Back to Journals » Journal of Pain Research » Volume 12
Peripheral nerve field stimulation to the preauricular area for intractable chronic migraine: a case report
Authors Li YF , Mao P , Zhu Q, Liu BT, Fan BF
Received 28 November 2018
Accepted for publication 15 March 2019
Published 24 May 2019 Volume 2019:12 Pages 1665—1671
DOI https://doi.org/10.2147/JPR.S196214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Yi-Fan Li,1,2 Peng Mao,2 Qian Zhu,2 Bo-Tao Liu,2 Bi-Fa Fan2
1Department of Graduate School, Beijing University of Chinese Medicine, Beijing 100029, People’s Republic of China; 2Department of Pain, China-Japan Friendship Hospital, Beijing 100029, People’s Republic of China
Objective: To report a successful attempt of peripheral nerve field stimulation (PNFS) in preauricular area to treat refractory chronic migraine (CM). This article focuses on novel utilization of PNFS and discusses its existing issues.
Case report: A 35-year-old woman diagnosed with CM complained about a 2-year history of severe pain at the occipitocervical and left auriculotemporal area, sometimes bilaterally, which did not benefit from conventional medical therapy. After failed attempts of occipital nerve stimulation and PNFS at the retroauricular area, we used exploratory PNFS at the preauricular area, which to our knowledge is the first reported case in literature, to such an area in refractory CM. The patient experienced satisfactory pain relief and obvious improvement in quality of life. And the amelioration on the severity of pain was validated by reduced scores (from 9 to 2) on the numerical rating scale. The clinical effects continued in the next 2-year follow-up after the implantation, without adverse events.
Conclusion: PNFS is a promising and safe neuromodulation therapy for refractory CM. Facial areas like preauricular region are applicable for lead implantation. Nevertheless, the underlying mechanisms are still unverified, and there is still a lack of standardized operation guides of PNFS. Large-scale randomized clinical trials should be conducted to further validate these findings.
Keywords: chronic migraine, neuromodulation, peripheral nerve field stimulation, occipital nerve stimulation, preauricular pain, case report
Introduction
Migraine is a common neurological disease that occurs frequently among young people, with the global morbidity around 12%. It is also ranked third in the burden of disease according to the 2010 global survey.1 About 2.5–3.0% of patients with migraine show an increasing number of attacks per year, indicating a “chronic” trend.2 Chronic migraine (CM) accounts for about 8% of the total number of migraine sufferers, with a morbidity of 1–2%.3,4 Research has shown that compared with episodic migraine patients, the quality of life of the patients with CM was lower; the medical treatment frequency, higher; time of hospitalization, longer; the drug abuse, more obvious; and the economic burden, higher than patients with paroxysmal migraine.5
The treatment for CM is dominated by prophylactic and therapeutic drugs. For intractable CM that does not benefit from drug treatment, multiple multicenter randomized controlled studies have shown that peripheral nerve electrical stimulation (occipital nerve stimulation; ONS) is effective.6–8
Peripheral nerve field stimulation (PNFS), also known as the subcutaneous stimulation (SubQ), is a newly emerging therapy for chronic pain in recent years.9 Unlike spinal cord stimulation (SCS) where leads are placed in the epidural space, leads of PNFS are placed into the subcutaneous tissue in the area of pain, thereby subcutaneously stimulating the peripheral nerves and their endings in the corresponding region to reach the spinal cord through the sensory afferent fibers and achieve analgesic effects.10
PNFS is mainly used for lower back pain, failed spinal surgery, head and face pain, and as an adjuvant therapy to SCS. For craniofacial pain, the location of the electrodes is mostly at the supraorbital, infraorbital, and occipital areas.
To the best of our knowledge, this is the first case report of PNFS applied successfully at the anterior auricular area for the treatment of ONS-ineffective CM. The detailed case report follows.
Clinical material
Case report
A 35-year-old policewoman came to our pain treatment center with the complaint of a 2-year-long history of headache resulting from childbirth. The pain presented as persistent distention and pulsating pain at the occipitocervical region and left auriculotemporal area, sometimes extending to the contralateral area. The pain was aggravated by menstruation and fatigue, and partly relieved by some rest. When the headache was severe, it was often accompanied by nausea and vomiting, without photophobia, phonophobia, or aura. The patient reported visual analog scale (VAS) scores of between 7 and 9. There was no obvious tenderness and hyperalgesia in the head and cervical paravertebral skin. Moreover, no obvious abnormalities were seen in head CT, cervical MRI, electroencephalogram, cervical vascular ultrasound, local cerebral blood flow imaging and so on, so we ruled out some organic lesion causes.
Treatment process
According to clinical symptoms and examinations, we diagnosed this case as CM without aura.11 The patient successively took variety of drugs, such as NSAIDs ibruprofen and celecoxib, flunarizine (maximum dose up to 20 mg/day), zomixetam, antidepressants and so on, which unfortunately were ineffective. Traditional Chinese medicine and acupuncture also did not bring benefits. Selective nerve block and radiofrequency pulse therapy at the occipital nerve, stellate ganglion, and pain point also did not achieve satisfactory clinical effects.
ONS was also performed. The lead (Pisces-Octad® lead, Medtronic Inc., Minneapolis, MN, USA) was punctured into the skin at the posterior occipital region at level C2, and implanted into the left mastoid in a horizontal direction. However, the patient only reported partial relief in the occipitocervical region and no improvement in the auriculotemporal region during the trial period.
Subsequently, we decided to try PNFS therapy at the pain area based on discussions with and the permission of the patient. PNFS procedure: The lead was led upward along the hairline of the left ear, and then to the temporal part through the tip of the ear, which was the patient’s pain area (also known as “pterion”). However, the patient was dissatisfied with the analgesic effect of the procedure in the following 1-week trial period. Hence, we tried another time to remove the lead from the front of the left ear to the upper part of the mandibular joint (Figures 1 and 2).
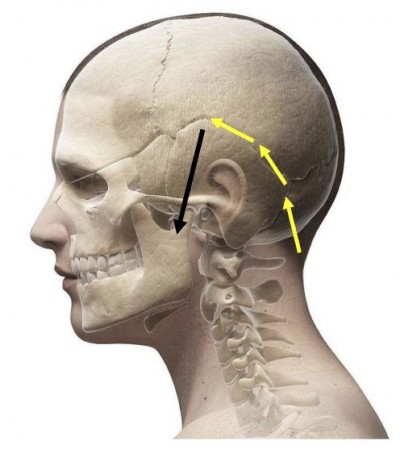
 | Figure 1 Schematic diagram of electrode placement: electrode implantation trajectory (yellow arrow), electrode final position (black arrow). |
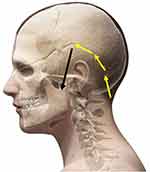
 | Figure 2 Establishment of a subcutaneous tunnel (extending the electrode along the hairline of the left ear, then leading to the temporal part through the tip of the ear). |
During the next 14-day trial period, a satisfactory analgesic effect was achieved. Pain in the temporal and occipital region was significantly relieved, reflected by VAS scores decreased by  50% (parameters: pulse width, 330 μs; frequency, 35 Hz; current, 1.98 mA). Ultimately after the trial, the patient decided to undergo permanent implantation. The stimulator (PrimeAdvanced® neurostimulator, Medtronic Inc.) was placed in the left posterior thoracic-axillary area. Postoperative radiographs confirmed appropriate electrode placement (Figure 3).
50% (parameters: pulse width, 330 μs; frequency, 35 Hz; current, 1.98 mA). Ultimately after the trial, the patient decided to undergo permanent implantation. The stimulator (PrimeAdvanced® neurostimulator, Medtronic Inc.) was placed in the left posterior thoracic-axillary area. Postoperative radiographs confirmed appropriate electrode placement (Figure 3).
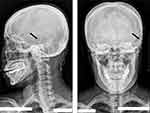 | Figure 3 Postoperative radiograph: peripheral nerve field stimulation (PNFS) in the anterior auricular region showing the position of the electrode (black arrow). |
Follow-up
We conducted follow-up evaluations at 3, 6, 12, 18, and 24 months after PNFS. The patient reported an obvious decrease on the numerical rating scale and continued to have approximately 50% pain relief (Figure 4). Apparent improvements on the onset, duration of the headache, and the impacts on daily life were also observed based on the feedback of the patient. The stimulation mode of IPG changed from continuous stimulation to intermittent mode 6 months after the implantation. The patient only needed stimulation around the time of menstruation. Furthermore, only under extreme circumstances did the patient need to take the adjuvant medications such as ibuprofen. Two years post-implantation, the baseline pain level fluctuated between 0 and 2, and the exacerbations were easily controlled.
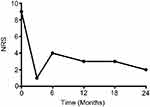 | Figure 4 Numerical rating scale (NRS) scores at 0, 3, 6, 12, 18, and 24 months. |
Discussion
There is quite a lot of research reporting that neuromodulation, such as with SCS and ONS, can be beneficial for refractory CM.12–18 Furthermore, the application and clinical effects of trigeminal nerve stimulation, transcranial magnetic stimulation, vagus nerve stimulation, and cerebellar stimulation, etc., are also under study and being explored.8
Different from SCS where electrodes are placed in the epidural space, and also unlike peripheral nerve stimulation (PNS) where electrodes are placed near a specific peripheral nerve, PNFS therapy does not target specific nerves that dominate the corresponding pain area. Instead, the electrodes are placed in the center of the pain region to stimulate the affected nerve in the area, including the skin sensory afferent nerves and segmental spinal nerve branches.19 PNFS is different from SCS and PNS in terms of therapeutic mechanisms, clinical application, characteristics, key points of operation, and parameter settings.
The mechanism of PNFS in the treatment of CM
At present, the mechanisms of CM are still unclear. It is currently accepted that it may be related to abnormal pain regulation, central sensitization, cortical hyperactivity, and neurogenic inflammatory response. As for the chronicity of migraine, it is a gradual process: recurrent headaches lead to the activation of the trigeminal vascular system and the weakening of the function of the brain stem descending inhibition system, resulting in increased cortical excitability, ultimately resulting in repeated episodes of pain.20
The mechanisms of PNFS treating pain are also unclear. Similar to SCS and PNS, it is believed to be based on the “Gate theory” put forth by Melzack and Wall,21 which suggest that by stimulating the myelinated A fibers, the glial inhibitory interneurons (SG cells) are stimulated, thus closing the gate and inhibiting the activity of the dorsal horn projecting neurons (T cells), and subsequently inhibiting the transmission of nociceptive information to the high center to alleviate pain.
In addition to these effects on the segmental regulation of the spinal cord, some researchers believe that PNFS may also affect the descending pain modulation system of the upper central nervous system by stimulating the subcutaneous electrode, thus exerting an analgesic effect. In addition, some studies have shown that local electrical stimulation can reduce the inflammation of the cutaneous nerve fibers, depolarizing the cell membrane, and reducing the sensitivity of catecholamine in the blood.22–24
Moreover, the possible mechanisms for PNFS also include influencing the excitability of the receptors and tissues in the pain area; affecting the local blood circulation; regulating the level of neurotransmitters; affecting the conduction of the spinal and thalamus pathway; and affecting the sympathetic efferent function.25 Although there is no unified conclusion on the mechanisms of PNFS, most researchers believe that PNFS may impact the endogenous enkephalin level, thus changing the threshold of nociceptive sensation in the target area. Therefore, further research into its basic mechanism is needed.26,27
Perhaps one of the most important reasons for successful PNFS treatment for CM, in this case, is because of the patient’s sufficient trust and active compliance during the treatment period. Regards the underlying mechanism, it may be that the relatively thin muscle fibers in the temporal to anterior auricular area made it easier for the electrode to stimulate the nerve branches in the superficial fascia. The therapeutic target of PNFS is the sensory afferent nerve in the subcutaneous tissue of the pain area. It is well known that the primary neuron of neuralgia and the thermal perception of the head and face are both attributed to the trigeminal ganglion; its anatomical position is the trigeminal nerve track in front of the petrous bone tip of the temporal bone, going through the nucleus of the trigeminal tract, and finally projecting to the dorsal-ventral nucleus of the dorsum thalamus. The location of our electrode is very close to the trigeminal ganglion, which may explain its mechanism of improving pain in the two regions of the temporal and occipital areas.
ONS likely yielded unsatisfactory curative effects because of the long-lasting moderate-to-severe headache induced by the patient’s extended massage to the neck and occipital muscle, which increased the tension of the occipital muscle, and inhibited the sensitivity and excitability of the afferent nerve fiber of the occipital nerve.
Clinical application of PNFS
PNFS is currently used in head and neck or occipital pain, trunk or medial axis pain, and stubborn pain in the limbs. D’Ammando et al28 employed PNFS on 22 patients with chronic refractory neuropathic pain and found that the clinical effect of PNFS was better on craniofacial postherpetic neuropathy (PHN) than on upper limb PHN; and of failed back surgery syndrome (FBSS) patients, pain in lumbosacral area achieved better analgesic effects than in perineal area. Granville et al29 used temporary bilateral sacral PNFS in the treatment of intractable coccyx pain and achieved good results.
Additionally, application of PNFS for head and neck pain or occipital region includes occipital neuralgia, neck headache, migraine, cluster headache, trigeminal neuralgia, and facial PHN. Levi et al30 used PNFS to treat facial neuropathic pain induced by radiation therapy in a patient with laryngeal cancer and achieved satisfactory effects. Verrills et al31 treated 83 patients with chronic headache by using PNFS and followed-up for 3–42 months; 60 of the 83 patients reported satisfactory clinical effects in terms of pain, analgesic usage, function, and mood status. Upadhyay et al32 reported a case of facial PHN treated by PNFS on the supraorbital to temporal region, which turned out to be effective. Klein et al33 reported successful PNFS in the trigeminal innervation area that resulted in excellent clinical outcome in a case of trigeminal neuralgia.
PNFS can be applied alone or as complementary therapy with SCS. Hamm-Faber et al34 used PNFS as complementary therapy with SCS on nine patients with lower back pain, and during the 4-year follow-up, the quality of life and pain intensity of these patients were improved significantly. Ejaa et al35 randomly compared SCS and SCS+PNFS on 52 FBSS patients, and after 12 months of follow-up, patients in the SCS+PNFS group showed superior results than those in the SCS group in terms of VAS, quality of life, anxiety and depression scale, and adjuvant analgesic usage. Zhou et al36 found that thoracic SCS did not achieve satisfactory effects on type I complex regional pain syndromes patients that underwent appendectomy, whereas PNFS attained >90% analgesia.
Advantages of PNFS
Compared with SCS, PNFS can reach areas that SCS cannot, such as the cranial nerve innervation area. Compared with PNS, PNFS can target pain areas that were not innervated by specific nerves. Besides, when the pain area is local, PNFS can accurately stimulate the pain area and is relatively easy to operate, with lesser trauma.9
Operation points of PNFS
The electrode of PNFS is placed in subcutaneous tissue. If the electrode is implanted in a too shallow location such as the dermis, it may cause burning or needling pain. Nevertheless, if the implantation is too deep, such as into deeper tissue, it may not achieve satisfactory treatment effects. If the electrode is implanted into the muscle, it may cause discomfort and affect the patient’s motion. Besides, an unfavorable electrode position may also cause muscle spasm. Currently, there are few studies on precautions to be taken in PNFS operation, which require further standardized key points of operation.37,38
In addition, we found that compared with SCS and PNS, literature reports on PNFS are mainly clinical case reports, and the large sample randomized clinical trials (RCTs) and related basic mechanisms are rarely studied, which provides us with future research direction.
Conclusion
The application of PNFS is very extensive. It is a choice for intractable CM characterized by temporal pain that can achieve ideal clinical effects and is still safe. However, the current circumstances call for advanced related mechanisms study, standardized operation guides, and large sample RCTs to promote the application of PNFS.
To the best of our knowledge, this is the first case to report on PNFS being successfully applied to the preauricular area to treat refractory CM, which was unresponsive to previous ONS treatment. We believe the details of this case report could provide a potential novel approach for the application of PNFS.
Ethics approval and coonsent to participate
This article was permitted by the patient who provided written informed consent. The study was approved by Beijing University of Chinese Medicine and China-Japan Friendship Hospital.
Acknowledgments
the authors would like to express their gratitude to the patient. The authors would also like to thank Prof. Ji-Sheng Han (Neuroscience Research Institute, Peking University, Beijing, PLC) for his advice for the clinical application of PNFS.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
2. Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurol. 2009;72(5 Suppl):3–7. doi:10.1212/WNL.0b013e3181974b19
3. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache J Head Face Pain. 2008;48(8):1157–1168. doi:10.1111/j.1526-4610.2008.01217.x
4. Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep. 2011;15(1):70–78. doi:10.1007/s11916-010-0157-z
5. Lantériminet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31(7):837–850. doi:10.1177/0333102411398400
6. Schwedt TJ. Chronic migraine. BMJ. 2014;348(12):1416. doi:10.1136/bmj.g1416
7. Beran RG. Management of chronic headache. Aus Fam Physician. 2014;43(3):106.
8. Roceanu A, Antochi F, Bajenaru O. Chronic migraine – new treatment options. Maedica. 2014;9(4):401–404.
9. Abejón D, Krames ES. Peripheral nerve stimulation or is it peripheral subcutaneous field stimulation; what’s in a moniker? Neuromodulation. 2009;12(1):1–4. doi:10.1111/j.1525-1403.2009.00192.x
10. Slavin KV, Yin D, Yin H, Gomez C. Subcutaneous peripheral nerve field stimulation for intractable pain. In: Neuromodulation. 2 nd ed. Academic Press; 2018;523–528.
11. Headache Classification Committee of the International Headache Society (IHS),The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
12. Young WB. Occipital nerve stimulation for chronic migraine. Curr Pain Headache Rep. 2014;18(2):396. doi:10.1007/s11916-013-0396-x
13. Perini F, De Boni A. Peripheral neuromodulation in chronic migraine. Neurol Sci. 2012;33(Suppl 1):S29–S31. doi:10.1007/s10072-012-1039-4
14. Mekhail NA, Estemalik E, Azer G, Davis K, Tepper SJ. Safety and efficacy of occipital nerves stimulation for the treatment of chronic migraines: randomized, double-blind, controlled single-center experience. Pain Pract. 2017;17:669–677. doi:10.1111/papr.12504
15. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86. doi:10.1186/1129-2377-14-86
16. De Agostino R, Federspiel B, Cesnulis E, Sandor PS. High-cervical spinal cord stimulation for medically intractable chronic migraine. Neuromodul. 2015;18(4):
17. Arcioni R, Palmisani S, Mercieri M, et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: a prospective, open-label, exploratory study. Eur J Pain. 2016;20(1):70–78. doi:10.1002/ejp.692
18. Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37(7):613–626. doi:10.1177/0333102417704187
19. Barolat G. Subcutaneous peripheral nerve field stimulation for intractable pain. In: Krames E, Peckham PH, Rezai A, editors. Neuromodulation. San Diego (CA): Elsevier; 2009:1017–1020.
20. Schwedt TJ, Larsonprior L, Coalson RS, et al. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014;15(1):154–165. doi:10.1111/pme.12267
21. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971. doi:10.1126/science.150.3699.971
22. Mørch CD, Nguyen GP, Wacnik PW, Andersen OK. Mathematical model of nerve fiber activation during low back peripheral nerve field stimulation: analysis of electrode implant depth. Neuromodulation. 2014;17:218–225. doi:10.1111/ner.12163
23. Reverberi C, Bonezzi C, Demartini L. Peripheral subcutaneous neurostimulation in the management of neuropathic pain: five case reports. Neuromodul. 2009;12:146–155. doi:10.1111/j.1525-1403.2009.00201.x
24. Krutsch JP, McCeney MH, Barolat G, Al Tamimi M, Smolenski A. A case report of subcutaneous peripheral nerve stimulation for the treatment of axial back pain associated with postlaminectomy syndrome. Neuromodulation. 2008;11:112–115. doi:10.1111/j.1525-1403.2008.00151.x
25. Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–1534. doi:10.1001/archneur.60.11.1524
26. Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: an electrophysiological study in healthy volunteers. Neuromodulation. 2005;8:225–232. doi:10.1111/j.1525-1403.2005.00029.x
27. O’Neill CW, Liu JJ, Leibenberg E, et al. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 2004;4:88–98. doi:10.1016/S1529-9430(03)00423-6
28. D’Ammando A, Messina G, Franzini A, Dones I. Peripheral nerve field stimulation for chronic neuropathic pain: a single institution experience. Acta Neurochir (Wien). 2016;158(4):767–772. doi:10.1007/s00701-016-2713-8
29. Granville M, Brennan P, Jacobson RE. Bilateral peripheral nerve field stimulation for intractable coccygeal pain: a case study using dual lead intercommunication. Cureus. 2017;9(11):e1832.
30. Levi V, Messina G, Franzini A, Zanin L, Castelli N, Dones I. Peripheral nerve field stimulation (PNFS) as a treatment opion for intractable radiation-induced facial neuropathic pain in a laryngeal cancer survivor: a case report. World Neurosurg. 2016;91:
31. Paul Verrills MD, Bsc RR, Bruce Mitchell MD, Vivian D, Barnard A. Peripheral nerve field stimulation for chronic headache: 60 cases and long-term follow-up. Neuromodulation. 2014;17(1):54. doi:10.1111/ner.12130
32. Upadhyay SP, Rana SP, Mishra S, Bhatnagar S. Successful treatment of an intractable postherpetic neuralgia (PHN) using peripheral nerve field stimulation (PNFS). Am J Hospice Pall Care. 2010;27(1):59–62. doi:10.1177/1049909109342089
33. Klein J, Sandi-Gahun S, Schackert G, Juratli TA. Peripheral nerve field stimulation for trigeminal neuralgia, trigeminal neuropathic pain, and persistent idiopathic facial pain. Cephalalgia. 2016;36(5):445. doi:10.1177/0333102415597526
34. Hamm‐Faber TE, Aukes H, Gorp E, Gültuna I. Subcutaneous stimulation as an additional therapy to spinal cord stimulation for the treatment of low back pain and leg pain in failed back surgery syndrome: four-year follow-up. Neuromodulation. 2015;18(7):618–622. doi:10.1111/ner.2015.18.issue-7
35. Ejaa VG, Teernstra O, Aukes HJ, et al. Long-term effect of peripheral nerve field stimulation as add-on therapy to spinal cord stimulation to treat low back pain in failed back surgery syndrome patients: a 12-month follow-up of a randomized controlled study. Neuromodulation. 2018. [Epub ahead of print].doi:10.1111/ner.12776
36. Zhou L, Chou H, Holder E. Abdominal wall Type-I complex regional pain syndrome treated effectively with peripheral nerve field stimulation: a case report. J Surg Case Rep. 2017;2017(1):222. doi:10.1093/jscr/rjw222
37. Mcroberts WP. Optimizing stimulation in a case of facial pain through “cross‐talk” of peripheral and central leads: a case report. Neuromodulation. 2016;19:8. doi:10.1111/ner.12443
38. Abejón D, Deer T, Verrills P. Subcutaneous stimulation: how to assess optimal implantation depth. Neuromodulation. 2011;14(4):343–347. doi:10.1111/j.1525-1403.2011.00357.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
