Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Pembrolizumab in Lymphopenic Metastatic Breast Cancer Patients Treated with Metronomic Cyclophosphamide: A Clinical and Translational Prospective Study
Authors Mery B , Ménétrier-Caux C, Montané L, Heudel PE, Ray-Coquard I, Bachelot T, Derbel O, Augereau P, Treilleux I, Berthet J, Nkodia A, Bardin-Dit-Courageot C, Attignon V, Ferrari A, Garin G, Perol D, Caux C, Dubois B, Trédan O
Received 2 December 2022
Accepted for publication 6 April 2023
Published 27 April 2023 Volume 2023:15 Pages 311—325
DOI https://doi.org/10.2147/BCTT.S400055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Benoîte Mery,1,2,* Christine Ménétrier-Caux,2,3,* Laure Montané,4 Pierre-Etienne Heudel,1 Isabelle Ray-Coquard,1 Thomas Bachelot,1 Olfa Derbel,5 Paule Augereau,6 Isabelle Treilleux,2,7 Justine Berthet,2,3 Axelle Nkodia,3 Christine Bardin-Dit-Courageot,3 Valery Attignon,8 Anthony Ferrari,9 Gwenaele Garin,4 David Perol,4 Christophe Caux,2,3 Bertrand Dubois,2,3,* Olivier Trédan1,2,*
1Department of Medical Oncology, Centre Léon Bérard, Lyon, France; 2Inserm U1052, CNRS 5286, Cancer Research Center of Lyon, Université Claude Bernard Lyon 1, Lyon, France; 3Laboratory of Cancer Immunotherapy of LYON (LICL), Centre Léon Bérard, Lyon, France; 4Clinical Research Platform (DRCI), Centre Léon Bérard, Lyon, France; 5Department of Medical Oncology, Hôpital Privé Jean-Mermoz, Lyon, France; 6Department of Medical Oncology, Institut de Cancérologie de L’ouest- Paul Papin, Angers, France; 7Biopathology Department, Centre Léon Bérard, Lyon, France; 8Genomic of Cancer Platform, Centre Léon Bérard, Lyon, France; 9Gilles Thomas Bioinformatics Platform, Synergie Lyon Cancer Foundation, Centre Léon Bérard, Lyon, France
*These authors contributed equally to this work
Correspondence: Benoîte Mery, Department of Medical Oncology, Centre Léon Bérard, 28 Rue Laennec, Lyon, 69008, France, Tel +33 4 78 78 26 44, Fax +33 4 78 78 27 15, Email [email protected]
Purpose: Metastatic endocrine-resistant breast cancer (MBC) is a disease with poor prognosis and few treatment options. Low lymphocyte count is associated with limited overall survival. In a prospective cohort of lymphopenic patients with HER-2 negative MBC, we assessed the clinical and biological impact of pembrolizumab combined with metronomic cyclophosphamide.
Experimental Design: This multicenter Phase II study evaluated the safety and clinical activity of pembrolizumab (intravenous (IV), 200mg, every 3 weeks) combined with metronomic cyclophosphamide (50mg/day, per os) in lymphopenic adult patients with HER2-negative MBC previously treated by at least one line of chemotherapy in this setting according to a Simon’s minimax two-stage design. Blood and tumor samples were collected to assess the impact of the combined treatment on circulating immune cells and the tumor immune microenvironment through multiparametric flow cytometry and multiplex immunofluorescence analyses. Primary endpoint was the clinical benefit rate at 6 months of treatment (CBR-6M). Secondary endpoints were objective response rate (ORR), duration of response, progression free survival (PFS), and overall survival (OS).
Results: Two out of the twenty treated patients presented clinical benefit (one Tumor Mutational Burden (TMB)-high patient with complete response (CR) and one patient with objective response (OR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST V1.1) associated with a strong increase of cytokine-producing and proliferating CD4+ T cells and higher CD8+ T cells to macrophage ratios in the tumor. This impact on CD4+ and CD8+ T cell polyfunctionality was still observed more than one year for the patient with CR. A decreased in their absolute number of CD4+ and CD8+ memory T cells was observed in other patients.
Conclusion: Pembrolizumab combined with metronomic cyclophosphamide was well tolerated, and displayed limited anti-tumoral activity in lymphopenic MBC. Correlative translational data of our trial advocates for additional studies with other chemotherapy combinations.
Keywords: metastatic breast cancer, lymphopenia, immunotherapy, chemotherapy, immunomonitoring
Introduction
Chemotherapy remains the cornerstone of first-line therapy in the metastatic setting for the triple-negative breast cancer (TNBC) subtype,1 as well as for endocrine-therapy resistant luminal breast cancers. The need to improve MBC patients’ outcomes has led to novel therapeutic approaches, including immune checkpoint inhibitors (ICPi) that have changed the treatment landscape for other solid tumors.2 If MBC is not historically considered as an immunogenic tumor, clinical studies have highlighted the prognostic value of tumor infiltrating lymphocytes (TILs). Higher TILs infiltration and higher PD-L1 expression have been reported in TNBC compared to hormone receptor (HR)-positive breast cancers.3,4 Such rationale led to clinical trials with monoclonal antibodies blocking the PD-1/PD-L1 axis, which however revealed low efficacy. This highlights the need to enhance the sensitivity to PD-1 blockade, notably through action on the tumor immune microenvironment.5,6 In the KEYNOTE-355 study, the combination of pembrolizumab (anti-PD-1) plus chemotherapy significantly improved PFS in comparison to chemotherapy plus placebo. This improvement was marked in the group of patients with PD-L1-positive tumors (CPS score ≥10), with median progression-free survival (PFS) of 9.7 months with pembrolizumab, compared to 5.6 months with placebo (hazard ratio [HR] for progression: 0.65, 95% CI 0.49–0.86; one-sided p=0.0012).7 In parallel, pembrolizumab has demonstrated promising results in early-stage TNBC.8
Cyclophosphamide, one of the most widely used alkylating agents in the treatment of solid malignancies, has potent cytotoxic and lympho-ablative activity at high dose, whereas it has immuno-stimulatory and anti-angiogenic effects at low doses with metronomic protocols. It may induce i) a marked inhibition of tumor angiogenesis by compromising the endothelial cell repair system, ii) immuno-stimulatory effects through the induction of a shift from Th2 toward Th1 and Th17 (increase of IFNγ and IL-17 secretion, decrease of IL-10 and TGFβ),9 iii) a quantitative depletion and functional alteration of regulatory T (Treg) cells associated with an enhanced T cell proliferation.10
Lymphopenia occurring in more than 20% of patients with advanced solid tumors, is an independent risk factor for disease progression, early death and may be a marker for immune dysfunction.11–13 Here, we report the prospective clinical and translational data of lymphopenic patients with HER-2 negative MBC treated with cyclophosphamide in combination with pembrolizumab.
Methods
Study Design and Participants
Chemoimmune (NCT03139851) was an open-label, single arm, multicenter, phase II study conducted according to a Simon’s minimax two stage design to evaluate the safety (safety run-in) and clinical activity of pembrolizumab combined with metronomic cyclophosphamide in lymphopenic patients with HER2-negative MBC. Eligible patients were women, ≥18-year-old, with histologically confirmed HER2-negative MBC regardless of PD-L1 expression of the tumor for whom one to three prior regimens of chemotherapy have failed. Key other eligibility criteria included adequate performance status according to Eastern Cooperative Oncology Group (PS ECOG) of 0 to 2, documented lymphopenia (lymphocyte count<1.5G/L within 15 days before treatment start), measurable disease as per RECIST V1.1 and adequate hematologic, renal, and hepatic functions. Patients were non-eligible if they had any contra-indication to pembrolizumab or cyclophosphamide, active infection or auto-immune disease, history of pneumonitis or evidence of interstitial lung disease or had received prior ICPi. This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization-and Good Clinical Practice. All patients provided written informed consent and ethics approval for this study was approved by the Ethics Committee of the Leon Berard Cancer Center.
Procedures
Eligible patients received combination of pembrolizumab given at 200mg intravenously every 3 weeks (Q3W) in combination with cyclophosphamide at 50mg per day orally. Both treatments were administered until symptomatic deterioration attributed to disease progression with integrated assessment of radiographic data, unacceptable toxicity, investigator decision to discontinue or withdrawal of patient consent. Adverse events were graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 during the treatment period and up to 90 days after the last dose of study drugs. Patients were monitored weekly during cycle 1 then every 3 weeks with safety lab tests and clinical exams.
Tumor lesions were assessed according to RECIST version 1.1 at baseline (within 4 weeks prior to cycle 1 day 1), every 6 weeks for the first 24 weeks, then every 12 weeks thereafter until disease progression or the start of another treatment. Following treatment discontinuation, survival data were collected approximately every 3 months, until death or loss to follow-up. Blood and tumor samples were collected at baseline and cycle 3 day 1 to assess the impact of combination on circulating immune cells and tumor microenvironment and to identify potential biomarkers.
Outcomes
For the safety run-in, the primary endpoint was the number of Severe Toxicities (ST) occurring during the first 6 weeks of treatment, considered by the Investigator to be related to study drugs and graded according to NCI-CTC-AE v4.03. ST included any Grade ≥ 3 non-hematological toxicity with interest for transaminase ASAT/ALAT increases, Type 1 diabetes mellitus (if new onset) or hyperglycemia, pneumonitis, renal failure or nephritis and any Grade ≥ 4 hematological and non-hematological adverse effects (AE) including in particular diarrhea and colitis, hypophysitis, hyperthyroidism, neutropenia only if the duration > 14 days and febrile neutropenia. At the end of the safety run-in, if ≤ 1/6 patients experienced a ST: the safety data will be considered acceptable and enrolment will continue.
Primary endpoint for the Phase II part was CBR defined as the proportion of patients with CR, partial response (PR) or prolonged stable disease (SD) at 6 months. Secondary endpoints were ORR at 6 months (ORR-6m), duration of response, PFS and OS.
Multiplex Immunofluorescence Tumor Sample Staining and Multispectral Imaging
Tumor biopsies were collected at baseline and at day 1 of cycle 3 and in case of disease progression. These samples were analyzed to characterize the impact of pembrolizumab combined with cyclophosphamide on the tumor microenvironment and to identify potential predictive biomarkers of clinical benefit. Formaldehyde-fixed and paraffin-embedded (FFPE) tumor specimens were tested for PD-L1 expression by immunohistochemistry (Monoclonal Mouse Anti-PD-L1, Clone 22C3).14 Tissues with a PD-L1 Combined Positive Score (CPS) ≥ 10 were considered positive. The CPS is defined as the number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) divided by total number of tumor cells multiplied by 100.15
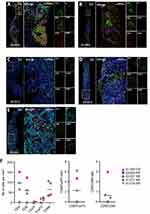
Fully automated seven-colors multiplex immuno-fluorescence (multi-IF) was performed with the OPAL system (Akoya Biosciences) using the BOND RX stainer (Leica Biosystems) for patients with available FFPE tumor tissue. Tumor sections (4µM) were deparaffinized, rehydrated and antigen retrieval treatment was performed using ER1 (for CD20, FOXP3, CD68 and CK) or ER2 (for CD4 and CD8) buffer. The sections were sequentially stained with each primary antibody, followed by OPAL-HRP secondary antibody incubation and then revealed with tyramide signal amplification and OPAL fluorophore, as follows: CD4 (clone EP204; Sigma Aldrich; 1/30 dilution)/OPAL 520; CD8 (clone C8/4B11; DAKO; 1/40 dilution)/OPAL 570; CD20 (clone L26; DAKO; 1/500 dilution)/OPAL 690; FOXP3 (clone D608R; Cell Signaling; 1/50 dilution)/OPAL 620; Cytokeratin (clone AE1/AE3; DAKO; 1/500 dilution)/OPAL 480 and CD68 (clone PG-M1; DAKO; 1/200 dilution)/OPAL 780. The sections were then counterstained with spectral DAPI (Akoya Biosciences) and mounted with a coverslip. Whole slides were imaged at a 40x magnification using the Vectra Polaris multispectral scanner (Akoya Biosciences) and digital images were visualized with the Phenochart viewer (Akoya Biosciences) and unmixed using the spectral library from the software. Data for 5 patients (2 patients with OR) and 3 patients in the PD group) with lymph node samples collected during the study are presented to illustrate the immune infiltrate. A representative hotspot area was chosen for each tumor sample, cells of each phenotype were manually counted and their density (number of cells by mm2) was calculated by dividing the number of cells by the surface of tissue.
Blood Sample Analysis
The impact of the therapy on immune cell subpopulations in blood was assessed by multiparametric flow cytometry analyses of fresh whole blood to investigate the modulations within the T cell subpopulations, their activation status (PD-1, ICOS) and their capacity to proliferate (Ki67). To investigate the impact of the treatment on peripheral immune cell functionality, patients’ whole blood samples were short-term (6h) in vitro stimulated with PMA/ionomycin to evaluate the capacity of T cell subsets to produce cytokines.
Whole Exome Sequencing Analysis
Whole exome was captured from 200 ng of tumor from FFPE tumor and paired constitutional DNA and libraries were prepared using Agilent SureSelect XTHS2 All Exon V8 (35 Mb). Libraries were sequenced of subsequent libraries was performed using the NovaSeq6000 Illumina sequencer in 100 bp paired-end.
Demultiplexing was made with Illumina’s software bcl2fastq-v2-20-0. Raw sequence fastq files from normal et tumoral samples were aligned to human reference genome GRCh38.p13 with bwa-mem-v0.7.17. Duplicates were then marked with biobambam-v2.0.89. Coverage distributions were computed with mosdepth-v0.2.9.
Somatic point mutations and small indels (<50bp) were called using Mutect2 from GATK-v4.1.2.0. A panel of normal samples to remove systematic sequencing artifacts was used. Cells were subsequently annotated with ensembl-vep-v98.3 using its internal annotation cache.
Tumor mutation burden was computed on CDS regions only (34,780,817 bp length) taking only HIGH and MODERATE impact variations from ensembl-vep annotations (and thus ignoring synonymous variations) with an allelic frequency greater than 0.1.
MSI status was computed using MSIsensor-v0.6. This tool computes both length distributions of microsatellites from the tumor and its matched normal data and then compare these distributions to infer putative instability of each site. We used a list of 2932 curated sites and a tumor was considered as MSI if the fraction of unstable sites was > 20%.
Statistical Analysis
The primary hypothesis was that the combination of pembrolizumab and cyclophosphamide would increase the CBR-6M from 20% to 40%. The sample size was calculated according to a Simon minimax two-stage design. Assuming a unilateral type I error rate of 5% and a power of 80%, enrolment of 33 evaluable patients was planned with 18 patients in stage 1 (expecting 5 non-progression as a minimum to carry on the enrolment in stage 2) and 22 patients in stage 2. A cut-off of 11 out of 33 evaluable patients not experiencing progression at 6 weeks was required to accept that further trial should be undertaken. Efficacy and safety analyses were planned for patients who received at least one dose of the combined treatment. Efficacy analysis included patients enrolled in the safety run-in phase.
The CBR-6M and ORR-6M were presented by percentages with 95% CIs. PFS and OS were analysed using the Kaplan–Meier method to estimate the median values with the corresponding 95% CIs. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). An Independent Data Monitoring Committee (IDMC) was established to review interim analyses as well to provide recommendations to the sponsor.
Results
Patients
Twenty patients from four French institutions were enrolled between September 2017 and June 2018. At the end of the safety run, 1/6 patient experienced a ST (a case of Grade 4 lymphopenia). According to defined stopping rules and following validation by the IDMC, the combination was considered safe and 14 additional patients were enrolled (Figure 1). At the end of Simon’s Stage 1, the recruitment was suspended according to stopping rules.
Patients’ characteristics at baseline are summarized in Table 1. Median age at inclusion was 58.5 years [38.0– 76.0]. Nineteen (19/20, [95%]) were ECOG PS 0 or 1. Eight patients (40%) had TNBC and twelve patients (60%) had HR-positive breast cancer. Median time between first metastases and inclusion was 2.2 years [0.5– 9.7]. Liver and bone were the main metastatic sites.
 |
Table 1 Patients’ Characteristics at Baseline |
Treatment and Responses
The median treatment duration with pembrolizumab and cyclophosphamide combination was 2.8 months, median number of pembrolizumab cycles was 4 [1–27]. All the patients discontinued study treatment, mainly due to disease progression (90%), one patient discontinued on her own decision, and one patient died on treatment. Treatment responses are presented in Table 2. Median follow-up was 21.7 months [17-23]. At the end of the stage I, the CBR-6M was 10% (2/20 patients, LCL=1.8%) with one CR and one long-lasting SD. The minimal number of successes required to proceed to Stage 2 according to the Simon design (ie ≥5 patients with a CBR-6m among 18 evaluable patients) was not reached and the study enrolment was prematurely ended. The ORR-6M was 5% [0.1%; 24.9%]. The best responses were as follows: CR n=1 (ER-, PR+), PR n= 1 (TNBC), SD n=7, PD n=10. For the 2 patients with OR, the duration of response was, respectively, 2.8 months and 21.7 months. A swimmer plot is presented in Figure 2. Median PFS and OS were 1.5 months [1.4–4.6] and 9.6 months [4.3–21.5], respectively. All deaths were due to disease progression.
 |
Table 2 Treatment and Responses |
The only CR was obtained after 2 months of treatment and no evidence of disease progression was still observed in June 2021, 3 years after inclusion (despite patient decision to discontinue treatment after almost 2 years of treatment). When entering in the trial, this patient suffering from an ER-, PR+ and MSS, had histologically proven lymph node recurrence (cervical, sub-clavicular and infra-clavicular lymph node metastasis) 3 years after adjuvant chemotherapy followed by breast and supraclavicular lymph nodes radiotherapy.
Safety
All patients experienced at least one AE: 14 patients (70%) had at least one event related to pembrolizumab and 16 patients (80%) had at least one event related to cyclophosphamide (Supplementary Table 1). At the end of the safety run, one Grade 4 lymphopenia was reported as a ST.
Ninety-five percent of patients (19/20) experienced at least an AE Grade ≥ 2 including 25% of patients (5/20 patients) with at least one AE related to pembrolizumab and 65% of patients (13/20) patients at least one AE related to cyclophosphamide. The main (≥10%) Grade ≥ 2 AE related to the combination were lymphocytes count decrease, fatigue and PAL increase. Sixty percent (12/20) of patients experienced at least one AE Grade ≥3; 15% of them was related to pembrolizumab and 40% related to cyclophosphamide. Three related serious AE were reported including one Grade 3 abdominal pain, one Grade 2 Infusion-related reactions, and one Grade 3 pneumopathy (Supplementary Table 1). No immune-related AE were reported.
Evaluation of PD-L1 Expression, TMB and Immune Infiltration in Tumor Samples by IHC and Multispectral if Tissue Imaging
Biological data are presented according to the best response to treatment with patients with OR (CR+PR, OR group), SD (SD group) and patients with PD (PD group). Among the 2 patients with OR, the patient with long-term CR (01–005) had PD-L1 CPS <10 and the patient with PR (02–003) had CPS >10. All patients in the PD group were CPS <10. Moreover, the WES analysis performed on several patients identified the long-term CR (01–005) as TMB-H (TMB: 12.43 mutations/Mb). Unfortunately, no information was available for the patient with PR (02–003) due to lack of material. All SD/PD patients tested (n=9/17) except one (01–018, 14.68 mutations/Mb) were diagnosed as TMB-L.
Figure 3A–3E showed multiplex IF stainings for 5 patients for which lymph node metastatic tissue was available for analysis (2 patients with OR and 3 patients with PD). Although the small size of the cohort did not allow to draw any firm conclusion nor perform statistics, it is noteworthy that the 2 patients with OR (01–005 and 02–003) displayed at baseline a high immune cell infiltration with more CD4+ and CD8+ T cells and a higher CD8+ T cell to macrophage (CD68+ cells) ratio compared to PD patients (Figure 3F). Furthermore, one of the 2 patients (02–003) also presented a strong B cell infiltrate (Figure 3A), not seen in any other patient biopsies.
Overall, the 2 patients with OR presented either TMB or PDL1high and a high CD8/CD68 ratio.
Blood T Cell Phenotype and Function
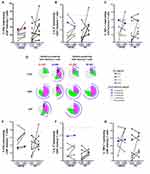
At inclusion, all patients presented lymphopenia (Supplementary Figure S1A) affecting both CD4+ and CD8+ T cells (Supplementary Figure S1B and S1C), including naive and memory T cell, as well as CD19+ B cells (Supplementary Figure S1D, S1E and S1F), whereas the FOXP3+ Treg cell population was not significantly altered (Supplementary Figure S1G). Although not statistically significant, the absolute numbers of total CD4+ and CD8+ T cells and of memory CD4+ and CD8+ T cells was higher in the group of patients with OR/SD compared to the group of patients with PD (Supplementary Figure S1B and S1C). Of note, the patient with long-term CR (01–005, red triangle) presented initially a drastic global lymphopenia.
Under treatment (C3D1), most patients presented a decrease in the absolute number of CD4+ and CD8+ memory T cells (Figure 4A and 4B), as well as a strong decrease in the number of B cells (Figure 4C). In contrary, the patient with long-term CR (01–005) presented an increase in the number of CD4+ and CD8+ memory T cells. In addition, a decrease in the percentage of PD-1 expression in CD4+ and CD8+ memory T cells was noticed for the majority of patients (Figure 4D and 4E). However, patient with long-term CR displayed an increased percentage of PD-1 expression in CD4+ memory T cells. ICOS was positively modulated in the majority of patients (Figure 4F), while, in contrast, Ki67 was overall not modulated (Figure 4G and 4H). However, the patient with PR (02–003, blue square) presented at inclusion and on treatment high level of Ki67 CD4+ and CD8+ memory T cells.
In terms of T cell functionality, in the majority of patients, CD4+ memory T cells were functionally altered with a reduced capacity to secrete IFNγ (Figure 5A), IL-2 (Figure 5B) and TNFα (Figure 5C) compared to healthy donors (dotted line).16 Patient with CR (01–005, red triangle) was the only patient with a strong increase in the absolute number of memory CD4+ T cells (Figure 4A), resulting in an absolute increase of IFNγ producing CD4+ memory T cells (8-fold versus 0.7 to 1.5 in the other patients) (Supplementary Figure S2), as well as of polyfunctional memory CD4+ T cells (co-secreting IFNγ, IL-2 ± TNFα= 50%) (Figure 5D).
For CD8+ memory T cells, we observed an overall increase in the percentage of cytokine producing cells for most patients in both groups. This upregulation was consistent for IFNγ (Figure 5E) and IL-2 (Figure 5F), but less clear for TNFα (Figure 5G), resulting in an overall increase in polyfunctional CD8+ memory T cells (Figure 5D).
For patient with CR (01–05, red triangle), the capacity of CD8+ memory T cells to secrete IFNγ (Figure 5E) but also IL-2 (Figure 5F) was not modulated by treatment, whereas TNFα secretion capacity was highly increased (6-fold, Figure 5G). Although not unique to this patient, this also led to an increased frequency of polyfunctional memory CD8+ T cells (Figure 5D). This impact on CD4+ and CD8+ T cell polyfunctionality was observed throughout the treatment and even more pronounced after more than one year of treatment (EOT) for patient with CR (Figure 5D).
In summary, in the 2 patients with either a complete or partial response, we observed features, unique to each patient, but both affecting the CD4+ T cell compartment, involving either a strong increase in polyfunctionality possibly related to the high TMB status of this patient favoring the generation of neoepitopes, or a high proliferation level from time of inclusion and through treatment.
Discussion
We investigated the anti-tumor and biological activity of pembrolizumab in combination with metronomic cyclophosphamide in HER-2 negative MBC patients regardless of PDL1 expression in the tumor presenting with low lymphocyte count (<1.5 G/L within 15 days before cyclophosphamide initiation). Lymphopenia is a marker for immune dysfunction11 and an independent risk factor for disease progression and early death.12,13 Consistent with our previous studies, the median OS of this current study population was less than 10 months [4.3–21.5]. Two out of the 20 treated patients have benefited from the treatment combination and appeared to display specific immune features. One out of the 2 responding patients (01–005, CR) was eligible for WES and was TMB-H. TMB has been previously reported to correlate with neoantigen load and response to anti-PD1/PDL1 treatment17,18 and may account for the priming/activation of T cells in this patient.
Quantitative and qualitative improvement have been observed with an increased number of CD4+ and CD8+ memory T cells associated with an enhanced polyfunctionality assessed by cytokine production.
The use of anti-PD-1 in MBC treatment has recently received much greater attention and especially for the TNBC subtype. Promising data have been recently released concerning the use of pembrolizumab in combination with chemotherapy both in first-line treatment for metastatic TNBC and in the neo-adjuvant setting. In pivotal Phase III Keynote-355 study, the combination of pembrolizumab plus chemotherapy significantly improved PFS in comparison to chemotherapy alone, among patients whose tumors were PD-L1 positive.18 In parallel, the combination of pembrolizumab and chemotherapy in neo-adjuvant setting (Keynote 522 trial) has provided a larger magnitude of pathological complete response (pCR) versus chemotherapy alone in patients with stage III and/or node positive TNBC (pCR rate: 64.8% with the pembrolizumab/chemotherapy regimen compared to 51.2% with placebo plus chemotherapy, with notably a higher rate of RCB 0–1.19
However, few MBC patients will benefit from PD-1/PD-L1 blockade with response rates around 5%.11,14 In order to enhance anticancer immune responses, previous studies have suggested the use of low-dose chemotherapy or irradiation.
Beyond these early settings, few MBC patients will benefit from PD-1/PD-L1 blockade, especially in the subpopulation of patients with aggressive disease who have biomarkers of reduced survival, such as lymphopenia.20,21 In order to enhance anticancer immune responses, previous studies have suggested the use of low-dose chemotherapy or irradiation.
We hypothesized that the use of low-dose chemotherapy would help to enhance the efficacy of ICPi. Indeed, peculiar properties of cyclophosphamide have been demonstrated with the ability to restore effector functions of T cells and natural killer cells, especially at low doses with a metronomic schedule.22 Among the previous clinical trials investigating ICPi treatment in combination with chemotherapy, the TONIC trial recently highlighted that the best responses were observed following doxorubicin and cisplatin induction (ORR in the doxorubicin: 35%, N = 17 and cisplatin group: 23%, N = 13), whereas lower responses were associated to cyclophosphamide induction (ORR: 8%, N = 12).23 Based on these results, only doxorubicin and cisplatin treatment induction have been selected in the phase II of the trial. However, the optimal treatment sequence to turn cold tumors into hot tumors is still debated. Consequently, cyclophosphamide may not be the best drug to enhance immunomodulation. Moreover, the duration of the treatment itself and the optimal sequence to get responses remain to be determined.
Furthermore, most of the current trials that evaluate ICP blockade are focused on TNBC, excluding HR-positive breast cancers. Our study encompassed both tumor subtypes and this has been a double edge-sword as patients with TNBC and HR-positive breast cancers constitute a heterogeneous population regarding the tumor microenvironment. Subsequently, this study draws additional attention to the need of patient’s selection, especially from an immunologic point of view. Indeed, it may be more relevant to focus on PDL1 positive breast cancers, as defined accurately by immunohistochemistry score, instead of considering the usual breast cancer subtypes nosology.
We have analyzed PD-L1 expression as well as T cell parameters at the tumor site and in the blood. All the patients from the PD group had CPS <10. This observation may be linked to i) the difficulty to capture PD-L1 expression within heterogeneous biopsies, ii) to PD-L1 expression modification upon previous chemotherapy treatments or iii) to pembrolizumab action in tumor-draining lymph nodes.
Both CR/PR patients had unique specific features, affecting the blood CD4+ T cell compartment and manifesting either as a strong increase of the proportion of polyfunctional cytokine-producing cells or a high proliferation level at inclusion and during treatment24,25 that could be related to the high TMB or PDL1 status. Furthermore, both patients presented a high CD4+ and CD8+ T cell infiltrate and an elevated CD8/macrophages ratio in the tumor at the time of inclusion. Recent studies have highlighted that higher baseline blood levels of CD4+ T cells or B cells and higher densities of tumor-infiltrating CD8+ T cells both constitute potential predictive biomarkers of efficacy for combinational therapeutic strategies involving immunotherapy in advanced TNBC.26 In particular, it has been recently shown that higher level of CD8+ T cells was the best predictive marker of response to the combination of camrelizumab (anti-PD-1) plus chemotherapy in TNBC, with response rates higher than 80%.27 In our analyses, we showed that, in the majority of patients, CD4+ memory T cells were functionally altered with a reduced capacity to secrete IFNγ and TNFα, an observation in line with our previous published data.16
This association between response to treatment and modulation of T cell functions described in our study is in line with previous reports.28,29 In particular, a recent publication reported early (day 7) changes occurring in blood of patients treated with anti-PD1, such as an increase in the percentage of Ki-67+ cells among PD-1+CD8+ T cells in thymic epithelial tumors and lung carcinoma that significantly predicted durable clinical benefit.30
Finally, within the limits of this small-sized phase II study, the combination of pembrolizumab with metronomic cyclophosphamide provides reassurance with regard to safety data. Lymphopenic MBC patients do not benefit from this combination in terms of CBR or survival. However, specific tumor and blood immune features select patients who may respond to immunotherapy-based treatment. Prospective, randomized clinical trials are sorely warranted to definitively answer which population of patients benefit from such combination beyond the tumor expression of PD-L1.
Translational Relevance
In this multicenter Phase II study, the combination of pembrolizumab with metronomic cyclophosphamide in lymphopenic patients with HER-2 negative MBC did not enhance the efficacy of PD-1 blockade. However, the two patients who benefited from treatment had immune characteristics in both tumor (a high preexisting CD8 T cell to macrophage ratio possibly related to a high TMB) and blood (high T cell proliferative capacity or increased T cell polyfunctionality under treatment) that could help in the future to predict patients who will respond to combination of chemotherapy and PD1 blockade. Despite that combination of pembrolizumab with metronomic cyclophosphamide demonstrated no sufficient anti-tumoral activity, larger studies are required to evaluate other chemotherapies to combine with anti-PD1 in order to enhance the response rate.
Data Sharing Statement
The data sets generated during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization-and Good Clinical Practice. All patients provided written informed consent and ethics approval for this study was approved by the Ethics Committee of the Leon Berard Cancer Center. Consent to publish was also approved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was an investigator-initiated trial (no grant number is applicable). CLB was the legal sponsor. MSD provided the investigational agent and funding, but had no role in the study design, data collection, analysis, interpretation, writing of the report, or decision to publish this report. The database is held by CLB, and CLB statisticians carried out the analysis. Research grants were perceived from “INCa” (PRT-K INCA 2009-113 (BreastImmun), Transla13-097 (Camel), Transla13-061 (TNBT)), “Lyrican” (LYRICAN INCa-DGOS-Inserm_12563), “Ruban Rose” for the blood immuno-monitoring and “Région Rhône-Alpes” (IRICE project) for the development of multiplex immunofluorescence staining.
Disclosure
Benoîte Mery and Christine Ménétrier-Caux are co-first authors for this study. Bertrand Dubois and Olivier Trédan are co-last authors for this study. Dr Pierre-Etienne Heudel reports personal fees, non-financial support from PFIZER, GILEAD, NOVARTIS, SEAGEN, LILLY; personal fees from MSD, during the conduct of the study. Professor Isabelle Ray-Coquard reports grants, personal fees from MSD and BMS; personal fees from Astra ZENECA, GSK, CLOVIS, ROCHE, DAICHI, MERSANA, Immunogen, Pharmamar, during the conduct of the study. Dr Thomas Bachelot reports grants, personal fees, non-financial support from Novartis, AstraZeneca, Daiichi, Pfizer; grants, personal fees from SeaGen, outside the submitted work. Dr Paule Augereau reports personal fees, non-financial support from AstraZeneca, GSK, Seagen; personal fees from Novartis, outside the submitted work. Dr David Perol reports personal fees, travel funding from ROCHE, personal fees from TAKEDA, ASTRAZENECA, BAYER, BOEHRINGHER-INGELHEIM, BRISTOL-MYERS SQUIBB, DAIICHI-SANKYO, ELI-LILLY, IPSEN, NOVARTIS, MERCK SHARP AND DOHME, JANSSEN, PFIZER, outside the submitted work. Dr Olivier Trédan reports grants, personal fees from MSD, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Eng J Med. 2010;363:1938–1948. doi:10.1056/NEJMra1001389
2. Kelly PN. The Cancer Immunotherapy Revolution. Science. 2018;359:1344–1345. doi:10.1126/science.359.6382.1344
3. van Rooijen JM, Stutvoet TS, Schröder CP, de Vries EGE. Immunotherapeutic options on the horizon in breast cancer treatment. Pharmacol Ther. 2015;156:90–101. doi:10.1016/j.pharmthera.2015.09.003
4. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi:10.1158/2326-6066.CIR-13-0127
5. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167:671–686. doi:10.1007/s10549-017-4537-5
6. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(74):74. doi:10.1001/jamaoncol.2018.4224
7. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Am Soc Clin Oncol. 2020;38:1000. doi:10.1200/JCO.2020.38.15_suppl.1000
8. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Annal Oncol. 2019;30:397–404. doi:10.1093/annonc/mdy517
9. Sistigu A, Viaud S, Chaput N, et al. Immunomodulatory Effects of Cyclophosphamide and Implementations for Vaccine Design. Springer; 2011. doi:10.1007/s00281-011-0245-0
10. Ghiringhelli F, Menard C, Puig PE, et al. Related papers Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25 + regulatory T cells and restores T and NK eVector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi:10.1007/s00262-006-0225-8
11. Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immuno Ther Cancer. 2019;7(85). doi:10.1186/s40425-019-0549-5
12. Trédan O, Ray-Coquard I, Chvetzoff G, et al. Validation of prognostic scores for survival in cancer patients beyond first-line therapy. BMC Cancer. 2011;11. doi:10.1186/1471-2407-11-95
13. Manuel M, Tredan O, Bachelot T, et al. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology. 2012;1(432):432–440. doi:10.4161/onci.19545
14. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, Phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi:10.1016/S0140-6736(20)32531-9
15. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi:10.5858/arpa.2018-0043-OA
16. Verronèse E, Delgado A, Valladeau-Guilemond J, et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology. 2015;5. doi:10.1080/2162402X.2015.1100791
17. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi:10.1126/science.aaa1348
18. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi:10.1016/j.cell.2014.12.033
19. Cortés J, Guo Z, Karantza V, Aktan G. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). Annals of Oncology. Available from: https://www.annalsofoncology.org/article/S0923-7534(19)56089-6/fulltext.
20. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi:10.1158/0008-5472.CAN-08-3845
21. Winer EP, Lipatov O, Im S-A, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511. doi:10.1016/S1470-2045(20)30754-3
22. Scurr M, Pembroke T, Bloom A, et al. Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clin Cancer Res. 2017;23:6771–6780. doi:10.1158/1078-0432.CCR-17-0895
23. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi:10.1038/s41591-019-0432-4
24. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi:10.1038/nature13954
25. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Catherine Sautès-Fridman. 1922;577(25):549–555.
26. Dieci MV, Miglietta F, Guarneri V. Immune infiltrates in breast cancer: recent updates and clinical implications. Cells. 2021;10:223. doi:10.3390/cells10020223
27. Liu J, Li Y, Li Q, et al. Biomarkers of response to camrelizumab combined with apatinib: an analysis from a phase II trial in advanced triple-negative breast cancer patients. Breast Cancer Res Treat. 2021;186:687–697. doi:10.1007/s10549-021-06128-4
28. Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. doi:10.1073/pnas.1705327114
29. Huang A, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. J Nat Disasters. 2017;26:60. doi:10.1038/nature22079
30. Kim KH, Cho J, Ku BM, et al. The first-week proliferative response of peripheral blood PD-1+CD8+ T cells predicts the response to Anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25:2144–2154. doi:10.1158/1078-0432.CCR-18-1449
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.