flow rate of 400 µL/min between Pumps A and B was maintained through the chromatographic separation. Column temperature was maintained at 40°C.
The mass spectrometer was operated using an electrospray ion source in the positive ion mode with the following instrument conditions: desolvation temperature at 100°C, heating block at 400°C, the gas flow at 3 L/min, with the drying gas flow of 15 L/min, and the capillary voltage set at 4,500 V. The collision gas pressure was at 230 kPa, and collision energy was −12 V for both the analyte and internal standards. Dwell time for the transition was set at 100 ms. Optimized multiple-reaction-monitoring transition for esomeprazole was m/z 346.10<198.10. Lansoprazole was used as an internal standard, and multiple-reaction-monitoring transition was m/z 370.10<252.10. Each sample was spiked with 100 ng/mL prior to protein precipitation.
Standards, quality control standards, and sample preparation
Dog plasma was the sample matrix used. Protein precipitation of canine plasma samples (test samples and quality control samples) was carried out by mixing, in equal parts, with a solvent mixture of methanol and acetone (4:1, v/v ratio) by vortexing for 30 seconds. This was followed by centrifugation at 20,000× g for 10 minutes, and samples were rested at 4°C for 30 minutes, followed by another centrifugation at 20,000× g for 10 minutes. The supernatant was transferred to high-performance liquid chromatography vials, and 2-µL samples were injected into the UHPLC column. Standard and quality control samples were assayed for every batch of five sets of test samples.
Stock solutions of Nexium® IV were prepared by dissolving the commercial formulation into Milli-Q water as per the manufacturer’s instructions and then diluted further with solvent mixture of methanol and acetone (4:1, v/v ratio). Quality control samples were independently prepared using blank plasma samples collected from untreated dogs (blank control samples) and spiked with 20 ng/mL for lower quantification, 1,000 ng/mL for mid-range, and 10,000 ng/mL upper limit quantification prior to protein precipitation; therefore, analytes were expected to be 10 ng/mL, 500 ng/mL, and 5,000 ng/mL, respectively.
Esomeprazole was analyzed from protein-precipitated dog plasma. Precipitated proteins were compacted by centrifugation as stated earlier, and the supernatant with analyte was decanted for analyses. The average absolute extraction analyte recovery from dog plasma (n=5) was 109%, 103%, and 95%,26 relative to the drug-free plasma spiked as described.
All samples were processed and analyzed within 2 weeks of the trial. The method was set up using six points of standards, over the linear calibration range of 5–5,000 ng/mL, in precipitated blank plasma matrix. Solvent mixture and dog plasma matrix (1:1 v/v) without drug was used for 0 ng/mL calibration standard and blanks. Reanalysis of calibration samples was carried out at the beginning, middle, and end of each of the three sample batches. Calibration assessments showed a linear response of r2>0.999. The average slopes and intercepts were 0.99563±0.02 and 698.541±6.93 (mean ± standard error of the mean), respectively. Instrument accuracy was calculated as 91.9%, 89.8%, 95.1%, 94.2%, 99.6%, and 99.1% over the six calibration points, ranging from 4.88 (lower limit of quantification) to 5,000 (higher limit of quantification) ng/mL. Internal standard (lansoprazole) recovery after precipitation (ie, extraction efficiency from samples) was 90.2%±0.07 (standard error of the mean) or 5.30435 (standard deviation).
Pharmacokinetic evaluation
Pharmacokinetic parameters including time to maximal concentration (Tmax) and maximal plasma concentration (Cmax) were determined directly from the data. AUC, terminal half-life (t½), volume of distribution (Vz), and clearance (Cl) were calculated by noncompartmental analysis using the software program PKSolver (PK Solver, China Pharmaceutical University, Nanjing, Jiangsu, People’s Republic of China).27 The elimination rate constant (λz) was estimated by log linear regression of concentrations observed during the linear phase of elimination,26 and the corresponding elimination t½ was calculated as 0.693/λz. The AUC0–∞ was calculated using the linear trapezoidal rule and po bioavailability (F%) calculated from the ratio of the areas under the plasma concentration curve (AUC0–∞), after po and IV administration, indexed to their respective dose:
|
AUC0–∞ and Cmax were also dose-normalized to 1 mg/kg (eg, AUC0–∞/dose, Cmax/dose) for both IV and po preparations. Lag time was calculated and defined as the time until esomeprazole was first detected in plasma samples after po administration of enteric-coated esomeprazole tablets. Upon examination of the data, it was determined to be nonnormally distributed; therefore, it was analyzed with nonparametric descriptive statistics (median and range).
Results
All dogs successfully completed the study. One dog experienced very mild clinical signs of nausea ~2–3 hours after po administration, which lasted for ~4 hours. This was observed as eructations and lip-smacking. No vomiting or regurgitation was observed. Neither episodes of diarrhea nor decrease in appetite was reported in any dog during the study period, or in the 24-hour observation period at home. Overall, both po and IV administration of esomeprazole appeared to be well tolerated.
The pharmacokinetics of esomeprazole for the IV and po (enteric-coated tablet) groups are summarized in Tables 1 and 2, respectively. Median (range) plasma concentrations over time for both IV and po esomeprazole groups are shown in Figure 1. The median (range) t1/2 for the IV formulation was determined to be 45.56 (39.43–64.20) minutes, whereas this value was 63.97 minutes after po administration. Median (range) dose-normalized Cmax was 4.06 µg/mL/mg after IV administration and 1.04 µg/mL/mg after po administration. The median dose-normalized AUC0–∞ after IV and po dosing was 246.65 µg/mL*min/mg and 140.57 µg/mL*min/mg, respectively. After po administration of esomeprazole, the median (range) Vz/F_obs was 0.64 (0.32–1.33) L/kg, and median (range) Cl_obs was 4.08 (2.94–5.26) mL/kg/min. The Tmax after po administration was varied, with a median value of 105 minutes and a wide range of 45–360 minutes.
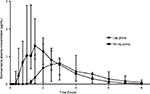
Visual observation of the data demonstrated two dog populations: those that demonstrated lag absorption of the po formulation and those that did not demonstrate a lag in the absorption of the po formulation. Lag times were calculated for each dog, which is defined as the time until esomeprazole was first detected in plasma after po administration. Median (range) lag time for all dogs was 45 minutes (5–75 minutes). Dogs were split into groups: no lag absorption group (Tmax<2 hours, n=4) and lag absorption group (Tmax≤2 hours, n=4), and the median (range) plasma concentration for each group is plotted against time in Figure 2. The median dose-normalized AUC0–∞ for both of these groups was calculated and found to be 128.13 µg/mL*min/mg for the lag absorption group and 195.52 µg/mL*min/mg for the no lag absorption group. Median (range) lag time for no lag absorption was 32.5 minutes (5–75 minutes) and 52.5 minutes (45–75 minutes) for the lag absorption group.
The median bioavailability of the orally administered, enteric-coated esomeprazole tablets was 63.33%. The highest po bioavailability achieved was 79.77% after a single po dosing, whereas the lowest bioavailability achieved was an outlier at 32.26%. The remaining six dogs had more consistent bioavailability ranging from 62% to 72%.
Discussion
This study was the first to evaluate pharmacokinetics of IV and enteric-coated po esomeprazole in healthy dogs. The t½ for esomeprazole was found to be ~45 minutes after IV administration and 64 minutes after po administration. A previous study on omeprazole in dogs demonstrated that the route of administration did not appear to impact the t½, and this was ~60 minutes for both po and IV routes.28 The cause of this longer t½ following po administration could reflect a delayed absorption as seen in some test subjects, with the rate of absorption affecting the elimination rate constant (ie, a flip-flop phenomenon).29 Indeed, in humans, an increase in t½ is observed after repeat administration due to a combination of decreased first-pass effect and decreased systemic clearance.25 Furthermore, omeprazole, and presumably other PPIs, will enhance the extent of their own absorption over several days, as the gastric pH increases.30 Irrespective, the clinical effect of PPIs is significantly longer than such a short t½ would suggest, which is due to covalent bonding of this class of antiacid agents at their receptor.1 Similarly in human medicine, most PPIs have a t½ of approximately 1 hour, yet they are administered once daily.
An advantage of the current study was the sensitive technique used to analyze esomeprazole in canine plasma. Although similar methods have been reported previously for humans31 and the dog,31,32 they have not been used for pharmacokinetic studies, and greater advantage is the lower limit of detection and high precision and accuracy available with UHPLC-MS. Consequently, the plasma concentration–time curves could be better characterized; therefore, a more accurate determination of the pharmacokinetic parameters became possible.
The po bioavailability of esomeprazole in the dog was relatively high (median 63.33%), which is substantially higher than that of omeprazole (15%28) in this species. This was a useful outcome and suggests that the po route of administration is viable and effective. However, direct comparison is difficult, as omeprazole was administered in a suspension with Methocel™ in the aforementioned study.28 This may have provided some buffering to the solution; however, it is unlikely to have given the same protection to the medication as enteric coating.28 This same study found that the bioavailability of omeprazole increased to 70% when directly administered into the duodenums of dog using Heidenhain pouches. Enteric coating of esomeprazole in the current study was therefore likely to be a major contributor to the high and clinically useful po bioavailability of esomeprazole, even after a single dose, and further emphasized the need for enteric coating of PPIs. Moreover, this bioavailability is expected to increase since, as reported previously, po absorption may increase over time for this class of drugs, as gastric pH rises in response to the drug.30 For example, the po bioavailability of esomeprazole in humans was initially ~50% after a single dose,33 which increased to ~68% by day 525 and 80% by day 734 following repeated administration; however, further studies are required to confirm the changes in po bioavailability with repeated dosing in dogs.
Despite this clinically favorable po bioavailability outcome for esomeprazole in dogs, there was still marked variability in Tmax with po dosing, ranging between 45 and 360 minutes (median of 105 minutes). This parameter in humans varies when using PPIs depending on the drug formulation and the presence of food,1 yet all dogs were fasted for a minimum of 12 hours prior to administration. This was not directly monitored by the investigators, and it is possible that some subjects may have scavenged food overnight, unbeknown to their owners. Much older studies on omeprazole in dogs showed delayed absorption of enteric-coated granules versus liquid suspensions (with Methocel), with Tmax reported to be 60–120 minutes in dogs, given enteric-coated preparations (granules).35 Enteric-coated tablets, rather than enteric coated granules were used in the current study, and dissolution of the whole tablet may have been a major factor in the notable lag time observed in some dogs. Long lag times can be observed in some patients after po administration of enteric-coated tablets due to delays in gastric emptying and the time in which the protective coating dissipates and the core medication is released.36 Irrespectively, there is a clear relationship between AUC and inhibition of stomach acid secretion,33,37 and this pharmacokinetic parameter is considered most relevant to determine the clinical efficacy of PPIs.38 A higher AUC equates to greater systemic exposure of the drug, and therefore, there is greater availability to bind to proton pumps in the parietal cells.39 Similarly in dogs, gastric acid inhibition was related to the total dose and AUC, not to the shape of the curve or Cmax.40 All dogs except one achieved a bioavailability of >60% after a single po dose, despite the presence of delayed absorption in some. The clinical significance of this may be important in which rapid gastric acid inhibition is preferred (ie, administration before a general anesthetic). IV preparations would have a more predictable and prompt onset of action. Furthermore, as observed earlier, repeated dosing increases the AUC due to decreased plasma clearance and a reduction in first-pass metabolism, leading to greater gastric acid inhibition.25,37 A similar effect may occur with repeated dosing of po esomeprazole in dogs; however, further studies would be required to confirm this.
One possible explanation for any interindividual variability in pharmacokinetic parameters for esomeprazole could relate to differences in the rate and extent of metabolism. In humans, omeprazole and to a lesser extent esomeprazole are extensively metabolized by CYP2C19 and CYP3A4, both parts of the cytochrome P450 system. Mutations to the CYP2C19 gene can lead to variations in the pharmacokinetics and the pharmacodynamics of these PPIs, and humans who rapidly metabolize these drugs are termed “extensive metabolizers.”33 Significant variability in the po bioavailability of omeprazole has been reported in the horse, and polymorphisms of the CYP2C19 gene in this species have been suggested as a potential cause.26 However, it has been reported that esomeprazole appears to be less sensitive to the CYP2C19 polymorphism and therefore provides more consistent suppression of gastric acid in humans.1 The role of CYP2C19 gene in the metabolism of PPIs in the dog is unknown, but variability in expression could potentially explain the low po AUC and subsequently low bioavailability (~32%) of esomeprazole in one of the dogs in the current study.
A further promising outcome from the current study was that esomeprazole appeared to be well tolerated in the dog following administration by either route. In humans, adverse effects are uncommon10,12 and generally relate to gastrointestinal complaints, including nausea, diarrhea, and abdominal pain.33,41 Mild diarrhea has also been reported to affect 10% of the dogs following IV esomeprazole,24 but it was not observed in the current study. One patient was observed to have mild and transient nausea, which included lip-smacking and eructations, but this was quickly resolved. This lack of observed adverse effects of esomeprazole by either route is a further clinical advantage over omeprazole, since a commercially available IV formulation of esomeprazole is available, which is desirable for inpatients when po products are not practical (eg, unconscious or vomiting).
Limitations of this study include the small number of cases. Furthermore, dogs enrolled in this particular study did not have a history of gastrointestinal disease, and future studies would be required to investigate the potential impact of gastrointestinal disease on the pharmacokinetics of this medication. In humans, the pharmacokinetic profile of po esomeprazole in patients with GER disease was similar to that in healthy volunteers.10 The effect of feeding was also not evaluated in this study. It has been reported in humans that feeding a high-fat meal within 15 minutes post-esomeprazole administration greatly reduces bioavailability.42 Interestingly, while another human study also found feeding reduced both AUC and Cmax, the percentage of time pH >4 was not affected over the 5-day study duration.43
In conclusion, we performed the first pharmacokinetic study of po and IV esomeprazole in healthy, fasted dogs. This study demonstrated good po bioavailability with no side effects after IV dosing and minimal side effects after po dosing. Future work should consider the effect of disease, feeding, repeated dosing, and different po preparations on po bioavailability in dogs.
Acknowledgment
This study was funded by the School of Veterinary Science Donor/Bequest Research funding scheme (No BEQECO2014).
Disclosure
The authors report no conflicts of interest in this work.
References
Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19(1):25–35. | ||
Kodama K, Fujisaki H, Kubota A, et al. E3710, a new proton pump inhibitor, with a long-lasting inhibitory effect on gastric acid secretion. J Pharmacol Exp Ther. 2010;334(2):395–401. | ||
Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260(25):13681–13684. | ||
Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med. 2011;25(1):47–54. | ||
Bersenas AM, Mathews KA, Allen DG, Conlon PD. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res. 2005;66(3):425–431. | ||
Williamson KK, Willard MD, Payton ME, Davis MS. Efficacy of omeprazole versus high-dose famotidine for prevention of exercise-induced gastritis in racing Alaskan sled dogs. J Vet Intern Med. 2010;24(2):285–288. | ||
Tolbert MK, Odunayo A, Howell RS, Peters EE, Reed A. Efficacy of intravenous administration of combined acid suppressants in healthy dogs. J Vet Intern Med. 2015;29(2):556–560. | ||
Johnson DA. Review of esomeprazole in the treatment of acid disorders. Expert Opin Pharmacother. 2003;4(2):253–264. | ||
Nichita C, Abdou AE, Maerten P, et al. A single dose of intravenous esomeprazole decreases gastric secretion in healthy volunteers. Aliment Pharmacol Ther. 2009;30(10):1022–1029. | ||
Lind T, Rydberg L, Kyleback A, et al. Esomeprazole provides improved acid control vs. omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14(7):861–867. | ||
Möschwitzer J, Achleitner G, Pomper H, Müller RH. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58(3):615–619. | ||
Wilder-Smith CH, Bondarov P, Lundgren M, et al. Intravenous esomeprazole (40 mg and 20 mg) inhibits gastric acid secretion as effectively as oral esomeprazole: results of two randomized clinical studies. Eur J Gastroenterol Hepatol. 2005;17(2):191–197. | ||
Stanton ME, Bright RM. Gastroduodenal ulceration in dogs. Retrospective study of 43 cases and literature review. J Vet Intern Med. 1989;3(4):238–244. | ||
Henderson AK, Webster CR. Disruption of the gastric mucosal barrier in dogs. Compend Contin Educ Vet. 2006;28:340–353. | ||
Cariou M, Lipscomb VJ, Brockman DJ, Gregory SP, Baines SJ. Spontaneous gastroduodenal perforations in dogs: a retrospective study of 15 cases. Vet. Rec. 2009;165(15):436–441. | ||
Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin of North Am Small Anim Pract. 2012;42(4):669–692, vi. | ||
Weisse C, Berent AC, Todd K, Solomon JA, Cope C. Endovascular evaluation and treatment of intrahepatic portosystemic shunts in dogs: 100 cases (2001-2011). J Am Vet Med Assoc. 2014;244(1):78–94. | ||
Panti A, Bennett RC, Corletto F, Brearley J, Jeffery N, Mellanby RJ. The effect of omeprazole on oesophageal pH in dogs during anaesthesia. J Small Anim Pract. 2009;50(10):540–544. | ||
Sellon RK, Willard MD. Esophagitis and esophageal strictures. Vet Clin North Am Small Anim Pract. 2003;33(5):945–967. | ||
Leib MS, Dinnel H, Ward DL, Reimer ME, Towell TL, Monroe WE. Endoscopic balloon dilation of benign esophageal strictures in dogs and cats. J Vet Intern Med. 2001;15(6):547–552. | ||
Munster M, Horauf A, Lubke-Becker A, Grest P, Rutten M. Idiopathic esophagopathies resembling gastroesophageal reflux disease in dogs. Tierärztl Prax Ausg K Kleintiere Heimtiere. 2013;41(3):173–179. | ||
Davis MS, Willard MD, Nelson SL, et al. Efficacy of omeprazole for the prevention of exercise-induced gastritis in racing Alaskan sled dogs. J Vet Intern Med. 2003;17(2):163–166. | ||
Mirzaeian S, Sarachahi AA, Shojaee Tabrizi A, Derakhshandeh A. Eradication of gastric Helicobacter spp. by triple therapy in dogs. Vet Med (Praha) 2013;58(11):582–586. | ||
Zacuto AC, Marks SL, Osborn J, et al. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med. 2012;26(3):518–525. | ||
Hassan-Alin M, Andersson T, Bredberg E, Rohss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56(9–10):665–670. | ||
Sykes BW, Underwood C, McGowan CM, Mills PC. Pharmacokinetics of intravenous, plain oral and enteric-coated oral omeprazole in the horse. J Vet Pharmacol Ther. 2015;38(2):130–136. | ||
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. | ||
Larsson H, Carlsson E, Junggren U, et al. Inhibition of gastric acid secretion by omeprazole in the dog and rat. Gastroenterology. 1983;85(4):900–907. | ||
Toutain PL, Bousquet-Melou A. Plasma terminal half-life. J Vet Pharmacol Ther. 2004;27(6):427–439. | ||
Andersson T, Andren K, Cederberg C, Lagerstrom PO, Lundborg P, Skanberg I. Pharmacokinetics and bioavailability of omeprazole after single and repeated oral administration in healthy subjects. Br J Clin Pharmacol. 1990;29(5):557–563. | ||
Hultman I, Stenhoff H, Liljeblad M. Determination of esomeprazole and its two main metabolites in human, rat and dog plasma by liquid chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(2):317–322. | ||
Yang X, Ma Q. Determination of esomeprazole in dog plasma by LC-MS-MS. J Pharmaceut Prac. 2013;(1):61–63. | ||
Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64(10):935–951. | ||
Simon B, Muller P, Pascu O, et al. Intra-oesophageal pH profiles and pharmacokinetics of pantoprazole and esomeprazole: a crossover study in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2003;15(7):791–799. | ||
Larsson H, Mattsson H, Carlsson E. Gastric acid antisecretory effect of two different dosage forms of omeprazole during prolonged oral treatment in the gastric fistula dog. Scand J Gastroenterol. 1988;23(8):1013–1019. | ||
Nerella NG, Block LH, Noonan PK. The impact of lag time on the estimation of pharmacokinetic parameters. I. One-compartment open model. Pharmaceut Res. 1993;10(7):1031–1036. | ||
Andersson T, Rohss K, Bredberg E, Hassan-Alin M. Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Aliment Pharmacol Ther. 2001;15(10):1563–1569. | ||
Andersson T. Single-isomer drugs: true therapeutic advances. Clinical Pharmacokinetics. 2004;43(5):279–285. | ||
Andersson T, Hassan-Alin M, Hasselgren G, Rohss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clinical Pharmacokinetics. 2001;40(6):411–426. | ||
Larsson H, Mattson H, Sundell G, Carlsson E. Animal pharmacodynamics of omeprazole. A survey of its pharmacological properties in vivo. Scand J Gastroenterol Supp. 1985;108:23–35. | ||
Metz DC, Miner PB, Heuman DM, Chen Y, Sostek M. Comparison of the effects of intravenously and orally administered esomeprazole on acid output in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005;22(9):813–821. | ||
Sostek MB, Chen Y, Andersson T. Effect of timing of dosing in relation to food intake on the pharmacokinetics of esomeprazole. Br J Clin Pharmacol. 2007;64(3):386–390. | ||
Junghard O, Hassan-Alin M, Hasselgren G. The effect of the area under the plasma concentration vs time curve and the maximum plasma concentration of esomeprazole on intragastric pH. Eur J Clin Pharmacol. 2002;58(7):453–458. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.