Back to Journals » Infection and Drug Resistance » Volume 16
Pharmacokinetics of Antituberculosis Drugs in Plasma and Cerebrospinal Fluid in a Patient with Pre-Extensive Drug Resistant Tuberculosis Meningitis
Authors Liang Z , Liao W , Chen Q, Li H , Ye M, Zou J, Deng G , Zhang P
Received 13 December 2022
Accepted for publication 6 March 2023
Published 23 March 2023 Volume 2023:16 Pages 1669—1676
DOI https://doi.org/10.2147/IDR.S401281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zhilin Liang,1,* Weiming Liao,2,* Qifu Chen,3 Hui Li,1 Meiling Ye,1 Jin Zou,4 Guofang Deng,1 Peize Zhang1
1Department of Pulmonary Medicine & Tuberculosis, The Third People’s Hospital of Shenzhen, National Clinical Research Center for Infectious Disease, Southern University of Science and Technology, Shenzhen, People’s Republic of China; 2Department of Thoracic Oncology, Jiangxi Provincial Cancer Hospital, Nanchang, People’s Republic of China; 3Department of Neurosurgery, The Third People’s Hospital of Shenzhen, National Clinical Research Center for Infectious Disease, Southern University of Science and Technology, Shenzhen, People’s Republic of China; 4Department of Clinical Laboratory, The Third People’s Hospital of Shenzhen, National Clinical Research Center for Infectious Disease, Southern University of Science and Technology, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peize Zhang; Guofang Deng, Tel +8613509650204 ; +8613530027001, Email [email protected]; [email protected]
Abstract: Drug-resistant tuberculous meningitis (TBM) is the most devastating and critical form of extrapulmonary tuberculosis. Here, we present a case of a 45-year-old male with pre-extensive drug-resistant tuberculosis meningitis (pre-XDR-TBM). He underwent emergency surgery for the long-tunneled external ventricular drainage (LTEVD). Molecular test and phenotypic drug sensitivity test (DST) of Mycobacterium tuberculosis in cerebrospinal fluid (CSF) showed that the isolate was resistant to both rifampin and fluoroquinolones. An anti-tuberculous regimen of isoniazid, pyrazinamide, cycloserine, moxifloxacin, clofazimine, and linezolid was tailored accordingly. We monitored the drug concentration in his plasma and CSF before (at 0-hour) and after anti-TB drugs administration (at 1-hour, 2-hour, 6-hour, and 12-hour) on 10th day after treatment initiation. We hope to provide reference values of drug exposures in plasma and CSF for patients with pre-XDR-TBM.
Keywords: pre-extensive drug resistant TB, tuberculous meningitis, pharmacokinetics, long-tunneled external ventricular drain, drug concentration
Introduction
Tuberculosis (TB) has afflicted humans since primordial times. In 2020, about ten million new TB cases and 1.4 million TB-related deaths were registered.1,2 Tuberculous meningitis (TBM) is the most devastating form of extrapulmonary tuberculosis, which accounts for 1–5% of all TB cases.3,4 The highest risk populations are children under 5-years, people living with HIV and immunocompromised individuals.5–7 Delayed diagnosis and treatment of TBM can result in over 50% fatality and severe neurological sequelae.8 Despite advances in anti-TB chemotherapy, mortality from TBM remains unacceptably high: up to 50% in patients with HIV-1 co-infection and 19.3% among children.9–11 The prevalence of rifampicin/multidrug-resistant tuberculous meningitis (RR/MDR-TBM) shows an increasing trend globally, the effect of which is catastrophic on prognosis with a mortality >80%.12–14
There is no optimal regimen for the treatment of pre-extensive drug resistant tuberculous meningitis (pre-XDR-TBM) as yet. Regimens are composed in accordance to the WHO’s disease management guideline for pulmonary drug-resistant TB. It is pivotal for drugs to have a good penetration to the brain to be effective for the treatment of TBM. There are only a few published clinical reports on tailored drug-resistant TBM regimen and their therapeutic drug concentration in the plasma and cerebrospinal fluid (CSF).15–17 Here, we present a case of pre-XDR-TBM with therapeutic monitoring of drug concentration in the CSF and plasma during treatment. We hope to provide more information on the effective agents and dosage references for treatment of pre-XDR-TBM.
Case Presentation
A 45-year-old Chinese male suffered with fever and in comatose was transferred to our hospital (The Third People’s Hospital of Shenzhen) for specialist treatment on December 14, 2021 (day 1). His medical records showed that he had gradually aggravated headache, nausea, fatigue, and fever of a 38.5–39.0 °C range for more than 2 weeks. He sought medical attention for his symptoms at a local clinic two weeks prior (December 1, 2021). His blood routine examination, rapid antigen test for type A/B flu virus, and routine urine examination were all normal. He was prescribed an empirical antibiotic therapy with Levofloxacin and Cefuroxime for five days. However, his fever, headache, and fatigue persisted, and his consciousness worsened. He is a smoker but does not consume alcohol. He has no history of chronic disease. He has no recent travelling history in the past 6 months. He was admitted to a local hospital and underwent a lumbar puncture (December 13, 2021). His CSF examination showed a prominent elevated protein level (2155 mg/dl; normal: 15–45 mg/dl) and a low glucose level (0.85 mmol/L; normal: 2.22–3.89 mmol/L). Chest CT showed nodules with calcification on the upper right lung, and consolidations in both lower lobes (Figure 1 A, B&C). Magnetic resonance imaging (MRI) showed ventriculomegaly, meningeal enhancement and hydrocephalus (Figure 2A-D). A diagnosis of TBM was highly suspected and he was transferred to our hospital. Upon admission, his physical examination showed body temperature of 37.1°C, pulse rate of 166 beats/min, breathing rate of 52 count/min, blood pressure of 159/119 mmHg, estimated body weight of 85 kg, and height of 180 cm. Neurological examination revealed confusion, neck stiffness, and positive Kernig sign with no motor, sensory, or cerebellar deficits. Laboratory findings in peripheral blood were as below: WBC 17.07×109/L, CRP 29.12 mg/L, ESR 59mm/h, normal electrolyte and normal liver and kidney function. Lumbar puncture was also performed at admission, which showed a CSF pressure of >330 mmH2O. CSF examination revealed WBC 375×106/L (reference: 0–8 ×106/L), protein 2610 mg/dl (reference: 15–45 mg/dl), glucose 1.95 mmol/L (reference: 2.22–3.89 mmol/L). The simultaneous serum glucose level was 9.4 mmol/L. The patient was classified as grade 7 in the Glasgow Coma Scale (E2V2M3) and grade 4 in the Vellore grading system for tuberculous meningitis and hydrocephalus (TBMH). The patient underwent an emergency surgery of long-tunneled external ventricular drain (LTEVD) and Ommaya reservoir implantation under general anesthesia to relieve elevated intracranial pressure (Figure 3). GeneXpert MTB/RIF results of CSF were positive (semi-quantitative result: Medium) for TB-DNA with resistance to rifampicin. Antituberculosis treatment was commenced with isoniazid 800 mg/d, pyrazinamide 1500 mg/d, cycloserine 500 mg/d, moxifloxacin 400 mg/d, clofazimine 100 mg/d, and linezolid 600 mg twice a day. Dexamethasone 10mg per day was prescribed according to treatment guidelines. All the antituberculosis drugs and corticosteroids were administered while fasting. The patient’s mental and athletic ability improved gradually. After ten days of treatment (December 23, 2021), we hypothesized that the drug concentration had reached a steady state. We monitored the drug concentration in his plasma and CSF before (at 0-hour) and after drugs administration (at 1-hour, 2-hour, 6-hour, and 12-hour) using High-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS, AB SCIEX 4500 Series of Instruments, Canada).18–20 Venous blood was collected and CSF was collected through LTEVD simultaneously. Pharmacokinetics of antituberculosis drugs in the plasma and CSF is shown in Figure 4. The CSF-to-plasma ratios, Cmax, Cmax/MIC in plasma and CSF were shown in Table S2. The maximum concentration of all anti-TB drugs met the expected concentration in plasma and in CSF except of moxifloxacin and clofazimine. As such, no adjustment was made to the dosage and composition of the antituberculosis regimen except that we lowered the dose of linezolid to 600 mg/d two weeks after treatment initiation and lowered the isoniazid dose to 600 mg/d.
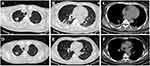 |
Figure 1 Chest CT scan at admission (A-C) and 2 months after antituberculosis therapy (D-F). |
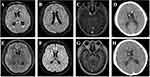 |
Figure 2 Brain MRI and CT scan at admission (A-D) and 2 months after antituberculosis therapy (E-H). |
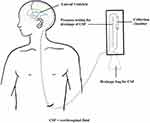 |
Figure 3 Long-tunneled external ventricular drain. |
 |
Figure 4 Concentration of various antituberculosis agents in the plasma and cerebrospinal fluid across time. |
The LTVED was removed on January 03, 2022 (day 21). The patient was no longer bed-bound and started doing some step-by-step mobility activities. Whole-genome sequencing (WGS) of strain from CSF showed rpoB (S450L) and gyrA (D94G) mutation which confirmed that the isolate was resistant to both rifampin and fluoroquinolones. The diagnosis of pre-XDR-TBM was confirmed. Accordingly, moxifloxacin was removed from his regimen. Isoniazid was kept in the anti-TB regimen as WGS of our patient’s CSF showed isoniazid susceptibility. The patient’s condition gradually improved, and he was discharged after 36 days (January 19, 2022) in the hospital. He was in good status without any neurological sequelae. CSF phenotypic DST of Mycobacterium tuberculosis on day 45 further confirmed resistance to rifampin and fluoroquinolones. The Minimum inhibitory concentration (MIC) values for anti-TB drugs were determined except for linezolid and clofazimine (Table S1). After two months of antituberculosis therapy, brain MRI and CT scan showed a remissive state of ventriculomegaly, meningeal enhancement, and hydrocephalus (Figure 2E-H). Chest CT showed that his lung lesions improved (Figure 1D-F). Laboratory results of his CSF over the course of hospitalization are shown in Table 1. The timeline depicting major clinical events is shown in Figure 5. Throughout the whole anti-TB treatment phase, we monitored his hepatic function regularly and it turned out normal. As of press time, the patient’s anti-TB treatment is still ongoing. He is in good physical condition with no disability. Anemia and peripheral neuritis were reported at the 6th month of anti-TB treatment and resolved after decreased dosage of linezolid to 600mg every other day and isoniazid to 300mg daily. He refused to do the therapeutic drug monitoring (TDM) so we could not get more details of his anti-TB drugs’ concentration in plasma or CSF.
 |
Table 1 Cerebrospinal Fluid Biochemical Testing Results |
 |
Figure 5 Timeline of major clinical events. |
Discussion
TBM is the most life-threatening extrapulmonary tuberculosis due to its high mortality.21 Early diagnosis and prompt initiation of TB treatment offer the best chance of survival and a good neurological outcome.22 Drug-resistant strains have further increased the challenge in the treatment of TBM and resulted in a high rate of fatality or sequela of severe disabilities.23 Current treatment recommendations for drug-resistant TBM are extrapolated from pulmonary TB disease management guidelines due to a lack of adequate supporting TBM clinical trial data.
Our patient was in a critical situation at admission. Emergency surgery of LTEVD and Ommaya reservoir implantation under general anesthesia were immediate steps taken to save his life. LTEVD reduces the risk of infections because external drainage catheter is tunneled underneath the skin to a site distant from the ventricle.24,25 The rapid molecular detection demonstrating RIF and FQ resistance confirmed the diagnosis of pre-XDR-TBM and effective anti-TB regimen increased the chance of successful control of mycobacteria. Moreover, we monitored the drug concentration in the CSF of anti-TB drugs, which provided a reference for therapeutic regimen design and dosage adjustment. Our findings presented pharmacokinetic data of a newly implemented second-line antituberculosis drug in the CSF for the treatment of drug-resistant TBM.
Cycloserine is currently listed as a class B drug for use in long-duration regimens for multidrug-resistant pulmonary TB and it is a potent agent for the treatment of TBM given its high central nervous system (CNS) penetration property.26 Existing TDM studies reported that cycloserine demonstrates sufficient bactericidal concentration in the CSF. In this case, cycloserine reached a maximum concentration of 20.62 ug/mL in the CSF and 36.28 ug/mL in plasma 2 hours after administration. Consistent with previous studies, we demonstrated that CSF-to-plasma ratio of cycloserine was about 60% and it has high penetration into the CSF.27,28
Pyrazinamide is being used worldwide as a standard first-line agent in the treatment of TBM because of its good penetration into the blood–brain barrier (BBB).10,29 The targeted Cmax of pyrazinamide in the plasma is between 20 and 60 mg/L.30 Cmax >45 mg/L has been shown to be associated with culture conversion and favourable treatment outcome. In this case, pyrazinamide reached Cmax 56.374 ug/mL in plasma 1 hour after administration and 45.227 ug/mL in the CSF 6 hours after administration. CSF-to-plasma ratio was more than 90%. Though a longer penetration time into the CSF was observed and no MIC of pyrazinamide was provided due to the difficulty of performing phenotypic susceptibility testing with pyrazinamide, we believed that pyrazinamide exposure was enough in plasma and CSF.
Linezolid is one of the key anti-tuberculosis agents for the treatment of MDR/XDR/RR-TB. The WHO revised the groupings of TB medicines and reclassified it as a group A drug for MDR/XDR-TB in 2018.31 Previous literatures reported that linezolid has 70–80% CNS penetration with variation among different individuals.16,32,33 In our patient, the linezolid dose was 600mg twice daily when treatment was initiated. The CSF-to-plasma ratio (calculated AUC0-12 in CSF and plasma) was 0.58. The time from drug administration to achieving peak concentration was 2 hours in both CSF and plasma. The Cmax was 31.808 mg/L in the plasma and 15.727mg/L in the CSF. Though both Cmax in plasma reached expected Cmax (12–26 mg/L),30 the drug exposure in CSF was only about half of that in plasma. These remind us that TDM of linezolid in the CSF is crucial for MDR/XDR-TBM patients in achieving effective bactericidal activity and mitigating possible induced drug resistance. But when anemia and peripheral neuritis were reported, we contributed these side effects to linezolid. After lowering linezolid to 600mg every other day, the toxicity resolved. As the narrow therapeutic window of linezolid, balancing efficacy and toxicity is always a clinical issue.
Isoniazid is known for its high CNS penetration property and a daily high-dose of 15–20 mg/kg has been recommended by the WHO for the treatment of MDR/XDR-TBM/PTB.34,35 In order to formulate an effective therapeutic regimen, we adopted high-dose isoniazid (800mg/d) and found that its CSF concentration reached a maximum (11.57 ug/mL) 6 hours after administration, which mean about 100% brain penetration and promised an effective bactericidal activity in the brain.29 After WGS of our patient’s CSF showed susceptibility of isoniazid, we lowered the isoniazid dose to 600 mg/d. Peripheral neuropathy is the most common toxicity from long-term use of isoniazid. When peripheral neuritis was reported at the 6th month of anti-TB treatment, we further decreased the dosage of isoniazid to 300mg daily.
Clofazimine is a class B drug in long-duration regimens for MDR/XDR pulmonary TB. Clofazimine is known not to distribute into CSF, there is no published study showed its CSF concentration in humans or animals. In our patient, the plasma concentration has reached a maximum of 0.35 ug/mL 6 hours after administration. However, its concentration in the CSF is below the limit of detection. We found no clofazimine in the CSF. It is believed that only unbound drugs can freely penetrate into an intact blood-brain and/or blood-CSF barrier. Clofazimine has a high plasma protein binding rate of >85%, which can be a principal factor limiting its penetration into the CNS.36 Even the high lipophilicity of clofazimine may be conducive to its CNS penetration, but it does not suffice in overcoming its high plasma protein binding rate and large molecular masses.37 In addition to the high plasma protein binding of clofazimine, human efflux transporters may also limit the drugs penetrating into the brain and CSF.38 CSF concentrations of clofazimine was found to be below the limit of detection across all sampling-time points in our patient. We believed that the use of clofazimine may have no benefit for the treatment of MDR- or pre-XDR-TB meningitis. This is a very important message although this finding requires further investigation.
Due to the difficulty in obtaining plasma and CSF, pharmacokinetics was only performed when the patient with external ventricular drainage. Hence, a full record of changes in CSF and plasma drug concentration over time could not be collected, which is a limitation of our study. We noticed that Tucker et al has adopted positron emission tomography (PET) in their study to reveal 11C-rifampicin drug biodistribution in TBM patients.39 We believe that it might be used as a noninvasive procedure for monitoring therapeutic concentration in CSF for TBM patients in future studies.
In conclusion, we presented a case of a pre-XDR-TBM patient who recovered from a critical disease with timely and effective treatment. We explored the concentration of several anti-tuberculosis drugs in his plasma and CSF to provide drug exposure and treatment strategies references for patients with pre-XDR-TBM, a life-threatening illness.
Ethics Approval and Consent to Participate
Written informed consent for publication of this report was obtained from the patient and his wife. Institutional research board of The Third People’s Hospital of Shenzhen approved the publication of this case.
Acknowledgments
We greatly appreciate our patient and his family for their trust and kind cooperation.
Funding
This work was supported by Summit Plan for Foshan High level Hospital Construction (No. FSSYKF-2020001), the Guangdong Provincial Clinical Research Center for Tuberculosis Project (No. 2020B1111170014) and Project of Shenzhen Third People’s Hospital (No. G2021023, No. G2021022) which are government fund for tuberculosis treatment and control.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642–1656. doi:10.1016/S0140-6736(19)30308-3
2. World Health Organization. Global Tuberculosis Report. Switzerland: World Health Organization; 2021.
3. Donovan J, Thwaites GE, Huynh J. Tuberculous meningitis: where to from here? Curr Opin Infect Dis. 2020;33(3):259–266. doi:10.1097/QCO.0000000000000648
4. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi:10.1016/S1474-4422(13)70168-6
5. du Preez K, Seddon JA, Schaaf HS, et al. Global shortages of BCG vaccine and tuberculous meningitis in children. Lancet Glob Health. 2019;7(1):e28–e9. doi:10.1016/S2214-109X(18)30474-1
6. Cecchini D, Ambrosioni J, Brezzo C, et al. Tuberculous meningitis in HIV-infected and non-infected patients: comparison of cerebrospinal fluid findings. Int J Tuberc Lung Dis. 2009;13(2):269–271.
7. Thwaites GE, Duc Bang N, Huy Dung N, Thi Quy H. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with Tuberculous meningitis. J Infect Dis. 2005;192(12):2134–2141. doi:10.1086/498220
8. Soria J, Metcalf T, Mori N, et al. Mortality in hospitalized patients with tuberculous meningitis. BMC Infect Dis. 2019;19(1):9. doi:10.1186/s12879-018-3633-4
9. Davis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ. The pathogenesis of tuberculous meningitis. J Leukoc Biol. 2019;105(2):267–280. doi:10.1002/JLB.MR0318-102R
10. Wilkinson RJ, Rohlwink U, Misra UK, et al. Tuberculous meningitis. Nat Rev Neurol. 2017;13(10):581–598. doi:10.1038/nrneurol.2017.120
11. Nataprawira HM, Gafar F, Risan NA, et al. Treatment Outcomes of Childhood Tuberculous Meningitis in a Real-World Retrospective Cohort, Bandung, Indonesia. Emerg Infect Dis. 2022;28(3):660–671. doi:10.3201/eid2803.212230
12. Thwaites GE, Lan NT, Dung NH, et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192(1):79–88. doi:10.1086/430616
13. Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–1751. doi:10.1056/NEJMoa040573
14. Seddon JA, Visser DH, Bartens M, et al. Impact of drug resistance on clinical outcome in children with tuberculous meningitis. Pediatr Infect Dis J. 2012;31(7):711–716. doi:10.1097/INF.0b013e318253acf8
15. Upton CM, Steele CI, Maartens G, Diacon AH, Wiesner L, Dooley KE. Pharmacokinetics of bedaquiline in cerebrospinal fluid (CSF) in patients with pulmonary tuberculosis (TB). J Antimicrob Chemother. 2022;77(6):1720–1724. doi:10.1093/jac/dkac067
16. Kempker RR, Smith AGC, Avaliani T, et al. Cycloserine and Linezolid for Tuberculosis Meningitis: pharmacokinetic Evidence of Potential Usefulness. Clin Infect Dis. 2021;72(7):1244–1246. doi:10.1093/cid/ciaa877
17. Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis. 2010;90(5):279–292. doi:10.1016/j.tube.2010.07.002
18. Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1–2):22–34. doi:10.1016/j.jchromb.2006.12.030
19. Jang M, Kim J, Han I, Yang W. Simultaneous determination of LSD and 2-oxo-3-hydroxy LSD in hair and urine by LC-MS/MS and its application to forensic cases. J Pharm Biomed Anal. 2015;115:138–143. doi:10.1016/j.jpba.2015.07.001
20. Yamada K, Watanabe A, Takeshita H, Matsumoto KI. A method for quantification of serum tenascin-X by nano-LC/MS/MS. Clin Chim Acta. 2016;459:94–100. doi:10.1016/j.cca.2016.05.022
21. Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6(5):e20077. doi:10.1371/journal.pone.0020077
22. Chin JH. Xpert MTB/RIF Ultra: the long-awaited game changer for tuberculous meningitis? Eur Respir J. 2017;50(4):1701201. doi:10.1183/13993003.01201-2017
23. Cresswell FV, Ssebambulidde K, Grint D, et al. High dose oral and intravenous rifampicin for improved survival from adult tuberculous meningitis: a Phase II open-label randomised controlled trial (the RifT study). Wellcome Open Res. 2018;3:83. doi:10.12688/wellcomeopenres.14691.1
24. George T, Moorthy RK, Rajshekhar V. Long tunnel external ventricular drain: an adjunct in the management of patients with infection associated hydrocephalus. Br J Neurosurg. 2019;33(6):659–663. doi:10.1080/02688697.2019.1667483
25. Zhou YJ, Wu JN, Chen LJ, Zhao HY. Comparison of infection rate with tunneled vs standard external ventricular drainage: a prospective, randomized controlled trial. Clin Neurol Neurosurg. 2019;184:105416. doi:10.1016/j.clineuro.2019.105416
26. WHO Guidelines Approved by the Guidelines Review Committee. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment - Drug-Resistant Tuberculosis Treatment. Geneva: World Health Organization; 2020.
27. DeVincenzo JP, Berning SE, Peloquin CA, Husson RN. Multidrug-resistant tuberculosis meningitis: clinical problems and concentrations of second-line antituberculous medications. Ann Pharmacother. 1999;33(11):1184–1188. doi:10.1345/aph.19008
28. Baron H, Epstein IG, Mulinos MG, Nair KG. Absorption, distribution, and excretion of cycloserine in man. Antibiot Annu. 1955;3:136–140.
29. Ruslami R, Gafar F, Yunivita V, et al. Pharmacokinetics and safety/tolerability of isoniazid, rifampicin and pyrazinamide in children and adolescents treated for tuberculous meningitis. Arch Dis Child. 2022;107(1):70–77. doi:10.1136/archdischild-2020-321426
30. Märtson AG, Burch G, Ghimire S, Alffenaar JC, Peloquin CA. Therapeutic drug monitoring in patients with tuberculosis and concurrent medical problems. Expert Opin Drug Metab Toxicol. 2021;17(1):23–39. doi:10.1080/17425255.2021.1836158
31. Rapid Communication: key Changes to Treatment of Multidrug- and RifampicinResistant Tuberculosis (MDR/RR-TB): world Health Organization. Available from: https://www.who.int/tb/publications/2019/WHO_RapidCommunicationMDRTB2019.pdf?ua=1.
32. Luque S, Grau S, Alvarez-Lerma F, et al. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int J Antimicrob Agents. 2014;44(5):409–415. doi:10.1016/j.ijantimicag.2014.07.001
33. Cabrera-Maqueda JM, Fuentes Rumí L, Valero López G, et al. Difusión de los antibióticos en el sistema nervioso central [Antibiotic diffusion to central nervous system]. Rev Esp Quimioter. 2018;31(1):1–12. doi:10.1007/s10096-017-2973-0. Spanish.
34. Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013;15(2):93–117. doi:10.1007/s40272-013-0017-5
35. Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997;41(12):2670–2679. doi:10.1128/AAC.41.12.2670
36. Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi:10.1128/CMR.00007-10
37. De Lange EC, Hammarlund-Udenaes M. Translational aspects of blood-brain barrier transport and central nervous system effects of drugs: from discovery to patients. Clin Pharmacol Ther. 2015;97(4):380–394. doi:10.1002/cpt.76
38. Te Brake LHM, de Knegt GJ, de Steenwinkel JE, et al. The Role of Efflux Pumps in Tuberculosis Treatment and Their Promise as a Target in Drug Development: unraveling the Black Box. Annu Rev Pharmacol Toxicol. 2018;58:271–291. doi:10.1146/annurev-pharmtox-010617-052438
39. Tucker EW, Guglieri-Lopez B, Ordonez AA, et al. Noninvasive (11) C-rifampinpositron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci Transl Med. 2018;10:470. doi:10.1126/scitranslmed.aau0965
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
