Back to Journals » Journal of Inflammation Research » Volume 14
Peripheral Lymphocyte Subsets Absolute Counts as Feasible Clinical Markers for Predicting Surgical Outcome in Gastric Cancer Patients After Laparoscopic D2 Gastrectomy: A Prospective Cohort Study
Authors Dan Zeng CD, Tong YX, Xiao AT, Gao C, Zhang S
Received 24 August 2021
Accepted for publication 15 October 2021
Published 29 October 2021 Volume 2021:14 Pages 5633—5646
DOI https://doi.org/10.2147/JIR.S335847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Ci Dian Dan Zeng, Yi Xin Tong, Ai Tang Xiao, Chun Gao, Sheng Zhang
Department of Gastrointestinal Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Chun Gao; Sheng Zhang Email [email protected]; [email protected]
Background: Immune function influenced patients’ recovery from major abdominal surgery. The aim of this study is to explore the clinical feasibility of peripheral lymphocyte absolute counts for predicting short-term surgical outcomes in gastric cancer patients after laparoscopic D2 gastrectomy.
Methods: This is a prospective cohort study from a single tertiary referral hospital. Patients diagnosed with gastric cancer who met the inclusion criteria were included in this study. We collected the demographic and clinicopathological characteristics of included patients. We monitored perioperative dynamics of absolute counts of peripheral lymphocyte subsets. Predictive factors for length of postoperative hospital stay and complications were investigated in univariate and multivariate analyses.
Results: A total of 137 gastric cancer patients were included. Decreased preoperative absolute counts of peripheral lymphocyte subsets were correlated with advanced clinical stage. In multivariate analysis, independent predictive factors for prolonged hospital stay were age (p=0.04), decreased preoperative B cell counts (p=0.05), decreased preoperative NK cell counts (p=0.05) and complications (p< 0.01). For postoperative complication, independent predictive factors were age (p=0.02), operation time (p=0.05), lymphocyte to C-reactive protein ratio (p=0.01) and decreased preoperative B cell counts (p=0.01).
Conclusion: Our findings for the first time revealed that absolute counts of peripheral lymphocyte subsets are independent predictive factors for surgical outcomes in gastric cancer patients after D2 gastrectomy. We suggested that patients with impaired immune state should receive both preoperative immune modulator and nutritional support.
Keywords: gastric cancer, absolute counts of peripheral lymphocyte subsets, immune function, surgical outcome, inflammatory cytokines
Introduction
Gastric cancer (GC) is the second most common cancer and a leading cause of cancer death in China, with the incidence rate of 40.3 cases per 100,000 people per year and the death rate of 29.1 cases per 100,000 people per year in 2015.1 Surgery and perioperative chemotherapy were the standard treatment for advanced gastric cancer patients.2–4 Postoperative complications after gastrectomy with lymphadenectomy was associated with heavy morbidity and mortality in gastric cancer patients. A recent large-scale investigation revealed that the incidence of overall complications was 29.8% in gastric cancer patients who underwent gastrectomy.5 Postoperative complications directly result in prolonged hospital stay, increased costs, and may be related to worse overall survival.
Recent advances in surgical technique as the minimal invasive operation and perioperative management as enhanced recovery after surgery (ERAS) have a profound contribution to improved recovery after gastrectomy.6,7 Risk factors such as old age, comorbidities, poor nutrition status, advanced stage, and systemic inflammatory response may be related to postoperative complications and prolonged hospital stay.8–10 Surgeons were investigating more markers to precisely predict the postoperative outcome and improve prognosis.
Systemic inflammatory response (SIR) has a close relationship with postoperative complications and short-term outcomes following surgery for cancer patients. Studies showed that pro-inflammatory markers such as C-reactive protein (CRP), Interleukin-6 (IL-6), lymphocyte to CRP ratio (LCR), neutrophil-to-lymphocyte ratio (NLR), etc were predictive factors for postoperative complications and unfavorable short-term outcome after surgery.11–13 Recent studies have reported that the immunological conditions of patients may be associated with surgical and oncological outcomes in various malignancies.10,14,15 Peripheral lymphocytes including CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells are important mediators of the immune system. They are the key players in innate and adaptive immune response.16 Lymphocyte subsets are correlated with immune surveillance and may be involved in tumorigenesis and predict outcomes in various malignancies.17 In addition, temporary inhibition of lymphocyte subsets after major surgery were reported and this may result in an unfavorable short-term outcome.18 The homeostasis of the immune system was modulated by pro-inflammatory and anti-inflammatory responses mediated by immune cells.19 Therefore, we aim to study the relationship between preoperative peripheral lymphocyte absolute counts and the short-term outcomes in gastric cancer patients.
The aim of this study was to investigate the impact of preoperative lymphocyte subsets on the recovery of gastric cancer patients who underwent laparoscopic gastrectomy with D2 lymphadenectomy. Further, we attempt to explore the perioperative dynamics of peripheral lymphocyte subsets.
Methods
Study Design and Participants
We performed a prospective cohort study in a single institution from April 2020 to April 2021. In total, 137 gastric cancer patients who underwent laparoscopic gastrectomy with D2 lymphadenectomy according to the Japanese Gastric Cancer Treatment Guidelines20 were included in our study. The inclusion criteria were: (1) patients aged from 18 to 75 years old; (2) patients with histopathological confirmed diagnosis of gastric cancer and underwent operation as laparoscopic gastrectomy with D2 lymph node dissection; (3) no previous history of malignancies; (4) no history of neoadjuvant or intraoperative chemotherapy. Exclusion criteria included: (1) Patients only received exploratory laparoscopy; (2) patients with poor general status (Karnofsky score <80, Eastern Cooperative Oncology Group (ECOG) >1 and American Society of Anesthesiologists (ASA) score >2); (3) patients with recent history of severe heart, lung, kidney, or liver failure. This study was approved by the Institutional Medical Ethics Committee (TJH20210109) and registered at Chinese Clinic Trial Registry (ChiCTR-IOR-17014139) with all aspects in this study complying with the 1964 Declaration of Helsinki and later versions. All participants provided written informed consent. This study was conducted and presented in compliance with the strengthening the reporting of cohort studies in surgery (STROCSS) guideline. (Supplemental Table 1).21
Surgical Procedure and Perioperative Management
We performed a comprehensive evaluation in demographic, clinical and nutritional profiles of gastric cancer patients after admission. The standard procedure of laparoscopic gastrectomy with D2 lymph node dissection in our institution was summarized and published previously.22,23 In brief, left gastroepiploic vessels (LGEVs), right gastroepiploic vessels (RGEVs), left gastric vein and artery, right gastric vessels (RGVs) were dissected, exposed, and ligated. D2 dissection of lymph node stations were performed according to Japanese Gastric Cancer Treatment Guidelines.20 Gastric mesenteries were dissected and separated from mesocolon. Omentectomy was performed in each patient and dissection of No. 14v lymph node was optional. Reconstruction of digestive tract was done by extracorporeal anastomosis as Billroth II or Roux-en-Y fashion, determined by surgeon’s experience.
In postoperative management, gastrointestinal function including digestive symptoms, time to first flatus and defecation was evaluated twice per day. The gastric tube was removed after first flatus. The patients started to intake a liquid diet and gradually transited into soft diet. Other details of perioperative ERAS program in this study were listed in Supplemental Table 2.
Data Collection and Flow Cytometry for Lymphocyte Subsets
Peripheral blood samples were collected immediately after admission and on postoperative day three and day seven. Peripheral blood mononuclear cells (PBMS) were isolated as previously report.24 Flow cytometry were performed to analyze the lymphocyte subpopulation. In brief, CD3+/CD4+/CD8+ T cell, CD19+ B cell, and CD16+CD56+NK cell counts (cells/μL) were measured by multiple-color flow cytometry with monoclonal anti-CD3-FITC, anti-CD4-APC, anti-CD8-PE, anti-CD19-APC, anti-CD16-PE, and anti-CD56-PE antibodies (BD Multitest) according to the manufacturer’s instructions. Flow cytometry (BD Biosciences) was used to detect the labeled cells and analyze the results.
The primary endpoint of this study was postoperative hospital stay and complication. To optimally separate patients into normal group and delayed recovery group, we applied 75% percentile (11 days) as a reference for definition of prolonged hospital stay. We prospectively collected the following demographic and clinical data for analysis: (1) demographic characteristics such as age, gender, body mass index (BMI), smoking, alcohol, comorbidities; (2) laboratory characteristics such as absolute lymphocyte count (ALC), hemoglobin, albumin, prealbumin, serum tumor markers as carcinoembryonic antigen (CEA); (3) clinical characteristics such as invasion depth (T), presence of lymph node metastases (N), tumor node metastasis stage (TNM); (4) postoperative outcomes such as first flatus, first defecation, length of hospital stay, postoperative in-hospital complication (according to Clavien–Dindo criteria).25.
Statistical Analyses
We present continuous variables as mean (standard deviation, SD)/medians (range) and analyzed with Student’s t-test or Mann–Whitney U-test. We present categorical variables were reported as whole numbers and percentages and compared using chi-squared test or Fisher’s exact test. The cutoff value for T, B and NK lymphocytes were determined by receiver operating characteristic (ROC) curve. The univariate logistic regression was used to evaluate potential predictive factors for prolonged hospital stay and postoperative complications. Only factors with p-value <0.1 in univariate analysis were included in the final multivariate analysis model. Multivariate logistic regression was employed to identify independent predictive factors for prolonged hospital stay and postoperative complications. All p-values were reported as two-sided with a significance level of 0.05. All statistical tests were performed in SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) and graphing were performed by GraphPad Prism version 8.00 software.
Results
Demographic and Clinical Characteristics of Included Patients
Between June 2020 and June 2021, 155 gastric cancer patients who underwent laparoscopic gastrectomy with D2 lymph node dissection were initially selected for this study. After screening based on inclusion and exclusion criteria, 137 gastric cancer patients were included in final study. The flow chart of patients’ inclusion and exclusion was shown in Supplemental Figure 1. Demographic and clinical parameters were summarized in Table 1. In general, the mean age of included patients was 58.0±11.5 comprising 86 (62.8%) men and 51 (37.2%) women. 23 (16.8%), 41 (29.9%), 63 (46.0%) and 10 (7.3%) patients were with stage I, II, III, and IV diseases respectively. Ten stage IV gastric cancer patients were all diagnosed with a single resectable liver metastatic lesion. They received combined gastrectomy and curative resection of liver lesion. On hundred and two (74.5%) patients underwent laparoscopic distal gastrectomy, and 25 (18.2%) patients underwent laparoscopic total gastrectomy. The median time to first flatus and defecation after surgery were 60 hours (48, 72 h) and 72 hours (48, 84 h) respectively. The median duration of postoperative hospital stay was nine days (IQR: 8–11). The details of postoperative length of hospital stay were list in Supplemental Table 3. Twenty (14.6%) patients suffered from Grade 1 and 2 postoperative complications and one (0.7%) patient suffered from Grade 3 postoperative complication. The details of postoperative complications were listed in Supplemental Table 4. We noted that patients with complications had decreased in peripheral T, B and NK cell absolute counts. However, the differences did not reach statistical significance (p >0.05).
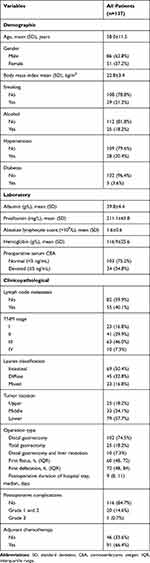 |
Table 1 The Demographic and Laboratory Characteristics of All Patients (n=137) |
Correlation Between Peripheral Lymphocyte Subsets and Clinicopathological Features
We explored the correlation between lymphocyte subsets and clinicopathological features. We discovered that peripheral lymphocyte subsets were correlated with age and nutritional status. The preoperative T cell, CD3+CD4+, CD3+CD8+, B cell count were significantly decreased in patients ≥60 years. Decreased T cell, CD3+CD4+, CD3+CD8+, B cell count were also significantly correlated with decreased serum albumin (<35 g/L) and decreased serum prealbumin (<200 mg/L) (Figure 1). Compared with patients with advanced stage (stage II, III, and IV), stage I gastric cancer had significant higher preoperative T cell, CD3+CD4+, CD3+CD8+, B cell and NK cell count (p <0.05) (Figure 2). We also found that T, B and NK cell counts have a positive correlation to serum interleukin-1 (IL-1) level. T cells absolute counts had a weak negative correlation between systemic cytokines such as serum IL-2R, IL-6, IL-8, TNF-A, C-reactive protein (CRP) and T cells. (Supplemental Figure 2).
 |
Figure 1 Correlation between absolute counts of peripheral lymphocyte subsets and gender (A), age (B), serum albumin level (C) and serum prealbumin level (D). *Represents significant differences. |
Perioperative Dynamic Change of Peripheral Lymphocyte Subsets
We monitored the perioperative dynamic change of peripheral lymphocyte subsets in 137 included gastric cancer patients. We showed that absolute count of peripheral T cell, CD3+CD4+, CD3+CD8+, B cell and NK cell declined after surgery on postoperative day three (POD3) and showed a slow growing trend on POD6 (Figure 3). We further divided the patients according to postoperative duration of hospital stay (>11 days, prolonged or ≤11 days, non-prolonged) and complications (with or without). On admission, absolute count of peripheral T cell, B cell and NK cell were significantly lower in prolonged hospital stay group compared to normal group. Patients in the prolonged hospital stay group showed a significantly lower T cell and B cell count on POD3 and NK cell count on POD6 compared to the normal group (Figure 4). Significant decreased T cell and B cell count were found in patients with postoperative complications on POD6 compared to patients without complications. However, we did not find significant difference in NK cell count between patients with/without complications (Figure 4).
 |
Figure 3 Perioperative dynamic change of T cell absolute count (A), B cell absolute count (B) and NK cell count (C) in gastric cancer patients. |
Absolute Count of Peripheral Lymphocyte Subsets and Postoperative Recovery
The rate of postoperative complication the average duration of hospital stay was correlated to preoperative peripheral T cell, B cell and NK cell count. The average duration of hospital stay and complication rate in patients with normal peripheral T, B and NK cell count were eight days and 7.9% respectively. The duration of hospital stay was significantly longer in patients with decreased T, B or NK cell count (Figure 5). We divided patients into prolonged (postoperative hospital stay >11 days) and non-prolonged (postoperative hospital stay ≤11 days) groups. Patients in the prolonged hospital stay group were significantly older (p=0.092) and with higher rate of complication (p <0.001). NLR was also significantly higher in prolonged group. In addition, patients in the prolonged group also demonstrated significant decreased peripheral T (p=0.06), B (p=0.001), and NK (p=0.027) cell count (Table 2). The complication rate was 12.2%, 22.2%, and 50.0% in patients with one, two, and three (both T, B, and NK cell) decreased (Figure 5). Details of postoperative complications were listed in Supplemental Table 4. We noted that patients with complications had decreased in peripheral T, B, and NK cell absolute counts. However, the differences did not reach statistical significance (p >0.05).
 |
Table 2 The Comparison Between Patients with Prolonged Hospital Stay and Control Group (n=137) |
Predictive Factors Associated with Postoperative Recovery
Predictive factors for postoperative prolonged hospital stay and complication were identified from univariate analysis (Table 3). The results revealed that age (≥60 years vs <60 years, hazard ratio, HR=3.47, p=0.02), decreased serum prealbumin (<190 mg/L, HR=2.51, p=0.05), peripheral lymphocyte count (p=0.03), NLR (p=0.01), operation time (>240 min vs ≤240 min, HR=2.60, p=0.05), decreased peripheral T cell count (≤720/µL, HR=3.37, p=0.01), decreased peripheral B cell count (≤90/µL, HR=7.19, p<0.01), decreased peripheral NK cell count (≤160/µL, HR=4.56, p<0.01) and complication (no vs yes, HR=38.9, p<0.01) were related to prolonged hospital stay. Age (≥60 years vs <60 years, HR=2.46, p=0.07), decreased serum prealbumin (<190 mg/L, HR=2.26, p=0.09), decreased hemoglobin (p=0.10), NLR (p=0.02), LCR (p=0.04), operation time (>240 min vs ≤240 min, HR=3.08, p=0.03), decreased peripheral T cell count (≤720/µL, HR=3.75, p<0.01), decreased peripheral B cell count (≤90/µL, HR=4.67, p<0.01), decreased peripheral NK cell count (≤160/µL, HR=2.48, p=0.05) were significantly related to postoperative complication. (Table 3).
 |
Table 3 Univariate and Multivariate Logistic Regression Analyses of Factors Associated with Prolonged Hospital Stay and Complications After Operation (n=137) |
Moreover, multivariate logistic regression analysis showed that age (≥60 years), peripheral B cell count (≤90/µL), peripheral NK cell count (≤160/µL) and postoperative complication were identified as independent predictive factors associated with prolonged hospital stay, with the HR of 4.34 (p=0.04, 95%CI 1.04–18.20), 3.61 (p=0.05, 95%CI 1.01–13.08), 4.28 (p=0.05, 95%CI 1.0–18.41), 29.0 (p<0.01, 95%CI 7.06–119.1) respectively. Age (≥60 years), LCR, operation time (>240 min) and peripheral B cell count (≤90/µL) were identified as independent predictive factors associated with postoperative complication, with the HR of 4.24 (p=0.02, 95%CI 1.26–14.3), 1.24 (p=0.01, 95%CI 1.08–1.43), 3.23 (p=0.05, 95%CI 1.0–10.5) and 7.05 (p=0.01, 95%CI 2.05–24.2), respectively. The details of univariate and multivariate analyses were listed in Table 3.
Discussion
Immune function influenced the short-term and long-term outcome in patients with gastric cancer. In this prospective cohort study from a single institution, we investigated the correlation between absolute counts of peripheral lymphocyte subsets and postoperative recovery and complications. We found that age, decreased peripheral B cell and NK cell count, and postoperative complications were independent risk factors of delayed recovery after laparoscopic gastrectomy in gastric cancer patients (Table 3).
Curative surgery was the standard treatment for advanced gastric cancer. However, gastrectomy is generally considered to be high risk with a relatively high rate of postoperative complications varying from 5% to 30%.5,26,27 Efforts, such as minimal invasive surgical technique and multimodal ERAS program, have been made to improve short-term outcome in gastric cancer patient underwent operation. Compared to open gastrectomy, laparoscopic gastrectomy had an advantage in decreased complications and enhanced postoperative recovery.27,28 In addition, preoperative malnutrition was associated with prolonged postoperative length of stay and postoperative complication after gastrectomy. Perioperative nutritional support may have a positive impact on postoperative recovery.29,30 Besides, the present study for the first time showed that preoperative decreased peripheral T, B or NK cell counts may associate with postoperative recovery and complication after laparoscopic gastrectomy. Immune function may be further impaired postoperatively in patients with delayed recovery and postoperative complications (Figure 4). Given the above findings, an immune modulator such as thymosin alpha 1 (Tα1) may be a potential treatment for gastric cancer patients with immune suppression and enhance postoperative recovery.31
Previous studies suggested that peripheral lymphocyte subpopulations may correlate with clinicopathological characteristics and prognosis in various malignancies. Reports revealed that peripheral T and B cell counts were significantly reduced in advanced stage patients compared to early stage or healthy individuals.32,33 The predictive value of peripheral NK cell count in cancer patients was controversial. Studies of colorectal cancer and non-small-cell lung cancer showed that decreased peripheral NK cell count was an independent risk factor for poor prognosis.33–35 However, Gong et al found that high baseline absolute count of peripheral NK cells was associated with a shorter survival in pancreatic neuroendocrine tumor (panNET) patients, indicating the potential multiply role of NK cells in carcinogenesis and cancer development.36 In the present study, we found that stage I gastric cancer had significant higher T cells, B cell, CD4+ and CD8+ cell counts than stage II, stage III, & IV patients. (Figure 2) However, NK cells count has no significant difference based on clinical stages (data not shown). Interestingly, we also observed that total T cells, CD4+ cells, CD8+ cells and B cell counts were negatively correlated with age, serum albumin and prealbumin level, suggesting immune function may closely relate to nutritional condition (Figure 1).
The interaction of immunity and systemic inflammation plays a pivotal role in tumorigenesis and cancer development.37,38 Various markers according to inflammatory and immune status such as neutrophil to lymphocyte ratio (NLR), CRP to albumin ratio, lymphocyte to C-reactive protein ratio (LCR) have been developed to predict short-term and long-term outcome in patients with cancer.39–41 Recent studies also showed that preoperative inflammatory markers can effectively predict postoperative complications such as pneumonia and anastomotic leakage in gastric cancer patients after gastrectomy.42,43 Moreover, a scoring system combined multifactors including inflammatory and nutritional showed promising role in predicting prognosis in gastric cancer. Hirahara et al found that the systemic immune-inflammation index (SII), integrated by peripheral lymphocyte, neutrophil, and platelet counts, was an independent prognostic factor for overall survival in gastric cancer patients.44 A recent study from Wang et al constructed a novel inflammatory-nutritional prognostic score (INPS) based on BMI, prealbumin, NLR, PLR, LMR, and PNI. The authors proved that INPS score has a good predicting performance for overall survival in stage III gastric cancer patients with radical surgery followed by adjuvant chemotherapy.45 In our study, our results also showed that NLR was significantly associated with prolonged hospital stay and complication in univariate analysis. However, this association did not reach statistical significance in multivariate analysis. Moreover, we found that LCR (lymphocyte to CRP ratio) was significantly associated with postoperative complication (p=0.01). Absolute count of peripheral lymphocyte was an indicator of host immunity. Previous studies have shown that preoperative absolute lymphocyte count was a predictor for oncological outcome in various malignancies.46,47 We explored the relationship between absolute counts of peripheral lymphocyte subsets and serum inflammatory cytokines. We found that T, B and NK cell counts were positively correlated to serum interleukin-1 (IL-1) level. This may be reasonable since IL-1 was a crucial mediator for T, B and NK cell proliferation and activation.48 It has been substantially reported that T cells may expose to persistent antigen signals in cancer. Higher level of serum inflammatory cytokine may be an indicator for potential higher tumor burden. This might be associated with a profound deterioration of T cell function and an exhaustion of T cell numbers.49,50 Furthermore, in our study B cell and NK cell count showed no significant correlation with inflammatory cytokines.
Clinical Implication and Limitations
This study provided novel observations and clinical feasibility of peripheral lymphocyte subset absolute counts in gastric cancer patients after laparoscopic gastrectomy with D2 lymphadenectomy. We discovered that preoperative absolute counts of peripheral lymphocyte subsets were predictive factors for postoperative recovery. Monitoring of lymphocyte subset counts might help physicians to identify patients with impaired immune function who might require perioperative immune and nutritional support.
We acknowledge that the present study has several limitations. First, the primary endpoints of this study were duration of hospital stay and postoperative complications. Various factors may have impacts on traditional clinical outcomes such as as hospital stay and complications. These outcomes may not precisely reflect patients’ recovery from surgery. In addition, varying from 10 to 17 days, there is no standard definition of prolonged hospital stay in gastric cancer patients after gastrectomy.51–53 We defined the prolonged hospital stay as 75% percentile (>11 days). However, lack of a common definition made it difficult to compare our findings with other studies. In addition, the correlation between counts of peripheral lymphocyte subsets and functional outcomes or long-term prognosis in gastric cancer patients was not clear. Second, the sample size of our study is relatively small and from a single institution. Selection bias may affect the credibility of this study. Third, the cutoff value for absolute count of lymphocyte subsets may vary according to different detection methods and included populations. To overcome these hurdles, prospective trials with a larger sample size and more clinical-pathological measurements are needed to validate the findings of our study. According to clinical guidelines for the diagnosis and treatment of gastric cancer from The Chinese Society of Clinical Oncology (CSCO),54 stage II and III gastric cancer patients suitable for surgery were recommended for D2 gastrectomy plus adjuvant chemotherapy (Evidence 1A). However, preoperative chemotherapy might affect the count of lymphocyte subsets, but it would improve long-term outcome in locally advanced gastric cancer.55 Studies with longer observation may also be valuable to explore the dynamic fluctuation and prognostic role of lymphocyte subsets in gastric cancer patients.
Conclusions
In summary, our study for the first time indicated that absolute counts of peripheral lymphocyte subsets are significantly associated with postoperative recovery in gastric cancer patients who underwent laparoscopic D2 gastrectomy. Second, peripheral lymphocyte subset absolute counts can be easily measured and assist physicians to identify patients with impaired immune function for perioperative immune modulator and nutritional support.
Data Sharing Statement
The database used and/or analyzed during the current study is not publicly available (to maintain privacy) but can be available from the corresponding author on reasonable request. Original data can be assessed by contacting corresponding author Sheng Zhang (Email: [email protected]).
Ethical Approval
Ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Acknowledgment
We thank Ms Cheng Chen for English grammatic correction of this manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Chinese Society of Clinical Oncology (no. Y-sy2018-029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun. 2020;40(5):205–210. doi:10.1002/cac2.12025
2. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252
3. Fuse N, Bando H, Chin K, et al. Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a Phase II study. Gastric Cancer. 2017;20(2):332–340. doi:10.1007/s10120-016-0606-4
4. Ajani JA, D’Amico TA, Bentrem DJ, et al. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 1.2020. Accessed October 30, 2020
5. Baiocchi GL, Giacopuzzi S, Reim D, Piessen G. Incidence and Grading of Complications After Gastrectomy for Cancer Using the GASTRODATA Registry: a European Retrospective Observational Study. Ann Surg. 2020;272(5):807–813. doi:10.1097/SLA.0000000000004341
6. Aoyama T, Yoshikawa T, Sato T, et al. Equivalent feasibility and safety of perioperative care by ERAS in open and laparoscopy-assisted distal gastrectomy for gastric cancer: a single-institution ancillary study using the patient cohort enrolled in the JCOG0912 Phase III trial. Gastric Cancer. 2019;22(3):617–623. doi:10.1007/s10120-018-0873-3
7. Kang SH, Lee Y, Min SH, et al. Multimodal Enhanced Recovery After Surgery (ERAS) Program is the Optimal Perioperative Care in Patients Undergoing Totally Laparoscopic Distal Gastrectomy for Gastric Cancer: a Prospective, Randomized, Clinical Trial. Gastric Cancer Ann Surg Oncol. 2018;25(11):3231–3238. doi:10.1245/s10434-018-6625-0
8. Coimbra FJF, de Jesus VHF, Franco CP, et al. Predicting overall and major postoperative morbidity in gastric cancer patients. J Surg Oncol. 2019;120(8):1371–1378. doi:10.1002/jso.25743
9. Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol. 2015;22(Suppl 3):S778–85. doi:10.1245/s10434-015-4820-9
10. Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg Today. 2020;50(1):3–11. doi:10.1007/s00595-019-01877-8
11. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23(9):2832–2840. doi:10.1245/s10434-016-5204-5
12. Plas M, Rutgers A, van der Wal-huisman H, et al. The association between the inflammatory response to surgery and postoperative complications in older patients with cancer; a prospective prognostic factor study. J Geriatr Oncol. 2020;11(5):873–879. doi:10.1016/j.jgo.2020.01.013
13. Bora Makal G, Yıldırım O. Are the C-reactive protein/albumin ratio (CAR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (NLR) novel inflammatory biomarkers in the early diagnosis of postoperative complications after laparoscopic sleeve gastrectomy? Obes Res Clin Pract. 2020;14(5):467–472. doi:10.1016/j.orcp.2020.07.003
14. Adiamah A, Skořepa P, Weimann A, Lobo DN. The Impact of Preoperative Immune Modulating Nutrition on Outcomes in Patients Undergoing Surgery for Gastrointestinal Cancer: a Systematic Review and Meta-analysis. Ann Surg. 2019;270(2):247–256. doi:10.1097/SLA.0000000000003256
15. Schwegler I, Von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92–97. doi:10.1002/bjs.6805
16. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
17. Milne K, Alexander C, Webb JR. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor infiltrating lymphocytes. J Transl Med. 2012;10:33. doi:10.1186/1479-5876-10-33
18. Vittimberga FJ
19. Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg Nutr. 2014;3(6):386–406.
20. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;142:113–123. doi:10.1007/s10120-011-0042-4
21. Agha RA, Borrelli MR, Vella-Baldacchino M, Thavayogan R, Orgill DP; STROCSS Group. The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int J Surg. 2017;46:198–202. doi:10.1016/j.ijsu.2017.08.586
22. Xie D, Yu C, Liu L, et al. Short-term outcomes of laparoscopic D2 lymphadenectomy with complete mesogastrium excision for advanced gastric cancer. Surg Endosc. 2016;30:5138–5139. doi:10.1007/s00464-016-4847-4
23. Xie D, Wang Y, Shen J, Hu J, Yin P, Gong J. Detection of carcinoembryonic antigen in peritoneal fluid of patients undergoing laparoscopic distal gastrectomy with complete mesogastric excision. Br J Surg. 2018;105:1471–1479. doi:10.1002/bjs.10881
24. Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983;220:868–871. doi:10.1126/science.6189183
25. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
26. Nicole van der W, Straatman J, Cuesta MA, Daams F, Donald L. Short-term outcomes in minimally invasive versus open gastrectomy: the differences between East and West. A systematic review of the literature. Gastric Cancer. 2018;21(1):19–30. doi:10.1007/s10120-017-0747-0
27. Inokuchi M, Nakagawa M, Tanioka T, Okuno K, Gokita K, Kojima K. Long- and short-term outcomes of laparoscopic gastrectomy versus open gastrectomy in patients with clinically and pathological locally advanced gastric cancer: a propensity-score matching analysis. Surg Endosc. 2018;32(2):735–742. doi:10.1007/s00464-017-5730-7
28. Lee HJ, Hyung WJ, Yang HK, et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270(6):983–991. doi:10.1097/SLA.0000000000003217
29. Fukuta A, Saito T, Murata S, et al. Impact of preoperative cachexia on postoperative length of stay in elderly patients with gastrointestinal cancer. Nutrition. 2019;58:65–68. doi:10.1016/j.nut.2018.06.022
30. Gharagozlian S, Mala T, Brekke HK, Kolbjørnsen LC, Åa U, Johnson E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer - A cross-sectional pilot study. Clin Nutr ESPEN. 2020;37:195–201. doi:10.1016/j.clnesp.2020.03.001
31. King R, Tuthill C. Immune Modulation with Thymosin Alpha 1 Treatment. Vitam Horm. 2016;102:151–178.
32. Fernandez SV, MacFarlane AW, Jillab M, et al. Immune phenotype of patients with stage IV metastatic inflammatory breast cancer. Breast Cancer Res. 2020;22(1):134. doi:10.1186/s13058-020-01371-x
33. Xia Y, Li W, Li Y, et al. The clinical value of the changes of peripheral lymphocyte subsets absolute counts in patients with non-small cell lung cancer. Transl Oncol. 2020;13(12):100849. doi:10.1016/j.tranon.2020.100849
34. Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–2328. doi:10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P
35. Tang YP, Xie MZ, Li KZ, Li JL, Cai ZM, Hu BL. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020;20(1):31. doi:10.1186/s12876-020-1177-8
36. Gong Y, Fan Z, Luo G, et al. Absolute counts of peripheral lymphocyte subsets correlate with the progression-free survival and metastatic status of pancreatic neuroendocrine tumour patients. Cancer Manag Res. 2020;12:6727–6737. doi:10.2147/CMAR.S257492
37. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073e81. doi:10.1093/carcin/bgp127
38. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/S1470-2045(14)70263-3
39. Saito H, Kono Y, Murakami Y, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric cancer patients. World J Surg. 2018;42(6):1819e25. doi:10.1007/s00268-017-4400-1
40. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44(5):607–612. doi:10.1016/j.ejso.2018.02.003
41. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2020;39(4):1209–1217. doi:10.1016/j.clnu.2019.05.009
42. Shi J, Wu Z, Wu X, et al. Early Diagnosis of Anastomotic Leakage After Gastric Cancer Surgery Via Analysis of Inflammatory Factors in Abdominal Drainage [published online ahead of print, 2021 Sep 22]. Ann Surg Oncol. 2021. doi:10.1245/s10434-021-10763-y
43. Mori M, Shuto K, Hirano A, et al. Preoperative Neutrophil-to-Lymphocyte Ratio may Predict Postoperative Pneumonia in Stage I-III Gastric Cancer Patients After Curative Gastrectomy: a Retrospective Study. World J Surg. 2021;45(11):3359–3369. doi:10.1007/s00268-021-06264-4
44. Hirahara N, Tajima Y, Matsubara T, et al. Systemic Immune-Inflammation Index Predicts Overall Survival in Patients with Gastric Cancer: a Propensity Score-Matched Analysis. J Gastrointest Surg. 2021;25(5):1124–1133. doi:10.1007/s11605-020-04710-7
45. Wang N, Xi W, Lu S, et al. A Novel Inflammatory-Nutritional Prognostic Scoring System for Stage III Gastric Cancer Patients With Radical Gastrectomy Followed by Adjuvant Chemotherapy. Front Oncol. 2021;11:650562. doi:10.3389/fonc.2021.650562
46. Zhang J, Huang SH, Li H, et al. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol. 2013;30(1):352. doi:10.1007/s12032-012-0352-3
47. Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB. 2007;9(6):456e60.
48. Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi:10.1038/nri2691
49. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862
50. Park JW, Chang HJ, Yeo HY, et al. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020;123(4):610–618. doi:10.1038/s41416-020-0924-5
51. Zhuang CL, Wang SL, Huang DD, et al. Risk factors for hospital readmission after radical gastrectomy for gastric cancer: a prospective study. PLoS One. 2015;10(4):e0125572. doi:10.1371/journal.pone.0125572
52. Guner A, Kim SY, Yu JE, et al. Parameters for Predicting Surgical Outcomes for Gastric Cancer Patients: simple Is Better Than Complex. Ann Surg Oncol. 2018;25(11):3239–3247. doi:10.1245/s10434-018-6684-2
53. Liu ZJ, Ge XL, Ai SC, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroenterol. 2017;23(27):4978–4985. doi:10.3748/wjg.v23.i27.4978
54. Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41(8):747–795. doi:10.1002/cac2.12193
55. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase 2/3 trial. Lancet. 2019;393(10184):1948–1957.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



